-
PDF
- Split View
-
Views
-
Cite
Cite
Leonardo De Luca, Pre-treatment with dual antiplatelet therapy in non-ST-segment elevation acute coronary syndromes: landing from guidelines recommendations to real-world ground, European Heart Journal - Cardiovascular Pharmacotherapy, Volume 7, Issue 5, September 2021, Pages 442–444, https://doi.org/10.1093/ehjcvp/pvab045
Close - Share Icon Share
Current European Society of Cardiology (ESC) guidelines for the management of acute coronary syndromes (ACS) without persistent ST-segment elevation do not routinely recommend pre-treatment, defined as oral P2Y12 inhibitor administration before coronary angiography, in patients scheduled for an invasive approach (class of recommendation III; level of evidence A).1
However, it should be noted that this recommendation varied significantly over the previous versions of ESC guidelines on non-ST-elevation (NSTE)-ACS (Figure 1).1,2 Indeed, pre-treatment has been used for years with the aim of protecting patients with a confirmed diagnosis of NSTE-ACS and long intervals to coronary angiography from fatal and recurrent ischaemic events and to perform percutaneous coronary intervention (PCI) with an ideal antithrombotic milieu.2 In this regard, the effectiveness of the pre-treatment strategy was evident with the first studies on clopidogrel, an oral P2Y12 inhibitor with a slow onset of action. In both CREDO (Clopidogrel for the Reduction of Events During Observation) and PCI-CURE (Percutaneous Coronary Intervention-Clopidogrel in Unstable angina to prevent Recurrent Events) trials, ACS patients pre-treated with clopidogrel for several hours or days before PCI benefitted from a significant reduction in recurrent ischaemic events.1
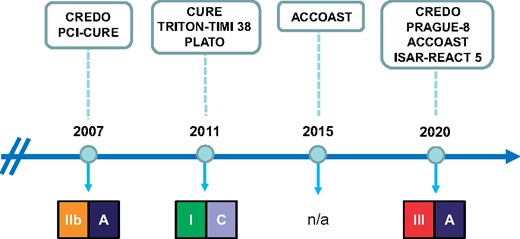
Recommendations on pre-treatment with dual antiplatelet therapy and study supporting these recommendations in the European Society of Cardiology guidelines for the management of non-ST-elevation-acute coronary syndromes published in the last two decades.
Later, ticagrelor and prasugrel, two oral P2Y12 inhibitors more effective and rapid than clopidogrel, were placed on the market. It is important to consider that, while in the PLATO (PLATelet inhibition and patient Outcomes) trial,2 which led to the approval of ticagrelor, pre-treatment for all included patients was allowed, in the TRITON-TIMI 38 (Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel–Thrombolysis in Myocardial Infarction) trial,3 pre-treatment with prasugrel was not permitted in case of NSTE-ACS. The ACCOAST (Comparison of Prasugrel at the Time of Percutaneous Coronary Intervention or as Pre-treatment at the Time of Diagnosis in Patients with Non-ST Elevation Myocardial Infarction) trial, which had the ambition to be the 1st study focused on pre-treatment, was therefore designed.4 In this study, 4033 NSTE-ACS patients were randomly assigned to receive prasugrel (a 30-mg loading dose) before the angiography (pre-treatment group) or placebo (control group).4 When PCI was indicated, an additional 30 mg of prasugrel was given in the pre-treatment group at the time of PCI and 60 mg of prasugrel was given in the control group. Several limitations were present in this study, probably leading to the increased risk of major bleeding at 7 days in the pre-treatment arm (hazard ratio, 1.90; 95% CI, 1.19–3.02; P = 0.006) with no effects on prevention of recurrent ischaemic events.5 Uncertainty about the benefits or possible harms of pre-treatment led to a substantial lack of recommendations about this strategy for NSTE-ACS patients scheduled for an invasive strategy in the 2015 ESC guidelines6 (Figure 1).
