-
PDF
- Split View
-
Views
-
Cite
Cite
Chun-Li Wang, Victor Chien-Chia Wu, Kuo-Hsuan Chang, Hui-Tzu Tu, Chang-Fu Kuo, Yu-Tung Huang, Pao-Hsien Chu, Chi-Ching Kuo, Shang-Hung Chang, Assessing major bleeding risk in atrial fibrillation patients concurrently taking non-vitamin K antagonist oral anticoagulants and antiepileptic drugs, European Heart Journal - Cardiovascular Pharmacotherapy, Volume 6, Issue 3, May 2020, Pages 147–154, https://doi.org/10.1093/ehjcvp/pvz035
Close - Share Icon Share
Abstract
This study compared the risk of major bleeding between atrial fibrillation (AF) patients who took non-vitamin K antagonist oral anticoagulants (NOACs) and antiepileptic drugs (AEDs) concurrently and those who took only NOACs.
We performed a retrospective cohort study using Taiwan National Health Insurance database and included AF patients who received NOAC prescriptions from 1 June 2012 to 31 December 2017. The major bleeding risks of person-quarters exposed to NOAC and 11 concurrent AEDs (carbamazepine, gabapentin, lamotrigine, levetiracetam, oxcarbazepine, phenobarbital, phenytoin, pregabalin, topiramate, valproic acid, and zonisamide) were compared with person-quarters exposed to NOAC alone. Adjusted incidence rate differences between NOAC with or without concurrent AEDs were estimated using Poisson regression models weighted by the inverse probability of treatment. Among 104 319 patients (age 75.0 ± 10.3 years; men, 56.2%), 8546 major bleeding events occurred during 731 723 person-quarters with NOAC prescriptions. Concurrent AED use was found in 15.3% of NOAC-treated patients. Concurrent use of NOAC with valproic acid, phenytoin, or levetiracetam increased adjusted incidence rates per 1000 person-years of major bleeding more significantly than NOAC alone: 153.49 for NOAC plus valproic acid vs. 55.06 for NOAC alone [difference 98.43, 95% confidence interval (CI) 82.37–114.49]; 135.83 for NOAC plus phenytoin vs. 54.43 for NOAC alone (difference 81.4, 95% CI 60.14–102.66); and 132.96 for NOAC plus levetiracetam vs. 53.08 for NOAC alone (difference 79.88, 95% CI 64.47–95.30).
For AF patients, the concurrent use of NOACs and valproic acid, phenytoin, or levetiracetam was associated with a higher risk of major bleeding.
Introduction
Atrial fibrillation (AF) is an important cause of ischaemic stroke, and oral anticoagulation is frequently indicated for stroke prevention in non-valvular AF patients.1 Non-vitamin K antagonist oral anticoagulants (NOACs) are used more frequently than warfarin in this context because of their better safety and efficacy profiles, as well as fewer drug–drug interactions.2 However, NOACs still pose clinically relevant bleeding risks especially in patients with multiple comorbidities, polypharmacy or those using high-risk medications.3 Significant drug–drug interactions are more likely to occur when NOACs are co-administered with medications that change the activity of permeability glycoprotein (P-gp) or cytochrome P450 3A4 (CYP3A4) system.4 Antiepileptic drugs (AEDs) that inhibit CYP3A4 or P-gp activity may increase NOAC levels and the risk of bleeding; conversely, AEDs that cause induction of CYP3A4 or P-gp activity may decrease NOAC levels and antithrombotic efficacy.4 In our previous study that evaluated the risk of major bleeding in patients taking NOACs and concurrent medications affecting P-gp and/or CYP3A4 system, the risk of major bleeding was significantly higher with concomitant use of NOAC with amiodarone, fluconazole, rifampin, or phenytoin than NOAC alone.3 Phenytoin is an AED with anticipated effects of CYP3A4/P-gp induction and P-gp competition.3,4
Antiepileptic drugs are commonly used as a long-term mono- or adjunctive-therapy in epilepsy or other applications, e.g. psychiatric illness, migraine, or neuropathic pain.5,6 Antiepileptic drugs as a group of drugs are susceptible to drug–drug interactions with NOACs, yet little is known about the clinical relevance of these interactions.7 There are limited clinical or pharmacokinetic data available, and recommendations in AF guidelines are primarily derived from observations in animal or in vitro studies and information provided in each of the NOAC summary of product characteristics.4,8 The effect of the concurrent use of AEDs other than phenytoin on the bleeding risk in NOAC users has not previously been assessed.
Hence, the aim of this study is to evaluate and compare the major bleeding risk between NOAC users who concurrently use AEDs and those who took NOAC alone in a nationwide retrospective cohort of AF patients.
Methods
Data source
This retrospective cohort study was approved by the institutional review board of Chang Gung Memorial Hospital, Linkou, Taiwan and complied fully with the existing national ethical and regulatory guidelines. The need to provide written informed consent was waived by the ethics committee because all data were anonymized by the Taiwan National Health Insurance (NHI) Administration. The Taiwan NHI system was established in 1995 as a single-payer compulsory insurance programme co-funded by the government, employers, and beneficiaries. By the end of 2017, more than 23 million beneficiaries, or more than 99.5% of the nation’s population, were registered under the NHI. Our analyses were based on the NHI claims database, which included outpatient, inpatient, and prescription records accessed through the Applied Health Research Data Integration Service Center from the NHI Administration in Taiwan. The whole database is linked by the encrypted patients’ identification. Diagnoses/procedures were recorded using the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes from 1997 through 2015 and the International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM) codes since 2016.
Study population
We identified 104 319 patients (outpatients and/or inpatients) with two or more consecutive records of AF diagnosis (ICD-9-CM code 427.31 or ICD-10-CM code I48) and NOAC (dabigatran, rivaroxaban, apixaban, or edoxaban) prescriptions for greater than 28 days from 1 June 2012 to 31 December 2017. The index date was defined as the first NOAC prescription. Patients were excluded if they had pulmonary embolism or deep vein thrombosis within 6 months before the index date, had received joint replacement or valvular surgery within 6 months before the index date, had had mitral stenosis <6 months before the index date, had had end-stage renal disease, or had been younger than 30 years of age. Patients were followed up until death, withdrawal from the NHI, or the end of the study (31 December 2017). The study flowchart is shown in Figure 1.
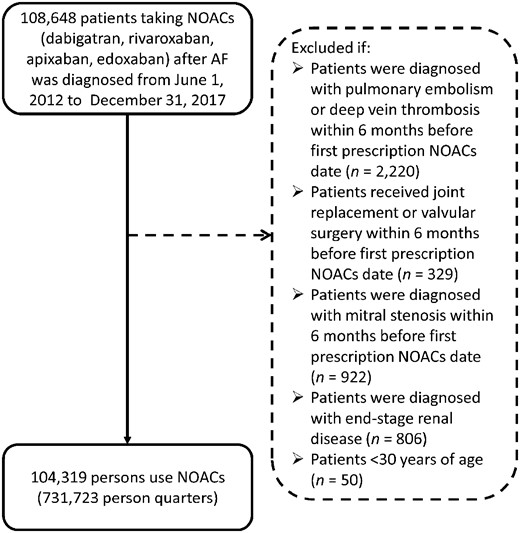
Enrollment of patients with non-valvular atrial fibrillation. From 1 June 2012 through 31 December 2017, this study evaluated a total of 108 648 atrial fibrillation patients using non-vitamin K antagonist oral anticoagulants.
