-
PDF
- Split View
-
Views
-
Cite
Cite
Sittinun Thangjui, Jakrin Kewcharoen, Ratdanai Yodsuwan, Angkawipa Trongtorsak, Harshith Thyagaturu, Bishesh Shrestha, Amanda R M Winans, Edward Bischof, Efficacy and safety of direct oral anticoagulant in morbidly obese patients with atrial fibrillation: systematic review and meta-analysis, European Heart Journal - Cardiovascular Pharmacotherapy, Volume 8, Issue 4, July 2022, Pages 325–335, https://doi.org/10.1093/ehjcvp/pvab026
Close - Share Icon Share
Abstract
We conducted a systematic review and meta-analysis on three outcomes. We assessed the efficacy and safety of direct oral anticoagulants (DOAC) compared to vitamin K antagonists (VKA) in morbidly obese patients with atrial fibrillation (AF). We compared the efficacy and safety of DOAC in obese patients and non-obese patients with AF. Finally, we updated the current knowledge of outcomes of AF patients with obesity compared with normal-weight patients regardless of anticoagulation type.
Using PubMed and Embase, we searched for literature published from inception to August 2020 for studies conducted in morbidly obese patients with AF who used DOACs and/or VKA for stroke or systemic embolism (stroke/SE) prevention that report efficacy and/or safety data. GRADE assessment was performed to determine the quality of the meta-analysis results. Direct oral anticoagulant was not statistically different from VKA in reducing stroke/SE with relative risk (RR) of 0.85 [95% confidence interval (CI): 0.56–1.29; very low certainty evidence]. Major bleeding risk was lower in the DOAC groups with RR of 0.62 (95% CI: 0.48–0.80; low certainty evidence). Obese patients with AF who used DOACs had lower risk of stroke/SE and similar major bleeding risk compared to non-obese patients with RR of 0.77 (95% CI: 0.70–0.84; low certainty evidence) and 1.02 (95% CI: 0.94–1.09; low certainty evidence), respectively. Obese patients with AF who used any type of anticoagulant had lower risk of stroke/SE compared to normal-weight patients with RR of 0.62 (95% CI: 0.57–0.69; low certainty evidence).
The use of DOACs in morbidly obese patients may be reasonable if needed, and more dedicated studies are needed to make a more robust recommendation.
Introduction
Direct oral anticoagulants (DOAC) continue to emerge as an alternative option to vitamin K antagonists (VKA) for systemic and venous thromboembolism prevention and treatment.1 After the US Food and Drug Administration (FDA) approved DOAC for stroke and systemic embolism (SE) prevention in patients with non-valvular AF, several guidelines, such as the 2019 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation and the 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS) recommended DOAC as a first-line therapy for stroke/SE prevention.2–6 These recommendations are based on major randomized controlled studies done on each DOAC compared with VKA.7–10 Direct oral anticoagulant offers a more favourable safety profile, faster onset, and more reliable therapeutic effect without the need for regular monitoring and fewer drug interactions.11,12 However, questions about safety and efficacy were raised in patients with morbid obesity due to the lack of supportive data and therapeutic drug level monitoring.13 Also, recent meta-analysis of DOAC trials suggested obese patients with AF had lower risk of SE and bleeding compared to normal-weight patients.14 This contradicts the pharmacodynamics (PK) and pharmacokinetics (PD) studies done in obese patients with DOAC, which suggested these patients may have suboptimal drug concentrations.15
Despite the increasing evidence of this medication class, data in patients with body mass index (BMI) >40 kg/m2 from both pharmacokinetic and clinical studies is limited.16 Moreover, the majority of the landmark DOAC trials underrepresented patients with low (<60 kg) or high (>120 kg) body weight.17 Because of this, the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis (SSC of the ISTH) in 2016 suggested that DOAC should not be used in patients with a BMI of >40 kg/m2 or a weight of >120 kg.16 Nevertheless, given that obesity is an established risk factor for VTE and AF, the use of DOAC in this population, especially those who cannot tolerate VKA, is not uncommon.18,19 Clinicians often find themselves in a clinical conundrum when treating obese patients intolerant to VKA. Therefore, the goals of this study were to conduct a systematic review and meta-analysis to (i) compare the efficacy (stroke/SE) and safety (major bleeding) of DOAC and VKA in morbidly obese patients with AF; (ii) compare the efficacy and safety of DOAC in obese patients compared to non-obese patients with AF; (iii) update the current meta-analysis on safety and efficacy outcomes of AF patients with obesity compared to normal-weight patients who used any form of oral anticoagulation.
Methods
The present analysis was conducted in accordance with published PRISMA guidance.20
Inclusion criteria
Studies were eligible if they reported outcome of stroke, systemic embolism, and bleeding in obese and/or normal weight patients with AF using DOAC and/or VKA. There were no restrictions based on either type of publication, gender, age, race, or language of publication.
Types of outcome measures
Primary outcome
Stroke/SE and major bleeding in morbidly obese patients (BMI ≥ 40 kg/m2) with AF who used DOAC or VKA.
Secondary outcome
Stroke/SE and major bleeding in obese (BMI ≥ 30 kg/m2) and non-obese (BMI < 30 kg/m2) patients with AF who used DOAC.
Stroke/SE and major bleeding risk between obese (BMI ≥ 30 kg/m2) and normal weight (BMI 18.5 to < 25 kg/m2) patients with AF who used any form of oral anticoagulation.
Definition
Obesity and normal weight status are defined by BMI. Body mass index of 18.5 to <25 is normal weight. Body mass index of 25 to <30 is overweight. Body mass index of 30 to <35 is obesity class I. Body mass index of 35 to <40 is obesity class II or severe obesity. Body mass index of 40 to <45 is obesity class III or morbid obesity. Body mass index of ≥45 is super obesity and consider as obesity class III. Major bleeding by ISTH definition is defined as clinically overt bleeding associated with any of the following: fatal outcome, involvement of a critical anatomic site (intracranial, spinal, ocular, pericardial, articular, retroperitoneal, or intramuscular with compartment syndrome), >2 g/dL reduction in haemoglobin concentration, transfusion of >2 units of whole blood or packed red blood cells, or permanent disability.21
Search strategy
We performed a systematic search of the MEDLINE (inception through August 2020), and Embase databases (inception through August 2020) for patients with AF using DOAC or VKA. Our search strings included (‘Factor Xa Inhibitors’[Mesh] OR apixaban OR edoxaban OR rivaroxaban OR dabigatran) AND (‘Obesity’[Mesh] OR BMI OR obesity[tiab]) AND (‘atrial fibrillation’[Mesh] OR AF[tiab] OR ‘atrial fibrillation’[tiab]) for PubMed. Embase search included (‘factor xa inhibitors’/exp OR ‘factor xa inhibitors’ OR doac OR noac OR ‘dabigatran’/exp OR dabigatran OR ‘apixaban’/exp OR apixaban OR ‘edoxaban’/exp OR edoxaban OR ‘rivaroxaban’/exp OR rivaroxaban) AND (‘obesity’/exp OR obesity OR ‘bmi’/exp OR bmi OR ‘morbid obesity’/exp OR ‘morbid obesity’) AND (‘atrial fibrillation’ OR af). We hand-searched the bibliographies of selected studies and meta-analyses to identify further eligible studies. Abstracts were reviewed for suitability and articles accordingly retrieved.
