-
PDF
- Split View
-
Views
-
Cite
Cite
Maria C Gorosabel, Nicolo Dubacher, Janine Meienberg, Gabor Matyas, Vascular Ehlers–Danlos syndrome: can the beneficial effect of celiprolol be extrapolated to bisoprolol?, European Heart Journal - Cardiovascular Pharmacotherapy, Volume 6, Issue 3, May 2020, Pages 199–200, https://doi.org/10.1093/ehjcvp/pvz067
Close - Share Icon Share
Vascular Ehlers–Danlos syndrome (vEDS; OMIM #130050) is a rare autosomal-dominant connective tissue disorder caused by pathogenic variants in the COL3A1 gene (OMIM *120180), leading among other features to an increased risk of potentially fatal arterial dissection and rupture. Recently, we have introduced a novel approach to measure the tensile force at rupture of the murine aorta, providing individual-level biomechanical read-out of the aortic wall integrity in contrast to cohort-level outcome of clinical trials limited to morbidity and mortality due to age- and other risk-factors-related arterial events (cf. incomplete penetrance and/or expressivity of arterial events). Our objective read-out allowed us to demonstrate that the beta-blocker celiprolol, but not the angiotensin-II-receptor-type-1-blocker losartan, significantly increases the reduced rupture force of the thoracic aorta in the Col3a1m1Lsmi mouse vEDS model.1 These findings explain the role of celiprolol in the only published clinical trial2 and long-term observational study in vEDS patients,3 both reporting fewer arterial events and improved survival upon celiprolol treatment. Thus, the clinically relevant question has been raised whether or not celiprolol’s beneficial effect can be extrapolated to other beta-blockers such as bisoprolol, also considering that celiprolol, unlike bisoprolol, is not available in the USA or Canada.4
To address this unmet need, we assessed the treatment efficacy of the beta-blocker bisoprolol in the same mouse model as tested for celiprolol.1 Accordingly, we treated 4-week-old heterozygous mice (Col3a1m1Lsmi/+, n = 10) for 4 weeks with bisoprolol supplemented in drinking water (333 mg/L ≈ 100 mg/kg/day bisoprolol, Bilol, Sandoz, Germany). Untreated heterozygous (Col3a1m1Lsmi/+, n = 12) and wild-type (Col3a1+/+, n = 12) littermates served as controls. Four treated heterozygous mice died due to an aortic rupture before the treatment concluded, which is comparable to the death rate of untreated heterozygous mice (Figure 1A). Maintenance and animal experimentation were in accordance with institutional and Swiss Federal Veterinary Office guidelines as reported elsewhere.1
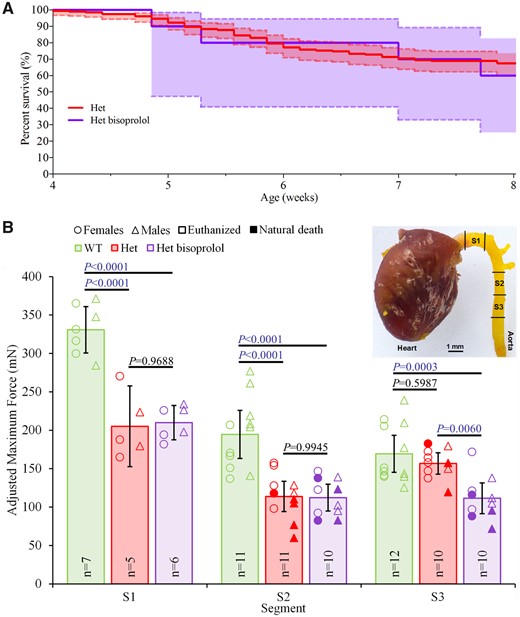
Bisoprolol does not increase the biomechanical integrity of the thoracic aorta in a mouse vascular Ehlers–Danlos syndrome model. (A) Survival curve of untreated (Het, n = 196, pooled from related experiments) and bisoprolol-treated (Het bisoprolol, n = 10) heterozygous (Col3a1m1Lsmi/+) mice. The shaded areas represent 95% confidence intervals. Note that none of the wild-type mice (WT) died during the experiment (data not shown). (B) Maximum tensile forces of three 1.5-mm-long thoracic aortic segments (S1–S3) of untreated WT and Het mice as well as Het mice treated with bisoprolol (Het bisoprolol). Note that the tensile force of segment S1 could not be measured in mice that died prematurely due to rupture of the ascending thoracic aorta. Segments damaged during sample preparation were excluded. Sample size (n) is displayed. Data are means with error bars indicating 95% confidence intervals. One-way ANOVA analysis with Tukey’s correction was performed using GraphPad Prism 8 (GraphPad Software, La Jolla, CA, USA) as previously described.1 Significant differences (P < 0.05) are shown in blue.
Using our novel read-out system as previously described,1 we measured the maximum tensile force at rupture of three uniaxially stretched thoracic aortic segments (S1–S3) per mouse (Figure 1B). Four weeks treatment with bisoprolol [S1: 209.9 mN; 95% confidence interval (CI), 187.5–232.3; S2: 112.3 mN; 95% CI, 94.9–129.7; S3: 111.5 mN; 95% CI, 91.4–131.6] did not increase the aortic rupture force when compared with untreated heterozygous mice (S1: 205.2 mN; 95% CI, 152.6–257.8; S2: 113.9 mN; 95% CI, 94.1–133.6; S3: 156.8 mN; 95% CI, 142.9–170.7). Furthermore, the aortic rupture forces of bisoprolol-treated heterozygous mice were significantly lower in comparison to untreated wild-type mice (S1: 330.7 mN; 95% CI, 300.5–360.9; S2: 194.6 mN; 95% CI, 163.4–225.9; S3: 169.3 mN; 95% CI, 145.2–193.5; Figure 1B).
Our data show that, unlike celiprolol,1 bisoprolol does not improve the biomechanical integrity of the murine vEDS thoracic aorta and thus does not have added value for treatment (i.e. beyond the beta-blocker-typical prevention of rise in blood pressure and heart rate) in our model of vEDS. Considering this lack of beneficial effect and the high dose of bisoprolol administered in our mice (far above the standard human equivalent), it is unlikely that in vEDS the beneficial effect of celiprolol observed in both mice and humans can be extrapolated to bisoprolol in humans, regardless of dose and the limitations of mouse-to-human data translation. The difference between the effect of celiprolol and bisoprolol may be because these beta-blockers belong to different generations, i.e. third-generation (vasodilating) and second-generation (non-vasodilating), respectively, and/or have different pharmacokinetic profiles4 and/or due to celiprolol’s postulated antioxidant properties.5 Consequently, although the added value of other beta-blockers in vEDS, if any, is unknown (especially for celiprolol-like third-generation beta-blockers such as carvedilol and nebivolol), celiprolol is currently the beta-blocker of choice for the medical therapy of vEDS, until further evidence emerges. Nevertheless, our findings indicate that beta-blocker does not equal beta-blocker in vEDS, challenging the paradigm that beta-blockers can be used interchangeably.
Funding
The Isaac Dreyfus-Bernheim Stiftung, the Gebauer Stiftung, and the Ernst Göhner Stiftung.
Conflict of interest: none declared.