Follow-up time and person-quarters
In this study, each calendar year was partitioned into four quarters for each patient and each year after the first NOAC prescription. The analytic unit was one person-quarter. Person-quarters were used because medications for chronic illnesses were refilled with a maximum length of 3 months under the NHI reimbursement policy. Medications and covariates were assessed for each person-quarter, which simplified the assessment of the complex prescription pattern of NOACs and concurrent drugs. We identified person-quarters exposed to NOACs with or without concurrent medications. The major bleeding risks of person-quarters exposed to NOACs and 11 concurrent AEDs (carbamazepine, gabapentin, lamotrigine, levetiracetam, oxcarbazepine, phenobarbital, phenytoin, pregabalin, topiramate, valproic acid, or zonisamide) were compared with person-quarters exposed to NOAC alone. We employed a blanking period of three months, the first person-quarter. Bleeding events occurring within the first person-quarter were not classified as outcome events.
Major outcomes
The primary outcome was major bleeding, defined as the hospitalization or the emergency department visit with a primary diagnosis of intracranial, gastrointestinal, or bleeding in other sites, as previously described.9 People with traumatic haemorrhage were excluded from analysis. Only one major-bleeding event was included in each person-quarter. Cases of major bleeding were identified by ICD codes. The sensitivity and specificity of ICD codes to detect major bleeding were 100% and 83.1%.10 Details of case definitions are listed in Supplementary material online, Table S1.
Covariates
Demographics, comorbidities, medications, and healthcare utilization of the patients were identified as covariates. These covariates were assessed for each person-quarter pertinent to the first date of the person-quarter. Patient demographics included age, sex, and socioeconomic factors. We assessed components of the Charlson comorbidity index,11 CHA2DS2-VASc score [congestive heart failure, hypertension, age ≥75 (doubled), diabetes mellitus, prior stroke or transient ischaemic attack [doubled], vascular disease, age 65–74, female],12 HAS-BLED score (hypertension, abnormal kidney or liver function, stroke, bleeding history, and alcohol use),13 comorbidities (dementia, chronic pulmonary disease, anaemia, kidney diseases, and hepatic diseases), and concurrent medications. The code lists of these covariates are shown in Supplementary material online, Table S1.
Models
We considered confounding by indication, which occurs from non-random treatment allocation for concurrent medications, as an essential feature when comparing bleeding risk among patients with concurrent NOAC and AED use vs. NOAC alone. The inverse probability of treatment weighting using the propensity score was performed to account for this bias. A propensity score was the probability that a patient was prescribed the concurrent AED during a person-quarter. For each person, a specific propensity score for a specific AED was calculated using logistic regression considering the aforementioned covariates pertinent to the first date of the person-quarter. Standardized differences were estimated to assess the balance of individual covariates before and after propensity score weighting. The balance of covariates was assessed using the absolute standardized mean difference. A negligible difference was defined as an absolute standardized mean difference <0.1. Supplementary material online, Tables S2A–K summarize the balance of covariates between users and nonusers for each AED.
Statistical analysis
We used a Poisson regression with a generalized estimating equations model to account for intra-individual correlation across person-quarters to calculate the adjusted incidence rate difference, incidence rate ratios, and 95% confidence intervals (CIs) that considered the inverse probability of treatment weighting using the propensity score. Person-quarters using NOAC alone were used as the reference category. Because 11 types of combinations were studied, the regression analysis was performed separately for each combination.
Three additional analyses were performed to ascertain the association between the combination of a NOAC plus concurrent AEDs and major bleeding: (i) the association between the combination of an NOAC and an angiotensin II receptor blocker (a medication to replace AED in the model as a negative control exposure) and major bleeding. (ii) The association between an NOAC plus a specific concurrent AED and acute pancreatitis, intestinal obstruction, or acute appendicitis (as negative control outcomes). (iii) The third sensitivity analysis evaluated whether including bleeding events occurred within the first person-quarter would affect the main result.
The Bonferroni correction was used to compensate for the possibility of an increase in Type I error due to multiple comparisons. Missing data were present among patients without a valid insurance status (estimated in <0.1% of NOAC users), and data associated with these patients were excluded. The entire analysis was performed using SAS (SAS Institute), version 9.4.
Results
Patient characteristics
We identified a total of 104 319 AF patients on NOAC therapy from 1 June 2012 to 31 December 2017. The characteristics of the AF patients at the first date of NOAC prescription are listed in Tables 1 and 2. The mean age was 75.0 ± 10.3 years, and 56.2% of the studied population were men. The baseline average CHA2DS2-VASc score was 4.3 ± 1.7, and the average HAS-BLED score was 2.4 ± 1.0. More than 40% of the included patients had been diagnosed with heart failure or cerebrovascular disease and more than one third with diabetes mellitus. A total of 15.3% of NOAC-treated AF patients received concurrent use of NOAC and AED.
| . | NOAC users (n = 104 319) . |
|---|---|
| Age (years) | 75.0 ± 10.3 |
| Men | 58 674 (56.2) |
| Residence | |
| Urban | 56 336 (54.0) |
| Suburban | 32 879 (31.5) |
| Rural | 14 485 (13.9) |
| Unknown | 619 (0.6) |
| Occupation | |
| Dependents of the insured individuals | 41 189 (39.5) |
| Civil servants, teachers, military personnel, and veterans | 1577 (1.5) |
| Non-manual workers and professionals | 6094 (5.8) |
| Manual workers | 33 285 (31.9) |
| Other | 22 174 (21.3) |
| Income | |
| Quintile 1 | 30 628 (29.4) |
| Quintile 2 | 11 792 (11.3) |
| Quintile 3 | 33 104 (31.7) |