Data extraction and assessment of risk of bias in included studies
Two authors (S.T. and H.T.) independently carried out selection of included studies and determined the risk of bias, with disputes resolved by consensus following discussion with a third author (B.S.). Assessment for risk of bias in included studies was done using two different quality assessment tools. Observational studies were assessed using the Newcastle-Ottawa Scale (NOS) for quality assessment.22 This scale has a full score of 9 and provides a protocol for judgement in these bias domains: selection (4), comparability (2), and exposure/outcome (3). Randomized controlled trials were assessed using the risk of bias tool (RoB).23 This tool comprises six main domains: two selection bias, one performance bias, one detection bias, one attrition bias, and one reporting bias. Reference manager version 5.4 was used to create the figure for the ROB.24
Assessment of publication bias
Tests for publication bias are performed in the event of 10 or more trials being included for analysis.25 Publication bias was assessed using funnel plot, Begg’s test, and Egger’s test.26,27 The P-value <0.05 in publication bias tests was suggestive of publication bias.
Statistical analysis
Three main analyses were planned to calculate relative risk of stroke/SE and major bleeding between each group using relative risk. To ensure class effect of all type of DOAC, pre-specified subgroup analysis was performed for each type of DOAC. Pre-specified sensitivity analysis was performed for the primary outcome using pooled relative risk. This was planned due to limited raw outcome data in studies that reported effect size only. Mean values are expressed as mean ± standard deviation (SD) unless otherwise stated. Q-statistic and I2 statistic was used to assess evidence of statistical heterogeneity.28 We considered an I2 statistic of 50% or more and Q-statistic with P-value <0.10 as indicative of a considerable level of statistical heterogeneity; if heterogeneity was present, the random effects model was used for the analysis.28 All analyses were conducted using STATA software (version 16 STATA Corp, College Station, TX, USA).
Summary of outcomes and assessment of the certainty of the evidence
Summary of outcomes was assessed by GRADE approach.29 Treatment effect estimates were rated as high, moderate, low, or very low depending on the certainty of the evidence. We used GRADEpro software to prepare a summary of findings’ tables describing the key outcomes in this study.30
Results
Literature search
The initial literature search identified 607 studies from MEDLINE and Embase. We found three additional studies from manual reference search. Twenty-five studies were relevant for evaluation. Twelve studies were excluded due to reasons given in Figure 1. A total of 13 studies were included for the analysis. Characteristics of the included studies in the first analysis are shown in Table 1.
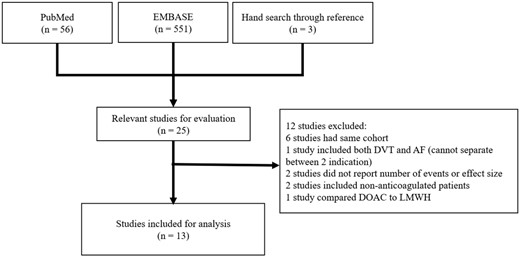
Flow diagram of the studies selection process. AF, atrial fibrillation; DOAC, direct oral anticoagulant; DVT, deep vein thrombosis; LMWH, low molecular weight heparin.
| Author (trial name), reference . | Year . | Country . | Study type . | Population (n) . | Follow-up (years as mean) . | DOAC . | Type of VKA . | Stroke/SE definition . | Major bleeding definition . | NOS . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All . | BMI 18.5–24.99 . | BMI ≥30 . | BMI ≥40 . | Type of DOAC (n, %) . | Usual dose . | Dose adjustment, criteria . | |||||||||
| Balla (ROCKET-AF),31 | 2017 | Multi-national | Post hoc analysis, RCT | 14 030 | 3289 | 5206 | N/A | 2 | Rivaroxaban | 20 mg OD | 15 mg OD, CrCl 30–49 mL/min | Warfarin | Stroke/SEa | ISTH definition | — |
| Bianco,32 | 2020 | USA | Retrospective cohort | 2957 | N/A | 1593 | 442 | N/A | DOAC | N/A | N/A | N/A | ICD codes | N/A | 7 |
| Boriani (ENGAGE-AF TIMI 48),33 | 2018 | Multi-national | Post hoc analysis, RCT | 19 879 | 4491 | 8457 | 1149 | Median 2.8 | Edoxaban (14 001, 70%) | 60 mg OD | 30 mg OD, CrCl of ≥ 50 mL/min, BW ≤ 60 kg, or use of verapamil, quinidine, or dronedarone | Warfarin | Stroke/SEa | ISTH definition | — |
| Connolly (RE-LY),7 | 2009 | USA | Post hoc analysis, RCT | 18 113 | 4697 | 6279 | N/A | Median 2 | Dabigatran (12 090, 66.7%) | 150 mg BID | 110 mg BID | Warfarin | Stroke/SEa | ISTH definition | — |
| Dietelzwig (ARISTHOPHANES),18 | 2020 | USA | Retrospective cohort | 88 461 | 0 | 88 461 | At least 16 707 | 0.5–0.67 | Dabigatran (7171, 8%) Apixaban (21 242, 24%) Rivaroxaban (29 146, 33%) | 150 mg BID 5 mg BID 20 mg OD | N/A | Warfarin | ICD codes | ICD codes of major bleeding in GI, ICH, ocular, and respiratory tract | 8 |
| Doucette,34 | 2020 | USA | Retrospective cohort | 398 | 60 | 137 | 36 | 2 | Apixaban (126, 44%) Rivaroxaban (127, 44%) Dabigatran (36, 13%) | 5 mg BID 20 mg OD 150 mg BID | 2.5 mg BID 15 mg OD or 10 mg OD 75 mg BID | No | N/A | Overt bleeding at a critical site with Hb drop of ≥ 2 g/dL or requiring 2 units of PRC | 8 |
| Kido,13 | 2018 | USA | Retrospective cohort | 128 | 0 | 0 | 128 | N/A | Dabigatran (20, 31%) Rivaroxaban (25, 39%) Apixaban (19, 30%) | N/A | N/A | warfarin | Diagnosed by neurology service | ISTH definition | 9 |
| Kushnir,35 | 2019 | USA | Retrospective cohort | 429 | 0 | 0 | 429 | Median 1 (IQR 0.4–1.6) | Apixaban (103, 24%) Rivaroxaban (174, 41%) | N/A | N/A | warfarin | Diagnosed by confirm brain imaging findings | ISTH definition | 8 |