So, what has changed in this 5-year period that has led the current ESC guidelines to consider pre-treatment a dangerous strategy in patients with NSTE-ACS? If we look at the references in support of this new recommendation,1 beside CREDO, ACCOAST, and the PRAGUE-8 (Primary Angioplasty in patients transferred from General community hospitals to specialized PTCA Units with or without Emergency thrombolysis-8) trial that included only patients with stable angina, the only new randomized study on ACS is represented by the multicentre, open-label, investigators-initiated ISAR-REACT 5 (Intracoronary Stenting and Antithrombotic Regimen: Rapid Early Action for Coronary Treatment-5) trial that failed to demonstrate the superiority of ticagrelor over prasugrel in terms of the composite ischaemic outcome at 1 year. A post hoc analysis of this study reported that in patients with NSTE-ACS, prasugrel given during PCI was associated with a reduced composite risk of death, myocardial infarction (MI), and stroke as compared with ticagrelor pre-treatment during 1-year follow-up, without increasing the risk of bleeding.7 These findings contrast with the ACCOAST trial4 and other observational or randomized studies8,9 published after the release of current ESC guidelines, which instead suggested a possible harm of routine pre-treatment with P2Y12 receptor antagonists.
Notably, current ESC guidelines allow the use of parenteral inhibitors of the P2Y12 receptor in patients not pre-treated10 and have also reduced the timing to coronary angiography in high-risk NSTE-ACS within the first 24 h of the patient admission, thus defining as the strategy of choice the referral of the patient to early invasive approach without pre-treatment with dual antiplatelet therapy.1 However, large nationwide or international observational studies have documented that the timing from hospital admission for NSTE-ACS to PCI is approximately 2 days.11–15 This interval has not changed in the last decade and probably for this reason the pre-treatment is still widely used in many European countries, although not adequately studied in randomized clinical trials after the advent of ticagrelor and prasugrel.16 In addition, recent studies such as the EARLY (Early or Delayed Revascularization for Intermediate- and High-Risk Non-ST-Segment Elevation Acute Coronary Syndromes?) trial have suggested that if patients with NSTE-ACS do not receive pre-treatment and must necessarily wait several hours to undergo invasive management in a contemporary clinical scenario, the incidence of ischaemic events is still very high and significantly increased compared with patients who can undergo an invasive fast-track management without pre-treatment.17 Therefore, the only potential advantage of postponing oral P2Y12 inhibitor administration at the time of coronary angiography is to avoid increased bleeding risk in patients with an uncertain diagnosis of ACS, a circumstance that is increasing with the wide use of high-sensitive troponins, or in those requiring emergency surgery18,19 (Figure 2).
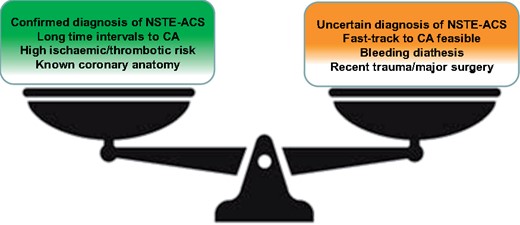
Factors favouring (green box) or opposing (red box) pre-treatment in non-ST-elevation-acute coronary syndromes. CA, coronary angiography.
In conclusion, the landing of these recommendations on the real-world ground appears complex, especially if we consider the scarce and controversial supporting evidences. It may still be reasonable to use common sense and clinical judgement by continuing to pre-treat patients with a confirmed diagnosis of NSTE-ACS who must not or cannot, for various organizational or clinical reasons, undergo an early invasive approach.
Conflict of interest: L.D.L. has received honoraria for advisory boards or as speaker/chairman at scientific congresses from Amgen, Aspen, AstraZeneca, Bayer, Boehringer Ingelheim, Chiesi, Daiichi Sankyo, Eli Lilly, Menarini, Pfizer/BMS, Sanofi, and Servier.
The opinions expressed in this article are not necessarily those of the Editors of the European Heart Journal-Cardiovascular Pharmacotherapy or of the European Society of Cardiology.