| Quintile 4 | 9347 (9.0) |
| Quintile 5 | 19 443 (18.6) |
| CHA2DS2-VASc score | 4.3 ± 1.7 |
| HAS-BLED score | 2.4 ± 1.0 |
| Charlson comorbidity index | 3.4 ± 2.4 |
| Cardiovascular diseases | |
| Hypertension | 86 579 (83.0) |
| Myocardial infarction | 4884 (4.7) |
| Congestive heart failure | 45 533 (43.7) |
| Peripheral vascular disease | 8452 (8.1) |
| Percutaneous coronary intervention | 6429 (6.2) |
| Coronary artery bypass surgery | 394 (0.4) |
| Diseases of the nervous system | |
| Cerebral vascular disease | 44 137 (42.3) |
| Ischaemic stroke | 33 096 (31.7) |
| Transient ischaemic attack | 8419 (8.1) |
| Hemiplegia and paraplegia | 4570 (4.4) |
| Dementia | 10 959 (10.5) |
| Metabolic disease | |
| Diabetes mellitus | 38 114 (36.5) |
| Diabetes with complications | 13 407 (12.9) |
| Pulmonary disease | |
| Chronic pulmonary disease | 37 454 (35.9) |
| Chronic obstructive pulmonary disease | 34 019 (32.6) |
| Chronic kidney disease | 22 348 (21.4) |
| Gastrointestinal and hepatic diseases | |
| Peptic ulcer disease | 37 776 (36.2) |
| Mild liver disease | 18 945 (18.2) |
| Moderate or severe liver disease | 266 (0.3) |
| . | NOAC users (n = 104 319) . |
|---|---|
| Age (years) | 75.0 ± 10.3 |
| Men | 58 674 (56.2) |
| Residence | |
| Urban | 56 336 (54.0) |
| Suburban | 32 879 (31.5) |
| Rural | 14 485 (13.9) |
| Unknown | 619 (0.6) |
| Occupation | |
| Dependents of the insured individuals | 41 189 (39.5) |
| Civil servants, teachers, military personnel, and veterans | 1577 (1.5) |
| Non-manual workers and professionals | 6094 (5.8) |
| Manual workers | 33 285 (31.9) |
| Other | 22 174 (21.3) |
| Income | |
| Quintile 1 | 30 628 (29.4) |
| Quintile 2 | 11 792 (11.3) |
| Quintile 3 | 33 104 (31.7) |
| Quintile 4 | 9347 (9.0) |
| Quintile 5 | 19 443 (18.6) |
| CHA2DS2-VASc score | 4.3 ± 1.7 |
| HAS-BLED score | 2.4 ± 1.0 |
| Charlson comorbidity index | 3.4 ± 2.4 |
| Cardiovascular diseases | |
| Hypertension | 86 579 (83.0) |
| Myocardial infarction | 4884 (4.7) |
| Congestive heart failure | 45 533 (43.7) |
| Peripheral vascular disease | 8452 (8.1) |
| Percutaneous coronary intervention | 6429 (6.2) |
| Coronary artery bypass surgery | 394 (0.4) |
| Diseases of the nervous system | |
| Cerebral vascular disease | 44 137 (42.3) |
| Ischaemic stroke | 33 096 (31.7) |
| Transient ischaemic attack | 8419 (8.1) |
| Hemiplegia and paraplegia | 4570 (4.4) |
| Dementia | 10 959 (10.5) |
| Metabolic disease | |
| Diabetes mellitus | 38 114 (36.5) |
| Diabetes with complications | 13 407 (12.9) |
| Pulmonary disease | |
| Chronic pulmonary disease | 37 454 (35.9) |
| Chronic obstructive pulmonary disease | 34 019 (32.6) |
| Chronic kidney disease | 22 348 (21.4) |
| Gastrointestinal and hepatic diseases | |
| Peptic ulcer disease | 37 776 (36.2) |
| Mild liver disease | 18 945 (18.2) |
| Moderate or severe liver disease | 266 (0.3) |
Values are expressed in n (%) or mean ± standard deviation.
| . | NOAC users (n = 104 319) . |
|---|---|
| Age (years) | 75.0 ± 10.3 |
| Men | 58 674 (56.2) |
| Residence | |
| Urban | 56 336 (54.0) |
| Suburban | 32 879 (31.5) |
| Rural | 14 485 (13.9) |
| Unknown | 619 (0.6) |
| Occupation | |
| Dependents of the insured individuals | 41 189 (39.5) |
| Civil servants, teachers, military personnel, and veterans | 1577 (1.5) |
| Non-manual workers and professionals | 6094 (5.8) |
| Manual workers | 33 285 (31.9) |
| Other | 22 174 (21.3) |
| Income | |
| Quintile 1 | 30 628 (29.4) |
| Quintile 2 | 11 792 (11.3) |
| Quintile 3 | 33 104 (31.7) |
| Quintile 4 | 9347 (9.0) |
| Quintile 5 | 19 443 (18.6) |
| CHA2DS2-VASc score | 4.3 ± 1.7 |
| HAS-BLED score | 2.4 ± 1.0 |
| Charlson comorbidity index | 3.4 ± 2.4 |
| Cardiovascular diseases | |
| Hypertension | 86 579 (83.0) |
| Myocardial infarction | 4884 (4.7) |
| Congestive heart failure | 45 533 (43.7) |
| Peripheral vascular disease | 8452 (8.1) |
| Percutaneous coronary intervention | 6429 (6.2) |
| Coronary artery bypass surgery | 394 (0.4) |
| Diseases of the nervous system | |
| Cerebral vascular disease | 44 137 (42.3) |
| Ischaemic stroke | 33 096 (31.7) |
| Transient ischaemic attack | 8419 (8.1) |
| Hemiplegia and paraplegia | 4570 (4.4) |
| Dementia | 10 959 (10.5) |
| Metabolic disease | |
| Diabetes mellitus | 38 114 (36.5) |
| Diabetes with complications | 13 407 (12.9) |
| Pulmonary disease | |
| Chronic pulmonary disease | 37 454 (35.9) |
| Chronic obstructive pulmonary disease | 34 019 (32.6) |
| Chronic kidney disease | 22 348 (21.4) |
| Gastrointestinal and hepatic diseases | |
| Peptic ulcer disease | 37 776 (36.2) |
| Mild liver disease | 18 945 (18.2) |
| Moderate or severe liver disease | 266 (0.3) |
| . | NOAC users (n = 104 319) . |
|---|---|
| Age (years) | 75.0 ± 10.3 |
| Men | 58 674 (56.2) |
| Residence | |
| Urban | 56 336 (54.0) |
| Suburban | 32 879 (31.5) |
| Rural | 14 485 (13.9) |
| Unknown | 619 (0.6) |
| Occupation | |
| Dependents of the insured individuals | 41 189 (39.5) |
| Civil servants, teachers, military personnel, and veterans | 1577 (1.5) |
| Non-manual workers and professionals | 6094 (5.8) |
| Manual workers | 33 285 (31.9) |
| Other | 22 174 (21.3) |
| Income | |
| Quintile 1 | 30 628 (29.4) |
| Quintile 2 | 11 792 (11.3) |
| Quintile 3 | 33 104 (31.7) |
| Quintile 4 | 9347 (9.0) |
| Quintile 5 | 19 443 (18.6) |
| CHA2DS2-VASc score | 4.3 ± 1.7 |
| HAS-BLED score | 2.4 ± 1.0 |
| Charlson comorbidity index | 3.4 ± 2.4 |
| Cardiovascular diseases | |
| Hypertension | 86 579 (83.0) |
| Myocardial infarction | 4884 (4.7) |
| Congestive heart failure | 45 533 (43.7) |
| Peripheral vascular disease | 8452 (8.1) |
| Percutaneous coronary intervention | 6429 (6.2) |
| Coronary artery bypass surgery | 394 (0.4) |
| Diseases of the nervous system | |
| Cerebral vascular disease | 44 137 (42.3) |
| Ischaemic stroke | 33 096 (31.7) |
| Transient ischaemic attack | 8419 (8.1) |
| Hemiplegia and paraplegia | 4570 (4.4) |
| Dementia | 10 959 (10.5) |
| Metabolic disease | |
| Diabetes mellitus | 38 114 (36.5) |
| Diabetes with complications | 13 407 (12.9) |
| Pulmonary disease | |
| Chronic pulmonary disease | 37 454 (35.9) |
| Chronic obstructive pulmonary disease | 34 019 (32.6) |
| Chronic kidney disease | 22 348 (21.4) |
| Gastrointestinal and hepatic diseases | |
| Peptic ulcer disease | 37 776 (36.2) |
| Mild liver disease | 18 945 (18.2) |
| Moderate or severe liver disease | 266 (0.3) |
Values are expressed in n (%) or mean ± standard deviation.