| Murakawa (XAPASS),36 | 2019 | Japan | Prospective cohort | 9578 | 4410 | 499 | N/A | 1 | Rivaroxaban (99578, 100%) | 15 mg OD | 10 mg OD, CrCl < 50 mL/min | warfarin | Stroke/SEa | ISTH definition | 9 |
| Peterson,37 | 2019 | USA | Retrospective cohort | 9474 | N/A | N/A | 4543 | 0.8 | Rivaroxaban (4543, 48%) | N/A | N/A | warfarin | Hospitalization or ER visit with a primary diagnosis of ischaemic stroke and systemic embolism | Algorithm Cunningham et al. that includes GI bleeding and ICH | 8 |
| Proietti (SPORTIF),38 | 2016 | Multi-national | Post hoc analysis, RCT | 3630 | 874 | 1310 | N/A | 1.6 ± 0.4 | Ximelagatran (0, 0%) | N/A | N/A | warfarin | ISTH definition | — | |
| Sandhu (ARISTOTLE),39 | 2016 | Multi-national | Post hoc analysis, RCT | 17 913 | 4038 | 7134 | 1003 | Median 1.8 | Apixaban (9201, 50%) | 5 mg BID | 2.5 mg BID, age ≥ 80 years, BW ≤ 60 kg, or serum creatinine level ≥ 1.5 mg/dL | warfarin | Stroke/SEa | ISTH definition | — |
| Tittl (Dresden NOAC registry),40 | 2018 | Germany | Prospective cohort | 2334 | 892 | 1077 | N/A | 2.7 ± 1.5 | Apixaban (N/A) Rivaroxaban (N/A) Dabigatran (N/A) Edoxaban (N/A | N/A | N/A | No | N/A | ISTH definition | 8 |
| Summary | 187 324 | >22 751 | >120 153 | >24 437 | 8.1 ± 0.6 | ||||||||||
| Author (trial name), reference . | Year . | Country . | Study type . | Population (n) . | Follow-up (years as mean) . | DOAC . | Type of VKA . | Stroke/SE definition . | Major bleeding definition . | NOS . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All . | BMI 18.5–24.99 . | BMI ≥30 . | BMI ≥40 . | Type of DOAC (n, %) . | Usual dose . | Dose adjustment, criteria . | |||||||||
| Balla (ROCKET-AF),31 | 2017 | Multi-national | Post hoc analysis, RCT | 14 030 | 3289 | 5206 | N/A | 2 | Rivaroxaban | 20 mg OD | 15 mg OD, CrCl 30–49 mL/min | Warfarin | Stroke/SEa | ISTH definition | — |
| Bianco,32 | 2020 | USA | Retrospective cohort | 2957 | N/A | 1593 | 442 | N/A | DOAC | N/A | N/A | N/A | ICD codes | N/A | 7 |
| Boriani (ENGAGE-AF TIMI 48),33 | 2018 | Multi-national | Post hoc analysis, RCT | 19 879 | 4491 | 8457 | 1149 | Median 2.8 | Edoxaban (14 001, 70%) | 60 mg OD | 30 mg OD, CrCl of ≥ 50 mL/min, BW ≤ 60 kg, or use of verapamil, quinidine, or dronedarone | Warfarin | Stroke/SEa | ISTH definition | — |
| Connolly (RE-LY),7 | 2009 | USA | Post hoc analysis, RCT | 18 113 | 4697 | 6279 | N/A | Median 2 | Dabigatran (12 090, 66.7%) | 150 mg BID | 110 mg BID | Warfarin | Stroke/SEa | ISTH definition | — |
| Dietelzwig (ARISTHOPHANES),18 | 2020 | USA | Retrospective cohort | 88 461 | 0 | 88 461 | At least 16 707 | 0.5–0.67 | Dabigatran (7171, 8%) Apixaban (21 242, 24%) Rivaroxaban (29 146, 33%) | 150 mg BID 5 mg BID 20 mg OD | N/A | Warfarin | ICD codes | ICD codes of major bleeding in GI, ICH, ocular, and respiratory tract | 8 |
| Doucette,34 | 2020 | USA | Retrospective cohort | 398 | 60 | 137 | 36 | 2 | Apixaban (126, 44%) Rivaroxaban (127, 44%) Dabigatran (36, 13%) | 5 mg BID 20 mg OD 150 mg BID | 2.5 mg BID 15 mg OD or 10 mg OD 75 mg BID | No | N/A | Overt bleeding at a critical site with Hb drop of ≥ 2 g/dL or requiring 2 units of PRC | 8 |
| Kido,13 | 2018 | USA | Retrospective cohort | 128 | 0 | 0 | 128 | N/A | Dabigatran (20, 31%) Rivaroxaban (25, 39%) Apixaban (19, 30%) | N/A | N/A | warfarin | Diagnosed by neurology service | ISTH definition | 9 |
| Kushnir,35 | 2019 | USA | Retrospective cohort | 429 | 0 | 0 | 429 | Median 1 (IQR 0.4–1.6) | Apixaban (103, 24%) Rivaroxaban (174, 41%) | N/A | N/A | warfarin | Diagnosed by confirm brain imaging findings | ISTH definition | 8 |
| Murakawa (XAPASS),36 | 2019 | Japan | Prospective cohort | 9578 | 4410 | 499 | N/A | 1 | Rivaroxaban (99578, 100%) | 15 mg OD | 10 mg OD, CrCl < 50 mL/min | warfarin | Stroke/SEa | ISTH definition | 9 |
| Peterson,37 | 2019 | USA | Retrospective cohort | 9474 | N/A | N/A | 4543 | 0.8 | Rivaroxaban (4543, 48%) | N/A | N/A | warfarin | Hospitalization or ER visit with a primary diagnosis of ischaemic stroke and systemic embolism | Algorithm Cunningham et al. that includes GI bleeding and ICH | 8 |
| Proietti (SPORTIF),38 | 2016 | Multi-national | Post hoc analysis, RCT | 3630 | 874 | 1310 | N/A | 1.6 ± 0.4 | Ximelagatran (0, 0%) | N/A | N/A | warfarin | ISTH definition | — | |
| Sandhu (ARISTOTLE),39 | 2016 | Multi-national | Post hoc analysis, RCT | 17 913 | 4038 | 7134 | 1003 | Median 1.8 | Apixaban (9201, 50%) | 5 mg BID | 2.5 mg BID, age ≥ 80 years, BW ≤ 60 kg, or serum creatinine level ≥ 1.5 mg/dL | warfarin | Stroke/SEa | ISTH definition | — |
| Tittl (Dresden NOAC registry),40 | 2018 | Germany | Prospective cohort | 2334 | 892 | 1077 | N/A | 2.7 ± 1.5 | Apixaban (N/A) Rivaroxaban (N/A) Dabigatran (N/A) Edoxaban (N/A | N/A | N/A | No | N/A | ISTH definition | 8 |
| Summary | 187 324 | >22 751 | >120 153 | >24 437 | 8.1 ± 0.6 | ||||||||||
BID, two times daily; BMI, body mass index; BW, body weight; CrCl, creatinine clearance; DOAC, direct oral anticoagulant; ER, emergency room; GI, gastrointestinal; Hb, haemoglobin; ICD, international classification of diseases; ICH, intracranial haemorrhage; ISTH, International Society on Thrombosis and Hemostasis; N/A, not applicable; NOS, Newcastle-Ottawa scale; OD, once daily; PRC, packed red cell; RCT, randomized control trial; SE, systemic embolism; VKA, vitamin K antagonist.
Stroke: acute focal neurodeficit in the distribution of single brain artery lasting ≥24 h; SE: arterial embolism resulted in clinical ischaemia (excluding brain and pulmonary arterial system).
| Author (trial name), reference . | Year . | Country . | Study type . | Population (n) . | Follow-up (years as mean) . | DOAC . | Type of VKA . | Stroke/SE definition . | Major bleeding definition . | NOS . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All . | BMI 18.5–24.99 . | BMI ≥30 . | BMI ≥40 . | Type of DOAC (n, %) . | Usual dose . | Dose adjustment, criteria . | |||||||||
| Balla (ROCKET-AF),31 | 2017 | Multi-national | Post hoc analysis, RCT | 14 030 | 3289 | 5206 | N/A | 2 | Rivaroxaban | 20 mg OD | 15 mg OD, CrCl 30–49 mL/min | Warfarin | Stroke/SEa | ISTH definition | — |
| Bianco,32 | 2020 | USA | Retrospective cohort | 2957 | N/A | 1593 | 442 | N/A | DOAC | N/A | N/A | N/A | ICD codes | N/A | 7 |
| Boriani (ENGAGE-AF TIMI 48),33 | 2018 | Multi-national | Post hoc analysis, RCT | 19 879 | 4491 | 8457 | 1149 | Median 2.8 | Edoxaban (14 001, 70%) | 60 mg OD | 30 mg OD, CrCl of ≥ 50 mL/min, BW ≤ 60 kg, or use of verapamil, quinidine, or dronedarone | Warfarin | Stroke/SEa | ISTH definition | — |
| Connolly (RE-LY),7 | 2009 | USA | Post hoc analysis, RCT | 18 113 | 4697 | 6279 | N/A | Median 2 | Dabigatran (12 090, 66.7%) | 150 mg BID | 110 mg BID | Warfarin | Stroke/SEa | ISTH definition | — |
| Dietelzwig (ARISTHOPHANES),18 | 2020 | USA | Retrospective cohort | 88 461 | 0 | 88 461 | At least 16 707 | 0.5–0.67 | Dabigatran (7171, 8%) Apixaban (21 242, 24%) Rivaroxaban (29 146, 33%) | 150 mg BID 5 mg BID 20 mg OD | N/A | Warfarin | ICD codes | ICD codes of major bleeding in GI, ICH, ocular, and respiratory tract | 8 |
| Doucette,34 | 2020 | USA | Retrospective cohort | 398 | 60 | 137 | 36 | 2 | Apixaban (126, 44%) Rivaroxaban (127, 44%) Dabigatran (36, 13%) | 5 mg BID 20 mg OD 150 mg BID | 2.5 mg BID 15 mg OD or 10 mg OD 75 mg BID | No | N/A | Overt bleeding at a critical site with Hb drop of ≥ 2 g/dL or requiring 2 units of PRC | 8 |
| Kido,13 | 2018 | USA | Retrospective cohort | 128 | 0 | 0 | 128 | N/A | Dabigatran (20, 31%) Rivaroxaban (25, 39%) Apixaban (19, 30%) | N/A | N/A | warfarin | Diagnosed by neurology service | ISTH definition | 9 |
| Kushnir,35 | 2019 | USA | Retrospective cohort | 429 | 0 | 0 | 429 | Median 1 (IQR 0.4–1.6) | Apixaban (103, 24%) Rivaroxaban (174, 41%) | N/A | N/A | warfarin | Diagnosed by confirm brain imaging findings | ISTH definition | 8 |
| Murakawa (XAPASS),36 | 2019 | Japan | Prospective cohort | 9578 | 4410 | 499 | N/A | 1 | Rivaroxaban (99578, 100%) | 15 mg OD | 10 mg OD, CrCl < 50 mL/min | warfarin | Stroke/SEa | ISTH definition | 9 |
| Peterson,37 | 2019 | USA | Retrospective cohort | 9474 | N/A | N/A | 4543 | 0.8 | Rivaroxaban (4543, 48%) | N/A | N/A | warfarin | Hospitalization or ER visit with a primary diagnosis of ischaemic stroke and systemic embolism | Algorithm Cunningham et al. that includes GI bleeding and ICH | 8 |
| Proietti (SPORTIF),38 | 2016 | Multi-national | Post hoc analysis, RCT | 3630 | 874 | 1310 | N/A | 1.6 ± 0.4 | Ximelagatran (0, 0%) | N/A | N/A | warfarin | ISTH definition | — | |
| Sandhu (ARISTOTLE),39 | 2016 | Multi-national | Post hoc analysis, RCT | 17 913 | 4038 | 7134 | 1003 | Median 1.8 | Apixaban (9201, 50%) | 5 mg BID | 2.5 mg BID, age ≥ 80 years, BW ≤ 60 kg, or serum creatinine level ≥ 1.5 mg/dL | warfarin | Stroke/SEa | ISTH definition | — |
| Tittl (Dresden NOAC registry),40 | 2018 | Germany | Prospective cohort | 2334 | 892 | 1077 | N/A | 2.7 ± 1.5 | Apixaban (N/A) Rivaroxaban (N/A) Dabigatran (N/A) Edoxaban (N/A | N/A | N/A | No | N/A | ISTH definition | 8 |
| Summary | 187 324 | >22 751 | >120 153 | >24 437 | 8.1 ± 0.6 | ||||||||||
| Author (trial name), reference . | Year . | Country . | Study type . | Population (n) . | Follow-up (years as mean) . | DOAC . | Type of VKA . | Stroke/SE definition . | Major bleeding definition . | NOS . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All . | BMI 18.5–24.99 . | BMI ≥30 . | BMI ≥40 . | Type of DOAC (n, %) . | Usual dose . | Dose adjustment, criteria . | |||||||||
| Balla (ROCKET-AF),31 | 2017 | Multi-national | Post hoc analysis, RCT | 14 030 | 3289 | 5206 | N/A | 2 | Rivaroxaban | 20 mg OD | 15 mg OD, CrCl 30–49 mL/min | Warfarin | Stroke/SEa | ISTH definition | — |
| Bianco,32 | 2020 | USA | Retrospective cohort | 2957 | N/A | 1593 | 442 | N/A | DOAC | N/A | N/A | N/A | ICD codes | N/A | 7 |
| Boriani (ENGAGE-AF TIMI 48),33 | 2018 | Multi-national | Post hoc analysis, RCT | 19 879 | 4491 | 8457 | 1149 | Median 2.8 | Edoxaban (14 001, 70%) | 60 mg OD | 30 mg OD, CrCl of ≥ 50 mL/min, BW ≤ 60 kg, or use of verapamil, quinidine, or dronedarone | Warfarin | Stroke/SEa | ISTH definition | — |
| Connolly (RE-LY),7 | 2009 | USA | Post hoc analysis, RCT | 18 113 | 4697 | 6279 | N/A | Median 2 | Dabigatran (12 090, 66.7%) | 150 mg BID | 110 mg BID | Warfarin | Stroke/SEa | ISTH definition | — |