| . | NOAC users (n = 104 319) . |
|---|---|
| Aspirin | 49 400 (47.4) |
| Clopidegrel | 13 354 (12.8) |
| Ticlopidine | 2756 (2.6) |
| Ticagrelor | 1331 (1.3) |
| Bisoprolol | 39 826 (38.2) |
| Amiodarone | 26 905 (25.8) |
| Digoxin | 22 892 (21.0) |
| Diltiazem | 22 221 (21.3) |
| Metoprolol | 1524 (1.5) |
| Labetalol | 3632 (3.5) |
| Propranolol | 13 672 (13.1) |
| Dronedarone | 3802 (3.6) |
| Cyclosporine | 73 (0.1) |
| Itraconazole | 108 (0.1) |
| Ketoconazole | 206 (0.2) |
| Erythromycin | 2856 (2.7) |
| Atorvastatin | 16 158 (15.5) |
| Rifampin | 294 (0.3) |
| Bisphosphonate | 794 (0.8) |
| Fluvastatin | 1809 (1.7) |
| Pravastatin | 2082 (2.0) |
| Pitavastatin | 4107 (3.9) |
| Ezetimibe | 124 (0.1) |
| Insulin | 9635 (9.2) |
| Irbesartan | 6144 (5.9) |
| Losartan | 6349 (6.1) |
| Olmesartan | 2654 (2.5) |
| Glucocorticoid | 10 745 (10.3) |
| Non-steroid anti-inflammatory drugs | 25 291 (24.2) |
| . | NOAC users (n = 104 319) . |
|---|---|
| Aspirin | 49 400 (47.4) |
| Clopidegrel | 13 354 (12.8) |
| Ticlopidine | 2756 (2.6) |
| Ticagrelor | 1331 (1.3) |
| Bisoprolol | 39 826 (38.2) |
| Amiodarone | 26 905 (25.8) |
| Digoxin | 22 892 (21.0) |
| Diltiazem | 22 221 (21.3) |
| Metoprolol | 1524 (1.5) |
| Labetalol | 3632 (3.5) |
| Propranolol | 13 672 (13.1) |
| Dronedarone | 3802 (3.6) |
| Cyclosporine | 73 (0.1) |
| Itraconazole | 108 (0.1) |
| Ketoconazole | 206 (0.2) |
| Erythromycin | 2856 (2.7) |
| Atorvastatin | 16 158 (15.5) |
| Rifampin | 294 (0.3) |
| Bisphosphonate | 794 (0.8) |
| Fluvastatin | 1809 (1.7) |
| Pravastatin | 2082 (2.0) |
| Pitavastatin | 4107 (3.9) |
| Ezetimibe | 124 (0.1) |
| Insulin | 9635 (9.2) |
| Irbesartan | 6144 (5.9) |
| Losartan | 6349 (6.1) |
| Olmesartan | 2654 (2.5) |
| Glucocorticoid | 10 745 (10.3) |
| Non-steroid anti-inflammatory drugs | 25 291 (24.2) |
Values are expressed in n (%) or mean ± standard deviation.
| . | NOAC users (n = 104 319) . |
|---|---|
| Aspirin | 49 400 (47.4) |
| Clopidegrel | 13 354 (12.8) |
| Ticlopidine | 2756 (2.6) |
| Ticagrelor | 1331 (1.3) |
| Bisoprolol | 39 826 (38.2) |
| Amiodarone | 26 905 (25.8) |
| Digoxin | 22 892 (21.0) |
| Diltiazem | 22 221 (21.3) |
| Metoprolol | 1524 (1.5) |
| Labetalol | 3632 (3.5) |
| Propranolol | 13 672 (13.1) |
| Dronedarone | 3802 (3.6) |
| Cyclosporine | 73 (0.1) |
| Itraconazole | 108 (0.1) |
| Ketoconazole | 206 (0.2) |
| Erythromycin | 2856 (2.7) |
| Atorvastatin | 16 158 (15.5) |
| Rifampin | 294 (0.3) |
| Bisphosphonate | 794 (0.8) |
| Fluvastatin | 1809 (1.7) |
| Pravastatin | 2082 (2.0) |
| Pitavastatin | 4107 (3.9) |
| Ezetimibe | 124 (0.1) |
| Insulin | 9635 (9.2) |
| Irbesartan | 6144 (5.9) |
| Losartan | 6349 (6.1) |
| Olmesartan | 2654 (2.5) |
| Glucocorticoid | 10 745 (10.3) |
| Non-steroid anti-inflammatory drugs | 25 291 (24.2) |
| . | NOAC users (n = 104 319) . |
|---|---|
| Aspirin | 49 400 (47.4) |
| Clopidegrel | 13 354 (12.8) |
| Ticlopidine | 2756 (2.6) |
| Ticagrelor | 1331 (1.3) |
| Bisoprolol | 39 826 (38.2) |
| Amiodarone | 26 905 (25.8) |
| Digoxin | 22 892 (21.0) |
| Diltiazem | 22 221 (21.3) |
| Metoprolol | 1524 (1.5) |
| Labetalol | 3632 (3.5) |
| Propranolol | 13 672 (13.1) |
| Dronedarone | 3802 (3.6) |
| Cyclosporine | 73 (0.1) |
| Itraconazole | 108 (0.1) |
| Ketoconazole | 206 (0.2) |
| Erythromycin | 2856 (2.7) |
| Atorvastatin | 16 158 (15.5) |
| Rifampin | 294 (0.3) |
| Bisphosphonate | 794 (0.8) |
| Fluvastatin | 1809 (1.7) |
| Pravastatin | 2082 (2.0) |
| Pitavastatin | 4107 (3.9) |
| Ezetimibe | 124 (0.1) |
| Insulin | 9635 (9.2) |
| Irbesartan | 6144 (5.9) |
| Losartan | 6349 (6.1) |
| Olmesartan | 2654 (2.5) |
| Glucocorticoid | 10 745 (10.3) |
| Non-steroid anti-inflammatory drugs | 25 291 (24.2) |
Values are expressed in n (%) or mean ± standard deviation.
Major bleeding events
During follow-up, 8546 major bleeding events occurred during 731 723 person-quarters with NOAC prescriptions. Table 3 summarizes the incidence rate, adjusted incidence rate, and adjusted incidence rate difference for major bleeding events among the 11 combinations of an NOAC and an AED. The most common AEDs prescribed with concurrent NOACs were valproic acid (1.48%), followed by levetiracetam (1.44%), gabapentin (0.85%), phenobarbital (0.78%), and phenytoin (0.71%). The combinations of an NOAC with valproic acid, phenytoin, and levetiracetam were significantly associated with higher risks of major bleeding than NOAC alone (Figure 2). Compared with person-quarters of NOAC use alone, the adjusted incidence rate differences per 1000 person-years of major bleeding for an NOAC combined with other medications were 98.43 (95% CI 82.37–114.49) with valproic acid, 81.40 (95% CI 60.14–102.66) with phenytoin, and 79.88 (95% CI 64.47–95.30) with levetiracetam. The combinations of an NOAC and either carbamazepine, gabapentin, lamotrigine, oxcarbazepine, phenobarbital, pregabalin, topiramate, or zonisamide were not associated with higher risks of major bleeding.
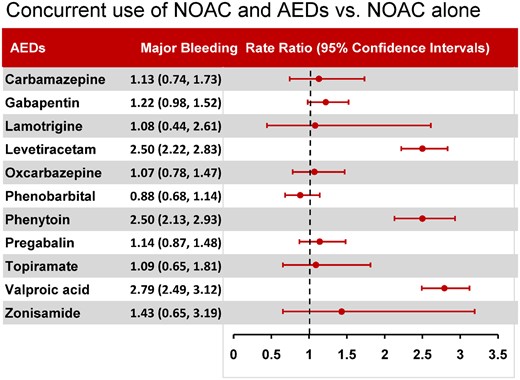
Major bleeding risk associated with concurrent use of non-vitamin K antagonist oral anticoagulant and antiepileptic drugs vs. non-vitamin K antagonist oral anticoagulant use alone.
Major bleeding risk among AF patients taking a non-vitamin K antagonist oral anticoagulant for atrial fibrillation with or without concurrent antiepileptic medications
| Concurrent antiepileptic medication . | Person-quarters with NOAC use . | Number of bleeding events . | Crude major bleeding incidence rate (95% CI) per 1000 person-years . | Adjusted incidence rate (95% CI) per 1000 person-yearsa . | Adjusted incidence rate difference (95% CI) per 1000 person-yearsa . | Adjusted rate ratio (95% CI)a . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Carbamazepine | ||||||||||
| With | 1530 | 21 | 56.04 (36.74–85.49) | 55.65 (36.49–84.88) | 6.47 (–17.06–30.01) | 1.13 (0.74–1.73) | ||||
| Withoutb | 730 193 | 8525 | 47.16 (46.07–48.28) | 49.18 (47.74–50.65) | 1 | |||||
| Gabapentin | ||||||||||
| With | 6227 | 108 | 68.75 (55.06– 85.85) | 69.74 (56.04–86.78) | 12.76 (–2.53–28.05) | 1.22 (0.98–1.52) | ||||
| Withoutb | 725 496 | 8438 | 46.99 (45.90–48.10) | 56.98 (55.32–58.69) | 1 | |||||
| Lamotrigine | ||||||||||
| With | 463 | 6 | 50.11 (20.03–125.31) | 51.36 (21.24–124.22) | 3.64 (–41.76–49.04) | 1.08 (0.44–2.61) | ||||
| Withoutb | 731 260 | 8540 | 47.18 (46.09–48.29) | 47.72 (45.37–50.20) | 1 | |||||
| Levetiracetam | ||||||||||
| With | 10 592 | 340 | 131.3 (116.95–147.42) | 132.96 (118.54–149.15) | 79.88 (64.47–95.30) | 2.5 (2.22–2.83) | ||||
| Withoutb | 721 131 | 8206 | 45.95 (44.87–47.05) | 53.08 (50.96–55.30) | 1 | |||||
| Oxcarbazepine | ||||||||||
| With | 2761 | 38 | 55.9 (40.80–76.58) | 55.95 (40.89–76.55) | 3.77 (–13.85–21.39) | 1.07 (0.78–1.47) | ||||
| Withoutb | 728 962 | 8508 | 47.15 (46.06–48.26) | 52.18 (50.52–53.90) | 1 | |||||
| Phenobarbital | ||||||||||
| With | 5702 | 60 | 42.09 (32.61–54.34) | 42.25 (32.74–54.52) | –5.55 (–16.37–5.27) | 0.88 (0.68–1.14) | ||||
| Withoutb | 726 021 | 8486 | 47.22 (46.13–48.34) | 47.8 (46.52–49.11) | 1 | |||||
| Phenytoin | ||||||||||
| With | 5190 | 171 | 134.78 (115.20–157.68) | 135.83 (116.23–158.72) | 81.4 (60.14–102.66) | 2.5 (2.13–2.93) | ||||
| Withoutb | 726 533 | 8375 | 46.55 (45.47–47.66) | 54.43 (52.35–56.59) | 1 | |||||
| Pregabalin | ||||||||||
| With | 4324 | 67 | 61.03 (46.67–79.80) | 62.71 (48.27–81.49) | 7.59 (–8.92–24.10) | 1.14 (0.87–1.48) | ||||
| Withoutb | 727 399 | 8479 | 47.1 (46.01–48.21) | 55.12 (53.40–56.91) | 1 | |||||
| Topiramate | ||||||||||
| With | 1110 | 15 | 55.16 (33.08–91.97) | 54.83 (33.02–91.04) | 4.4 (–23.47–32.26) | 1.09 (0.65–1.81) | ||||
| Withoutb | 730 613 | 8531 | 47.17 (46.08–48.28) | 50.43 (48.68–52.24) | 1 | |||||
| Valproic acid | ||||||||||
| With | 10 848 | 401 | 151.3 (136.30–167.96) | 153.49 (138.40–170.23) | 98.43 (82.37–114.49) | 2.79 (2.49–3.12) | ||||
| Withoutb | 720 875 | 8145 | 45.62 (44.54–46.72) | 55.06 (52.77– 57.45) | 1 | |||||
| Zonisamide | ||||||||||
| With | 336 | 6 | 72.83 (32.63–162.56) | 72.24 (32.58–160.19) | 21.87 (–35.74–79.47) | 1.43 (0.65–3.19) | ||||
| Withoutb | 731 387 | 8540 | 47.17 (46.08–48.28) | 50.38 (47.71–53.19) | 1 |
| Concurrent antiepileptic medication . | Person-quarters with NOAC use . | Number of bleeding events . | Crude major bleeding incidence rate (95% CI) per 1000 person-years . | Adjusted incidence rate (95% CI) per 1000 person-yearsa . | Adjusted incidence rate difference (95% CI) per 1000 person-yearsa . | Adjusted rate ratio (95% CI)a . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Carbamazepine | ||||||||||
| With | 1530 | 21 | 56.04 (36.74–85.49) | 55.65 (36.49–84.88) | 6.47 (–17.06–30.01) | 1.13 (0.74–1.73) | ||||
| Withoutb | 730 193 | 8525 | 47.16 (46.07–48.28) | 49.18 (47.74–50.65) | 1 | |||||
| Gabapentin | ||||||||||
| With | 6227 | 108 | 68.75 (55.06– 85.85) | 69.74 (56.04–86.78) | 12.76 (–2.53–28.05) | 1.22 (0.98–1.52) | ||||
| Withoutb | 725 496 | 8438 | 46.99 (45.90–48.10) | 56.98 (55.32–58.69) | 1 | |||||
| Lamotrigine | ||||||||||
| With | 463 | 6 | 50.11 (20.03–125.31) | 51.36 (21.24–124.22) | 3.64 (–41.76–49.04) | 1.08 (0.44–2.61) | ||||
| Withoutb | 731 260 | 8540 | 47.18 (46.09–48.29) | 47.72 (45.37–50.20) | 1 | |||||
| Levetiracetam | ||||||||||
| With | 10 592 | 340 | 131.3 (116.95–147.42) | 132.96 (118.54–149.15) | 79.88 (64.47–95.30) | 2.5 (2.22–2.83) | ||||
| Withoutb | 721 131 | 8206 | 45.95 (44.87–47.05) | 53.08 (50.96–55.30) | 1 | |||||
| Oxcarbazepine | ||||||||||
| With | 2761 | 38 | 55.9 (40.80–76.58) | 55.95 (40.89–76.55) | 3.77 (–13.85–21.39) | 1.07 (0.78–1.47) | ||||
| Withoutb | 728 962 | 8508 | 47.15 (46.06–48.26) | 52.18 (50.52–53.90) | 1 | |||||
| Phenobarbital | ||||||||||