| Dietelzwig (ARISTHOPHANES),18 | 2020 | USA | Retrospective cohort | 88 461 | 0 | 88 461 | At least 16 707 | 0.5–0.67 | Dabigatran (7171, 8%) Apixaban (21 242, 24%) Rivaroxaban (29 146, 33%) | 150 mg BID 5 mg BID 20 mg OD | N/A | Warfarin | ICD codes | ICD codes of major bleeding in GI, ICH, ocular, and respiratory tract | 8 |
| Doucette,34 | 2020 | USA | Retrospective cohort | 398 | 60 | 137 | 36 | 2 | Apixaban (126, 44%) Rivaroxaban (127, 44%) Dabigatran (36, 13%) | 5 mg BID 20 mg OD 150 mg BID | 2.5 mg BID 15 mg OD or 10 mg OD 75 mg BID | No | N/A | Overt bleeding at a critical site with Hb drop of ≥ 2 g/dL or requiring 2 units of PRC | 8 |
| Kido,13 | 2018 | USA | Retrospective cohort | 128 | 0 | 0 | 128 | N/A | Dabigatran (20, 31%) Rivaroxaban (25, 39%) Apixaban (19, 30%) | N/A | N/A | warfarin | Diagnosed by neurology service | ISTH definition | 9 |
| Kushnir,35 | 2019 | USA | Retrospective cohort | 429 | 0 | 0 | 429 | Median 1 (IQR 0.4–1.6) | Apixaban (103, 24%) Rivaroxaban (174, 41%) | N/A | N/A | warfarin | Diagnosed by confirm brain imaging findings | ISTH definition | 8 |
| Murakawa (XAPASS),36 | 2019 | Japan | Prospective cohort | 9578 | 4410 | 499 | N/A | 1 | Rivaroxaban (99578, 100%) | 15 mg OD | 10 mg OD, CrCl < 50 mL/min | warfarin | Stroke/SEa | ISTH definition | 9 |
| Peterson,37 | 2019 | USA | Retrospective cohort | 9474 | N/A | N/A | 4543 | 0.8 | Rivaroxaban (4543, 48%) | N/A | N/A | warfarin | Hospitalization or ER visit with a primary diagnosis of ischaemic stroke and systemic embolism | Algorithm Cunningham et al. that includes GI bleeding and ICH | 8 |
| Proietti (SPORTIF),38 | 2016 | Multi-national | Post hoc analysis, RCT | 3630 | 874 | 1310 | N/A | 1.6 ± 0.4 | Ximelagatran (0, 0%) | N/A | N/A | warfarin | ISTH definition | — | |
| Sandhu (ARISTOTLE),39 | 2016 | Multi-national | Post hoc analysis, RCT | 17 913 | 4038 | 7134 | 1003 | Median 1.8 | Apixaban (9201, 50%) | 5 mg BID | 2.5 mg BID, age ≥ 80 years, BW ≤ 60 kg, or serum creatinine level ≥ 1.5 mg/dL | warfarin | Stroke/SEa | ISTH definition | — |
| Tittl (Dresden NOAC registry),40 | 2018 | Germany | Prospective cohort | 2334 | 892 | 1077 | N/A | 2.7 ± 1.5 | Apixaban (N/A) Rivaroxaban (N/A) Dabigatran (N/A) Edoxaban (N/A | N/A | N/A | No | N/A | ISTH definition | 8 |
| Summary | 187 324 | >22 751 | >120 153 | >24 437 | 8.1 ± 0.6 | ||||||||||
BID, two times daily; BMI, body mass index; BW, body weight; CrCl, creatinine clearance; DOAC, direct oral anticoagulant; ER, emergency room; GI, gastrointestinal; Hb, haemoglobin; ICD, international classification of diseases; ICH, intracranial haemorrhage; ISTH, International Society on Thrombosis and Hemostasis; N/A, not applicable; NOS, Newcastle-Ottawa scale; OD, once daily; PRC, packed red cell; RCT, randomized control trial; SE, systemic embolism; VKA, vitamin K antagonist.
Stroke: acute focal neurodeficit in the distribution of single brain artery lasting ≥24 h; SE: arterial embolism resulted in clinical ischaemia (excluding brain and pulmonary arterial system).
Quality assessment tool
Mean NOS scale is 8.1 ± 0.6. NOS is presented in Table 1. Risk of bias tool was used for five randomized control trials. Three RCTs, including ARISTOTLE, ENGAGE TIMI-AF 48, and ROCKET-AF, had low risk in all area of bias.31,33,39 Dabigatran’s trail, RE-LY, had high risk of performance and detection biases due to lack of warfarin blinding and independent committee to detect the outcomes.7 SPORTIF trial for ximelagatran had high risk of performance bias and attrition bias because of lack of warfarin blinding and nearly 20% rate of loss to follow-up.38
Efficacy and safety analysis of DOAC vs. VKA in morbidly obese patients with AF
The first analysis compared efficacy and safety between DOAC and VKA in BMI ≥40 kg/m2 shown in Figure 2. Five studies had complete raw data of outcome and were included with a total of 18,548 patients.13,32,33,35,37 Incidence of stroke/SE in DOAC group was 2.4% and 2.6% in VKA group. There was no statistically significant difference between DOAC and VKA in preventing stroke/SE in morbidly obese patients with RR of 0.85 [95% confidence interval (CI): 0.56–1.29; very low-certainty evidence]. The incidence of major bleeding in DOAC and VKA group was 3.4% and 4.5%, respectively. Morbidly obese patients who used DOAC for stroke prevention with AF had statistically less major bleeding compared to VKA with RR of 0.62 (95% CI: 0.48–0.80; low-certainty evidence). The summary of findings was presented in Table 2. Direct oral anticoagulants in this analysis included edoxaban, apixaban, rivaroxaban, and dabigatran. Bianco et al.32 did not specify the type of DOAC used in their study. We did not perform a publication bias analysis due to the inadequate number of included studies.
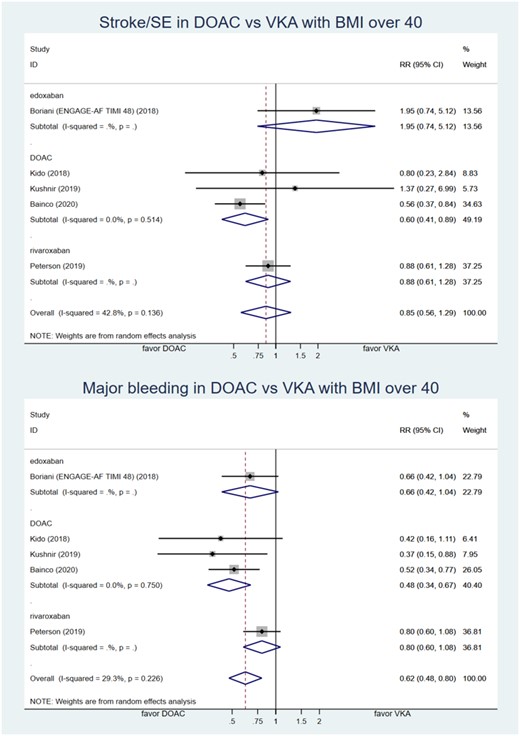
Forest plot of relative risk of stroke/SE and major bleeding comparing DOAC and VKA in patient with atrial fibrillation with BMI ≥40 kg/m2. BMI, body mass index; DOAC, direct oral anticoagulant; ENGAGE-AF TIMI 48, Global study to assess the safety and effectiveness of edoxaban (DU-176b) vs. standard practice of dosing with warfarin in patients with atrial fibrillation trial; RR, relative risk; SE, systemic embolism; VKA, vitamin K antagonist.