| With | 5702 | 60 | 42.09 (32.61–54.34) | 42.25 (32.74–54.52) | –5.55 (–16.37–5.27) | 0.88 (0.68–1.14) | ||||
| Withoutb | 726 021 | 8486 | 47.22 (46.13–48.34) | 47.8 (46.52–49.11) | 1 | |||||
| Phenytoin | ||||||||||
| With | 5190 | 171 | 134.78 (115.20–157.68) | 135.83 (116.23–158.72) | 81.4 (60.14–102.66) | 2.5 (2.13–2.93) | ||||
| Withoutb | 726 533 | 8375 | 46.55 (45.47–47.66) | 54.43 (52.35–56.59) | 1 | |||||
| Pregabalin | ||||||||||
| With | 4324 | 67 | 61.03 (46.67–79.80) | 62.71 (48.27–81.49) | 7.59 (–8.92–24.10) | 1.14 (0.87–1.48) | ||||
| Withoutb | 727 399 | 8479 | 47.1 (46.01–48.21) | 55.12 (53.40–56.91) | 1 | |||||
| Topiramate | ||||||||||
| With | 1110 | 15 | 55.16 (33.08–91.97) | 54.83 (33.02–91.04) | 4.4 (–23.47–32.26) | 1.09 (0.65–1.81) | ||||
| Withoutb | 730 613 | 8531 | 47.17 (46.08–48.28) | 50.43 (48.68–52.24) | 1 | |||||
| Valproic acid | ||||||||||
| With | 10 848 | 401 | 151.3 (136.30–167.96) | 153.49 (138.40–170.23) | 98.43 (82.37–114.49) | 2.79 (2.49–3.12) | ||||
| Withoutb | 720 875 | 8145 | 45.62 (44.54–46.72) | 55.06 (52.77– 57.45) | 1 | |||||
| Zonisamide | ||||||||||
| With | 336 | 6 | 72.83 (32.63–162.56) | 72.24 (32.58–160.19) | 21.87 (–35.74–79.47) | 1.43 (0.65–3.19) | ||||
| Withoutb | 731 387 | 8540 | 47.17 (46.08–48.28) | 50.38 (47.71–53.19) | 1 |
Adjusted by inverse probability of treatment weighting using the propensity score (sex, age, medical utilization, chronic kidney disease stage, anaemia, myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, dementia, chronic pulmonary disease, rheumatic disease, peptic ulcer disease, mild liver disease, diabetes, hemiplegia or paraplegia, moderate or severe liver disease, percutaneous coronary intervention, coronary artery bypass surgery, transient ischaemic attack, hypertension, aspirin, clopidogrel, ticagrelor, ticlopidine, warfarin, glucocorticoids, insulin, lipid-lowering agents, hypoglycaemic agents, antihypertensive, non-steroid anti-inflammatory drugs, proton pump inhibitors, residence, income level, and occupation).
Without indicates NOAC alone.
Major bleeding risk among AF patients taking a non-vitamin K antagonist oral anticoagulant for atrial fibrillation with or without concurrent antiepileptic medications
| Concurrent antiepileptic medication . | Person-quarters with NOAC use . | Number of bleeding events . | Crude major bleeding incidence rate (95% CI) per 1000 person-years . | Adjusted incidence rate (95% CI) per 1000 person-yearsa . | Adjusted incidence rate difference (95% CI) per 1000 person-yearsa . | Adjusted rate ratio (95% CI)a . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Carbamazepine | ||||||||||
| With | 1530 | 21 | 56.04 (36.74–85.49) | 55.65 (36.49–84.88) | 6.47 (–17.06–30.01) | 1.13 (0.74–1.73) | ||||
| Withoutb | 730 193 | 8525 | 47.16 (46.07–48.28) | 49.18 (47.74–50.65) | 1 | |||||
| Gabapentin | ||||||||||
| With | 6227 | 108 | 68.75 (55.06– 85.85) | 69.74 (56.04–86.78) | 12.76 (–2.53–28.05) | 1.22 (0.98–1.52) | ||||
| Withoutb | 725 496 | 8438 | 46.99 (45.90–48.10) | 56.98 (55.32–58.69) | 1 | |||||
| Lamotrigine | ||||||||||
| With | 463 | 6 | 50.11 (20.03–125.31) | 51.36 (21.24–124.22) | 3.64 (–41.76–49.04) | 1.08 (0.44–2.61) | ||||
| Withoutb | 731 260 | 8540 | 47.18 (46.09–48.29) | 47.72 (45.37–50.20) | 1 | |||||
| Levetiracetam | ||||||||||
| With | 10 592 | 340 | 131.3 (116.95–147.42) | 132.96 (118.54–149.15) | 79.88 (64.47–95.30) | 2.5 (2.22–2.83) | ||||
| Withoutb | 721 131 | 8206 | 45.95 (44.87–47.05) | 53.08 (50.96–55.30) | 1 | |||||
| Oxcarbazepine | ||||||||||
| With | 2761 | 38 | 55.9 (40.80–76.58) | 55.95 (40.89–76.55) | 3.77 (–13.85–21.39) | 1.07 (0.78–1.47) | ||||
| Withoutb | 728 962 | 8508 | 47.15 (46.06–48.26) | 52.18 (50.52–53.90) | 1 | |||||
| Phenobarbital | ||||||||||
| With | 5702 | 60 | 42.09 (32.61–54.34) | 42.25 (32.74–54.52) | –5.55 (–16.37–5.27) | 0.88 (0.68–1.14) | ||||
| Withoutb | 726 021 | 8486 | 47.22 (46.13–48.34) | 47.8 (46.52–49.11) | 1 | |||||
| Phenytoin | ||||||||||
| With | 5190 | 171 | 134.78 (115.20–157.68) | 135.83 (116.23–158.72) | 81.4 (60.14–102.66) | 2.5 (2.13–2.93) | ||||
| Withoutb | 726 533 | 8375 | 46.55 (45.47–47.66) | 54.43 (52.35–56.59) | 1 | |||||
| Pregabalin | ||||||||||
| With | 4324 | 67 | 61.03 (46.67–79.80) | 62.71 (48.27–81.49) | 7.59 (–8.92–24.10) | 1.14 (0.87–1.48) | ||||
| Withoutb | 727 399 | 8479 | 47.1 (46.01–48.21) | 55.12 (53.40–56.91) | 1 | |||||
| Topiramate | ||||||||||
| With | 1110 | 15 | 55.16 (33.08–91.97) | 54.83 (33.02–91.04) | 4.4 (–23.47–32.26) | 1.09 (0.65–1.81) | ||||
| Withoutb | 730 613 | 8531 | 47.17 (46.08–48.28) | 50.43 (48.68–52.24) | 1 | |||||
| Valproic acid | ||||||||||
| With | 10 848 | 401 | 151.3 (136.30–167.96) | 153.49 (138.40–170.23) | 98.43 (82.37–114.49) | 2.79 (2.49–3.12) | ||||
| Withoutb | 720 875 | 8145 | 45.62 (44.54–46.72) | 55.06 (52.77– 57.45) | 1 | |||||
| Zonisamide | ||||||||||
| With | 336 | 6 | 72.83 (32.63–162.56) | 72.24 (32.58–160.19) | 21.87 (–35.74–79.47) | 1.43 (0.65–3.19) | ||||
| Withoutb | 731 387 | 8540 | 47.17 (46.08–48.28) | 50.38 (47.71–53.19) | 1 |
| Concurrent antiepileptic medication . | Person-quarters with NOAC use . | Number of bleeding events . | Crude major bleeding incidence rate (95% CI) per 1000 person-years . | Adjusted incidence rate (95% CI) per 1000 person-yearsa . | Adjusted incidence rate difference (95% CI) per 1000 person-yearsa . | Adjusted rate ratio (95% CI)a . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Carbamazepine | ||||||||||
| With | 1530 | 21 | 56.04 (36.74–85.49) | 55.65 (36.49–84.88) | 6.47 (–17.06–30.01) | 1.13 (0.74–1.73) | ||||
| Withoutb | 730 193 | 8525 | 47.16 (46.07–48.28) | 49.18 (47.74–50.65) | 1 | |||||
| Gabapentin | ||||||||||
| With | 6227 | 108 | 68.75 (55.06– 85.85) | 69.74 (56.04–86.78) | 12.76 (–2.53–28.05) | 1.22 (0.98–1.52) | ||||
| Withoutb | 725 496 | 8438 | 46.99 (45.90–48.10) | 56.98 (55.32–58.69) | 1 | |||||
| Lamotrigine | ||||||||||
| With | 463 | 6 | 50.11 (20.03–125.31) | 51.36 (21.24–124.22) | 3.64 (–41.76–49.04) | 1.08 (0.44–2.61) | ||||
| Withoutb | 731 260 | 8540 | 47.18 (46.09–48.29) | 47.72 (45.37–50.20) | 1 | |||||
| Levetiracetam | ||||||||||
| With | 10 592 | 340 | 131.3 (116.95–147.42) | 132.96 (118.54–149.15) | 79.88 (64.47–95.30) | 2.5 (2.22–2.83) | ||||
| Withoutb | 721 131 | 8206 | 45.95 (44.87–47.05) | 53.08 (50.96–55.30) | 1 | |||||
| Oxcarbazepine | ||||||||||
| With | 2761 | 38 | 55.9 (40.80–76.58) | 55.95 (40.89–76.55) | 3.77 (–13.85–21.39) | 1.07 (0.78–1.47) | ||||
| Withoutb | 728 962 | 8508 | 47.15 (46.06–48.26) | 52.18 (50.52–53.90) | 1 | |||||
| Phenobarbital | ||||||||||
| With | 5702 | 60 | 42.09 (32.61–54.34) | 42.25 (32.74–54.52) | –5.55 (–16.37–5.27) | 0.88 (0.68–1.14) | ||||
| Withoutb | 726 021 | 8486 | 47.22 (46.13–48.34) | 47.8 (46.52–49.11) | 1 | |||||
| Phenytoin | ||||||||||
| With | 5190 | 171 | 134.78 (115.20–157.68) | 135.83 (116.23–158.72) | 81.4 (60.14–102.66) | 2.5 (2.13–2.93) | ||||
| Withoutb | 726 533 | 8375 | 46.55 (45.47–47.66) | 54.43 (52.35–56.59) | 1 | |||||
| Pregabalin | ||||||||||
| With | 4324 | 67 | 61.03 (46.67–79.80) | 62.71 (48.27–81.49) | 7.59 (–8.92–24.10) | 1.14 (0.87–1.48) | ||||
| Withoutb | 727 399 | 8479 | 47.1 (46.01–48.21) | 55.12 (53.40–56.91) | 1 | |||||
| Topiramate | ||||||||||
| With | 1110 | 15 | 55.16 (33.08–91.97) | 54.83 (33.02–91.04) | 4.4 (–23.47–32.26) | 1.09 (0.65–1.81) | ||||
| Withoutb | 730 613 | 8531 | 47.17 (46.08–48.28) | 50.43 (48.68–52.24) | 1 | |||||
| Valproic acid | ||||||||||
| With | 10 848 | 401 | 151.3 (136.30–167.96) | 153.49 (138.40–170.23) | 98.43 (82.37–114.49) | 2.79 (2.49–3.12) | ||||
| Withoutb | 720 875 | 8145 | 45.62 (44.54–46.72) | 55.06 (52.77– 57.45) | 1 | |||||
| Zonisamide | ||||||||||
| With | 336 | 6 | 72.83 (32.63–162.56) | 72.24 (32.58–160.19) | 21.87 (–35.74–79.47) | 1.43 (0.65–3.19) | ||||
| Withoutb | 731 387 | 8540 | 47.17 (46.08–48.28) | 50.38 (47.71–53.19) | 1 |
Adjusted by inverse probability of treatment weighting using the propensity score (sex, age, medical utilization, chronic kidney disease stage, anaemia, myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, dementia, chronic pulmonary disease, rheumatic disease, peptic ulcer disease, mild liver disease, diabetes, hemiplegia or paraplegia, moderate or severe liver disease, percutaneous coronary intervention, coronary artery bypass surgery, transient ischaemic attack, hypertension, aspirin, clopidogrel, ticagrelor, ticlopidine, warfarin, glucocorticoids, insulin, lipid-lowering agents, hypoglycaemic agents, antihypertensive, non-steroid anti-inflammatory drugs, proton pump inhibitors, residence, income level, and occupation).
Without indicates NOAC alone.
Separate analyses for dabigatran, rivaroxaban, and apixaban are summarized in Supplementary material online, Table S3. Because edoxaban was introduced in Taiwan after 2016, we did not study edoxaban in the separate analyses. The patterns of major bleeding risk associated with concurrent medications of dabigatran, rivaroxaban, or apixaban were similar to the primary results. Concurrent medications of an NOAC plus valproic acid, phenytoin, or levetiracetam were associated with a higher risk of major bleeding than NOAC alone.
Subgroup analyses were performed separately in patients with and without a history of ischaemic stroke or intracranial haemorrhage (Supplementary material online, Tables S4–S7). We observed a higher risk of intracranial haemorrhage associated with the combinations of an NOAC with valproic acid, phenytoin, or levetiracetam in patients with and without a history of ischaemic stroke or intracranial haemorrhage.