Summary of findings of DOAC compared to VKA in morbidly obese patient (BMI ≥ 40 kg/m2) with AF
| Outcomes . | No. of participants (studies) Follow-up . | Certainty of the evidence (GRADE) . | Relative effect (95% CI) . | Anticipated absolute effects . | |
|---|---|---|---|---|---|
| Risk with VKA . | Risk difference with DOAC . | ||||
Stroke/SE Follow-up: range 0.8 years to 2.8 years | 9274 (5 observational studies) | ⨁◯◯◯ VERY LOWa,b | RR 0.85 (0.56–1.29) | 26 per 1000 | 4 fewer per 1000 (11 fewer to 7 more) |
Major bleeding Follow-up: range 0.8 years to 2.8 years | 9274 (5 observational studies) | ⨁⨁◯◯ LOWb,c | RR 0.62 (0.48–0.80) | 45 per 1000 | 17 fewer per 1000 (23 fewer to 9 fewer) |
Stroke/SE (sensitivity analysis) assessed with: pooled effect size Follow-up: range 0.5 years to 2.8 years | (7 observational studies) | ⨁⨁◯◯ LOWb,d,e,f | RR 0.86 (0.77–0.96) | No data | No data |
Major bleeding (sensitivity analysis) assessed with: pooled effect size Follow-up: range 0.5 years to 2.8 years | (7 observational studies) | ⨁◯◯◯ VERY LOWb,d,f,g | RR 0.73 (0.67–0.79) | No data | No data |
| Outcomes . | No. of participants (studies) Follow-up . | Certainty of the evidence (GRADE) . | Relative effect (95% CI) . | Anticipated absolute effects . | |
|---|---|---|---|---|---|
| Risk with VKA . | Risk difference with DOAC . | ||||
Stroke/SE Follow-up: range 0.8 years to 2.8 years | 9274 (5 observational studies) | ⨁◯◯◯ VERY LOWa,b | RR 0.85 (0.56–1.29) | 26 per 1000 | 4 fewer per 1000 (11 fewer to 7 more) |
Major bleeding Follow-up: range 0.8 years to 2.8 years | 9274 (5 observational studies) | ⨁⨁◯◯ LOWb,c | RR 0.62 (0.48–0.80) | 45 per 1000 | 17 fewer per 1000 (23 fewer to 9 fewer) |
Stroke/SE (sensitivity analysis) assessed with: pooled effect size Follow-up: range 0.5 years to 2.8 years | (7 observational studies) | ⨁⨁◯◯ LOWb,d,e,f | RR 0.86 (0.77–0.96) | No data | No data |
Major bleeding (sensitivity analysis) assessed with: pooled effect size Follow-up: range 0.5 years to 2.8 years | (7 observational studies) | ⨁◯◯◯ VERY LOWb,d,f,g | RR 0.73 (0.67–0.79) | No data | No data |
Sensitivity analysis was performed using pooled effect size from studies that reported the outcome of interest without complete raw data. GRADE Working Group grades of evidence: High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect.
AF, atrial fibrillation; BMI, body mass index; CI, confidence interval; DOAC, direct oral anticoagulant; RCT, randomized controlled trial; RR, Risk ratio; stroke/SE, stroke and systemic embolism.
Two studies with apixaban showed a non-significant increase in the risk of stroke/SE while the other three studies suggest that DOAC decreased the risk of stroke/SE. This resulted in moderate heterogeneity with I2 = 42.8%.
Post hoc analysis of RCT for edoxaban was done to assess for outcomes in morbidly obese patients with AF but this is not the primary focus to include morbid obesity group.
Low heterogeneity was detected with I2 = 29.3%.
NOS was used to assess the selection bias of five observational studies with scores of 4/4. Two post hoc analysis of RCT has low-risk selection bias in ROB.
Low heterogeneity was detected in pooled effect size analysis with I2 = 17.5%.
Post hoc analysis of RCT for apixaban was done to assess for outcomes in morbidly obese patients with AF but this is not the primary focus to include morbid obesity group.
High heterogeneity was detected in pooled effect size analysis with I2 = 79.9%.
Summary of findings of DOAC compared to VKA in morbidly obese patient (BMI ≥ 40 kg/m2) with AF
| Outcomes . | No. of participants (studies) Follow-up . | Certainty of the evidence (GRADE) . | Relative effect (95% CI) . | Anticipated absolute effects . | |
|---|---|---|---|---|---|
| Risk with VKA . | Risk difference with DOAC . | ||||
Stroke/SE Follow-up: range 0.8 years to 2.8 years | 9274 (5 observational studies) | ⨁◯◯◯ VERY LOWa,b | RR 0.85 (0.56–1.29) | 26 per 1000 | 4 fewer per 1000 (11 fewer to 7 more) |
Major bleeding Follow-up: range 0.8 years to 2.8 years | 9274 (5 observational studies) | ⨁⨁◯◯ LOWb,c | RR 0.62 (0.48–0.80) | 45 per 1000 | 17 fewer per 1000 (23 fewer to 9 fewer) |
Stroke/SE (sensitivity analysis) assessed with: pooled effect size Follow-up: range 0.5 years to 2.8 years | (7 observational studies) | ⨁⨁◯◯ LOWb,d,e,f | RR 0.86 (0.77–0.96) | No data | No data |
Major bleeding (sensitivity analysis) assessed with: pooled effect size Follow-up: range 0.5 years to 2.8 years | (7 observational studies) | ⨁◯◯◯ VERY LOWb,d,f,g | RR 0.73 (0.67–0.79) | No data | No data |
| Outcomes . | No. of participants (studies) Follow-up . | Certainty of the evidence (GRADE) . | Relative effect (95% CI) . | Anticipated absolute effects . | |
|---|---|---|---|---|---|
| Risk with VKA . | Risk difference with DOAC . | ||||
Stroke/SE Follow-up: range 0.8 years to 2.8 years | 9274 (5 observational studies) | ⨁◯◯◯ VERY LOWa,b | RR 0.85 (0.56–1.29) | 26 per 1000 | 4 fewer per 1000 (11 fewer to 7 more) |
Major bleeding Follow-up: range 0.8 years to 2.8 years | 9274 (5 observational studies) | ⨁⨁◯◯ LOWb,c | RR 0.62 (0.48–0.80) | 45 per 1000 | 17 fewer per 1000 (23 fewer to 9 fewer) |
Stroke/SE (sensitivity analysis) assessed with: pooled effect size Follow-up: range 0.5 years to 2.8 years | (7 observational studies) | ⨁⨁◯◯ LOWb,d,e,f | RR 0.86 (0.77–0.96) | No data | No data |
Major bleeding (sensitivity analysis) assessed with: pooled effect size Follow-up: range 0.5 years to 2.8 years | (7 observational studies) | ⨁◯◯◯ VERY LOWb,d,f,g | RR 0.73 (0.67–0.79) | No data | No data |
Sensitivity analysis was performed using pooled effect size from studies that reported the outcome of interest without complete raw data. GRADE Working Group grades of evidence: High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect.
AF, atrial fibrillation; BMI, body mass index; CI, confidence interval; DOAC, direct oral anticoagulant; RCT, randomized controlled trial; RR, Risk ratio; stroke/SE, stroke and systemic embolism.
Two studies with apixaban showed a non-significant increase in the risk of stroke/SE while the other three studies suggest that DOAC decreased the risk of stroke/SE. This resulted in moderate heterogeneity with I2 = 42.8%.
Post hoc analysis of RCT for edoxaban was done to assess for outcomes in morbidly obese patients with AF but this is not the primary focus to include morbid obesity group.
Low heterogeneity was detected with I2 = 29.3%.
NOS was used to assess the selection bias of five observational studies with scores of 4/4. Two post hoc analysis of RCT has low-risk selection bias in ROB.
Low heterogeneity was detected in pooled effect size analysis with I2 = 17.5%.
Post hoc analysis of RCT for apixaban was done to assess for outcomes in morbidly obese patients with AF but this is not the primary focus to include morbid obesity group.
High heterogeneity was detected in pooled effect size analysis with I2 = 79.9%.
Sensitivity analysis was done with seven studies.13,18,32,33,35,37,39 Direct oral anticoagulants may prevent more stroke/SE when compared with VKA with RR of 0.86 (95% CI: 0.77–0.96; low-certainty evidence). Direct oral anticoagulants may cause less bleeding with RR of 0.73 (95% CI: 0.67–0.79; very low-certainty evidence) when compared with VKA (see Supplementary Figure S1 and Table 2).
Efficacy and safety of DOAC in obese vs. non-obese patient with AF
The second analysis compared risk of stroke/SE and major bleeding between obese (BMI ≥ 30 kg/m2) and non-obese (BMI < 30 kg/m2) patients who used DOAC for AF. Eight studies with a total of 53,278 patients were included.7,31–34,36,39,40 Types of DOAC included were dabigatran, apixaban, rivaroxaban, and edoxaban. Bianco et al.32 did not identify the type of DOAC. Obese patient with AF who used DOAC had lower risk of stroke/SE with RR of 0.77 (95% CI: 0.70–0.84; low-certainty of evidence).
Obese patients who used DOAC for stroke prevention with AF had no statistically different major bleeding risk compared to non-obese group with RR of 1.02 (95% CI: 0.94–1.09; low-certainty evidence) (see Figure 3 and supplementary Table S1). No publication bias test was performed in this analysis because the number of included studies was <10.
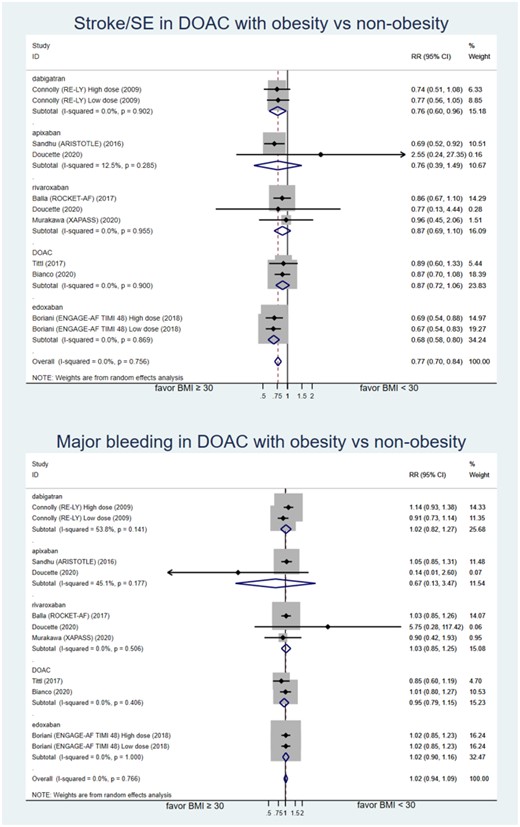
Forest plot of relative risk of stroke/SE and major bleeding comparing obesity and non-obesity patient with atrial fibrillation who used DOAC. ARISTOTLE, apixaban for the prevention of stroke in subjects with atrial fibrillation trial; DOAC, direct oral anticoagulant; ENGAGE-AF TIMI 48, Global study to assess the safety and effectiveness of edoxaban (DU-176b) vs. standard practice of dosing with warfarin in patients with atrial fibrillation trial; RE-LY, randomized evaluation of long-term anticoagulant therapy with dabigatran etexilate trial; ROCKET-AF, rivaroxaban vs. warfarin in non-valvular atrial fibrillation trial; SE, systemic embolism.
Efficacy and safety outcome in obese vs. normal weight patient with AF
The final analysis compared the risk of stroke/SE and major bleeding between obese and normal-weight patients with AF who used any form of oral anticoagulation. Seven studies with a total of 50 831 patients were included.7,33,34,36,38,39 This analysis included anticoagulation of dabigatran, edoxaban, rivaroxaban, apixaban, and warfarin. Obese patients had lower risk of stroke/SE compared to normal-weight patients who used any type of anticoagulation with RR of 0.62 (95% CI: 0.57–0.69; low-certainty evidence). Major bleeding risk was not statistically significant between obese and normal-weight patients regardless of the type of oral anticoagulation with RR of 1.00 (95% CI: 0.76–1.31; very low-certainty evidence) (see Figure 4 and supplementary Table S1). No publication bias test was performed in this analysis because the number of included studies was <10.
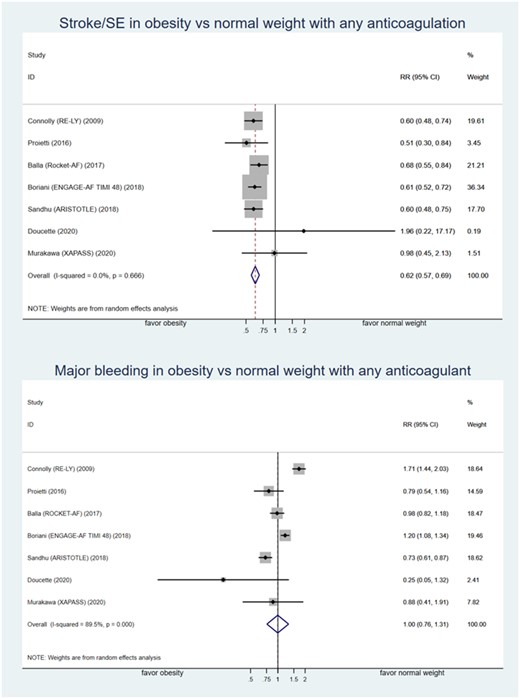
Forest plot of relative risk of stroke/SE and major bleeding comparing obesity and normal weight patient with atrial fibrillation who used any type of oral anticoagulation. ARISTOTLE, apixaban for the prevention of stroke in subjects with atrial fibrillation trial; ENGAGE-AF TIMI 48, Global study to assess the safety and effectiveness of edoxaban (DU-176b) vs. standard practice of dosing with warfarin in patients with atrial fibrillation trial; RE-LY, randomized evaluation of long-term anticoagulant therapy with dabigatran etexilate trial; ROCKET-AF, rivaroxaban vs. warfarin in non-valvular atrial fibrillation trial; RR, relative risk; SE, systemic embolism.