The first sensitivity analysis was to examine the associations between the concurrent use of an NOAC and an angiotensin receptor blocker (a medication to replace AED in the model as a negative control exposure) and major bleeding. None of the combinations was associated with a higher risk of major bleeding (Supplementary material online, Table S8). The second sensitivity analysis evaluated whether the concurrent use of an NOAC and one of the 11 AEDs was associated with unrelated events as negative control outcomes, such as acute pancreatitis, intestinal obstruction, and acute appendicitis. None of the combinations was associated with a higher risk of unrelated events (Supplementary material online, Table S9). The third sensitivity analysis evaluated whether including bleeding events occurred within the first person-quarter would affect the main results, and the result was similar to the main finding (Supplementary material online, Table S10).
Discussion
This nationwide population-based cohort study presents the following findings. First, notwithstanding a recent guideline to the contrary,4 some specific AEDs, including valproic acid, phenytoin, levetiracetam, phenobarbital, and phenytoin, were commonly co-prescribed AEDs for AF patients using NOACs in our study cohort. Second, valproic acid and levetiracetam, similar to phenytoin, were associated with more major bleeding events when they were co-prescribed with an NOAC.
Valproic acid, levetiracetam, phenobarbital, and phenytoin were often co-prescribed with NOACs in this study. A recent guideline recommends that valproic acid and levetiracetam not be prescribed to NOAC users due to significant anticipated effects on the CYP3A4 or P-gp system.4 In a study that used Swedish national registry data during 2007–2013, valproic acid was one of the most commonly used AEDs, and levetiracetam was the most common second-line monotherapy AED.14 Similar to their findings, our study also found that valproic acid and levetiracetam were the two most commonly used AEDs in our study cohort.
Among the 11 AED and NOAC combinations investigated, valproic acid was associated with the highest bleeding risk, with an adjusted incidence rate difference of 98.15 events per 1000 person-years compared to NOAC alone. Valproic acid is a widely used anticonvulsant agent, and one of its most frequently reported side effects is bleeding.15 Valproic acid per se has been shown to have disturbed haemostasis, including thrombocytopenia, platelet dysfunction, decreased fibrinogen plasma levels, and low von Willebrand factors.16–19 In addition, valproic acid can interfere with the pharmacokinetics of several drugs, including phenytoin, phenobarbital, and NOACs by inhibiting their metabolism through different cytochrome P450 isoforms.20 These coagulation disturbances and inhibitions of drug metabolism may have clinical relevance in NOAC-treated patients. In the present study, patients concurrently using valproic acid and an NOAC had experienced significantly more major bleeding than those using NOAC alone. Hence, concurrent use NOAC and valproic acid is not recommended.
Levetiracetam was also associated with more major bleeding when they were co-prescribed with an NOAC. Levetiracetam is considered as a newer and safer AED.21 Pharmacokinetic data of levetiracetam on the activity of CYP3A4 or P-gp system were mainly obtained from animal or in vitro studies: levetiracetam showed no effect on CYP3A4 activity in vitro and inconclusive effect on P-gp metabolism.22 Levetiracetam induced P-gp activity in mice and acted as P-gp substrates competing for P-gp metabolism in dogs.23,24 In contrast, levetiracetam showed no effect on P-gp activity in rats and humans.24–26 The mechanistic background regarding the effects of levetiracetam on CYP3A4 and P-gp metabolism does not fully explain the higher bleeding risk associated with levetiracetam-NOAC combination in the present study. A possible association between levetiracetam use and acute renal failure, and more specifically, interstitial nephritis may be related to the increased bleeding.27 In general, AF patients are older and more likely to have declining renal function, multiple comorbidity, and polypharmacy than non-AF patients. Since each of the NOACs undergoes renal elimination to some extent, patients with acute renal dysfunction may be at a higher risk of bleeding, especially if they are potentially subject to drug–drug interactions. If clinical use of levetiracetam is deemed necessary in NOAC-treated patients, careful monitoring of bleeding as a side effect is necessary, and in some cases, serum drug level measurements of the NOAC or levetiracetam may be needed.
To our knowledge, this is the first nationwide population-based cohort study to quantify the major bleeding risk associated with drug–drug interaction with NOACs. The person-quarter model using the propensity score helped to overcome confounding by indication biases and the complex prescription patterns in clinical settings. The design focused on a short-term risk of adverse events and addressed the unstable complex prescription behaviour. We considered complex prescription decision making for using concurrent medications in the model, with the probability of treatment weighting using the propensity score for each person-quarter. In addition, negative control exposure and negative control outcome were used to detect bias due to unmeasured confounding and strengthen the main finding of this study. The observed association between the use of NOACs concurrently with specific AEDs and increased risks of major bleeding is unlikely to have been related to unmeasured confounding.
Limitations
This study had several limitations. First, laboratory data were not available in this nationwide dataset and renal or liver function may have adversely affected bleeding risk, drug–drug interaction, and medication dosage. However, some proxy indicators (such as diagnosis of liver disease or chronic kidney disease) were used in the model to represent the severity of renal or liver disease. Second, dosages of NOACs and AEDs were not considered in the model as this would have added unnecessary complexity to the already complex model. Third, it is possible that the results may have been biased by unmeasured confounders. However, in the present study, we incorporated an extensive list of covariates that represent clinical factors typically considered by clinicians prescribing NOACs. Because all absolute standardized mean differences were below 0.1, indicating a suitably descriptive covariate, our data should be interpreted as the differences in bleeding risk associated with a specific AED with minimal indication bias. Third, data concerning thromboembolic outcome was not available in the current dataset. Future studies are well-advised to explore the effect of drug–drug interaction between NOAC and AED on thromboembolic events. Fourth, it is difficult to detect an increased bleeding risk associated with drug–drug interactions between NOAC and AED when the number of bleeding events is small. Fifth, it is difficult to identify reasons for AED treatment from insurance claims data. However, this study mainly evaluated the effect of NOAC-drug interactions on major bleeding following the use of AED. The reason for AED treatment will not affect the main finding. Lastly, bleeding risk of oral anticoagulant therapy differs among Asian and non-Asian populations,28 and P-gp and CYP3A4 activities might be different between the races. The external generalizability of study results to non-Asian populations or other ethnicities may be limited. Further clinical data are needed to validate our findings in non-Asian populations and other ethnicities.
Conclusions
For AF patients taking AEDs, the combination of an NOAC and valproic acid, phenytoin, or levetiracetam was associated with a higher risk of major bleeding.
Acknowledgements
The authors thank the statistical assistance and wish to acknowledge the support of the Maintenance Project of the Center for Big Data Analytics and Statistics (Grant CLRPG3D0045) at Chang Gung Memorial Hospital for study design and monitoring, data analysis, and interpretation. This study is based in part on data from the Chang Gung Research Database provided by Chang Gung Memorial Hospital. The interpretation and conclusions contained herein do not represent the position of Chang Gung Memorial Hospital. S.-H.C. and C.-C.K. acknowledge the Ministry of Science and Technology, Taiwan, and National Taipei University of Technology and Chang Gung Memorial Hospital Joint Research Programme, (NTUT-CGMH-108-07) NTUT-CGMH Joint Research Programme, for financial support.
Funding
This work was supported by the grants from the Chang Gung Memorial Hospital [CMRPG3I0091, CORPG3F0561] and Ministry of Science and Technology of Taiwan [107-2314-B-182A-159-MY2].
Conflict of interest: none declared.
References
Newsletter—World Health Organization. https://www.who.int/medicines/publications/PharmaNewsletter2_16.pdf (8 August 2019).