Discussion
This meta-analysis is the first to compare efficacy and safety between DOAC and VKA in patients with AF and BMI ≥40 kg/m2 and include results from major trials. To date, a guideline by SSC of the ISTH in 2016 suggested avoidance of DOAC use in patients with a BMI of >40 kg/m2 or a weight of >120 kg due to limited data.16 Also, the 2018 European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation had a similar suggestion, and referenced the ISTH guideline.6 Other guidelines have not specified recommendations on the usage of DOAC for AF in patients with obesity. Dose adjustments were mentioned in groups with impaired kidney function, which was based on adjusted dose studied in individual pivotal RCTs.41 Our primary analysis showed that there was no statistically significant difference in risk of stroke/SE between DOAC and VKA used in morbidly obese patients. Sensitivity analysis showed that DOACs significantly reduced the risk of stroke/SE in AF patients compared with VKA with low-certainty evidence. Still, decisions to use DOAC over VKA in morbidly obese patients have to be made cautiously given that the primary analysis showed low-certainty of evidence. The true effect may be substantially different from the estimate of the effect.29 Additionally, edoxaban may cause more stroke when compared with the rest of the DOACs with RR of 1.95 (95% CI 0.74–5.12). There was a statistically significant lower risk of major bleeding in DOAC group compared to VKA group with relative risk reduction of 27%. The incidence of major bleeding was ∼3.4% in the DOAC group and 4.5% in the VKA group. None of the included studies mentioned measuring drug levels in morbidly obese patients. One study mentioned increasing the dose of DOAC in one patient, but it was for the indication of venous thromboembolism treatment,40 otherwise no dose adjustment was mentioned in the morbidly obese patient group in these studies. Per ISTH suggestion, patients with a BMI >40 kg/m2 or a weight >120 kg who use a DOAC should have peak and trough levels monitored to ensure they fall within the expected range.16 However, these tests are difficult to perform, not widely available, and do not determine the efficacy of the DOAC.12 Studies on PK/PD have shown that obesity can affect the peak concentrations and reduce the half-lives of the medications, but these effects are modest and it is difficult to determine the clinical significance in real clinical practice.15 Our analysis gave some insight on how these medications work in a real clinical setting, regardless of drug level testing.
A recent meta-analysis on the dilemma of outcome in obese patients with AF which used either DOAC or warfarin showed that obese patients (BMI ≥ 30 kg/m2) had a lower risk of stroke, systemic embolism, and bleeding compared to normal-weight patients.14 This study mainly focused on obesity as a factor dictating the outcome in obese patients with AF, therefore the included studies used a combination of both DOAC and warfarin in AF patients from major DOAC trials including the ROCKET-AF trial (rivaroxaban or warfarin), RE-LY trial (dabigatran or warfarin), and ARISTOTLE (apixaban or warfarin). This result contradicted the assumption based on PK/PD studies that DOAC may have less therapeutic effect in the setting of obesity.15,42,43 We did an updated meta-analysis on this matter where we included all major DOAC trials (warfarin or DOAC included dabigatran, rivaroxaban, apixaban, or edoxaban), one warfarin only study, one study with apixaban and rivaroxaban, and one rivaroxaban only study. The result was similar. Risk of stroke and systemic embolism was statistically lower in obese patients with a combined prevalence of events in the obese group of 2.8% compared with 3.9% in the normal-weight group. Interestingly, patients in the obese group were younger compared with the normal-weight group despite having similar CHA2DS2-VASc scores. At least four of seven studies reported baseline age for the normal-weight group compared to the obesity group. Murakawa et al. reported mean age of 69.5 years in obesity group compared to 78 years in normal-weight group.36 ROCKET-AF and ARISTOTLE’s post hoc analyses showed similar results with mean age of 75 and 71.3 years in normal-weight group compared with 70 and 66.8 years in obesity group, respectively.31,39 Looking at individual studies in the analysis, the ARISTOTLE trial (apixaban or warfarin) showed less major bleeding in the obese group,39 whereas the RE-LY trial (dabigatran or warfarin) and ENGAGE-AF TIMI 48 trial (edoxaban or warfarin) had less major bleeding in the normal-weight group.14,33 This finding raised a point about the safety profile of each DOAC in the obese patient group: not all types of DOAC have the same bleeding risk, despite having similar efficacy in preventing stroke and systemic embolism as a class effect. However, the updated analysis was difficult to interpret due to the combination of both warfarin and DOAC in the study.
Therefore, we conducted an analysis comparing AF patients with obesity (BMI ≥ 30 kg/m2) and non-obesity (BMI < 30 kg/m2) treated with DOAC exclusively. This study included all of the pivotal DOAC trials for AF patients. The result showed that risk of stroke/SE was statistically lower in the obese patients compared to non-obese AF patients who used DOAC, with low-certainty evidence. Major bleeding risk was similar between obese and non-obese patients with AF who used DOAC, with low-certainty evidence. This minimized the concern of unfavourable bleeding of different DOAC from the prior analysis, and reassured that the class effect of DOACs had similar safety and efficacy profiles in obese patients with AF.
Limitation
We acknowledge several limitations in our meta-analysis. Firstly, this meta-analysis was based on post hoc analysis of RCTs and observational studies. This carries a risk of potential biases due to the nature of the study design. Secondly, in analysis of DOAC vs. VKA in morbidly obese patients, two large studies were excluded in the initial analysis due to lack of raw data.18,39 These two studies were included in a sensitivity analysis by using adjusted effect size from multivariate analyses. Some studies did not report adjusted effect size, therefore, univariate effect size was used. The adjusted effect size may help reduce some confounding variables, but not entirely. Thirdly, a study from Bianco et al.32 is an abstract. This has not yet been peer-reviewed and may need further assessment of the quality of data, but our meta-analysis tried to include as many studies as possible to avoid publication bias. The results should be interpreted with caution because the true effects are likely to be substantially different from the study result when the certainty of the evidence was rated as low to very low rating by GRADE assessment. Other cause of very low certainty of the evidence in this analysis is due to study limitations and indirectness. Some studies did not directly focus on obesity as the main outcome. Also, our analysis found only two studies that mention body weight of >120 kg as the cut-off point.13,37 This prevented our results from addressing the missing data in the ISTH recommendation. Recently, post hoc analysis of the edoxaban trial discussed patients with extreme weight (≤55 kg and ≥120 kg). Further analysis can be conduct to address the missing data on this weight group.44
Conclusion
The efficacy of DOAC in patients with morbid obesity (BMI ≥ 40 kg/m2) and AF may not be different to VKA in preventing stroke and systemic embolism. In addition, major bleeding risk may be lower in morbidly obese patients with AF who are treated with DOAC compared to standard treatment of warfarin. Safety and efficacy of DOAC may be similar between obese and non-obese patients. However, the true treatment effects are likely to be substantially different from our meta-analysis estimates. A dedicated study with direct design for this BMI group to determine the true efficacy and safety of the medication may be useful to develop evidence-based recommendations on the use of DOAC in morbidly obese patients with AF.
Supplementary material
Supplementary material is available at European Heart Journal – Cardiovascular Pharmacotherapy online.
Acknowledgements
Matt Roslund, Bassett Healthcare Network’s librarian for proofreading the manuscript and help with extensive literature searching.
Conflict of interest: None declared.
References



