-
PDF
- Split View
-
Views
-
Cite
Cite
Leslie Marisol Lugo, José Luis Ferreiro, Ticagrelor in patients with myocardial infarction: is the treatment strategy crystal clear?, European Heart Journal - Cardiovascular Pharmacotherapy, Volume 5, Issue 4, October 2019, Pages 207–209, https://doi.org/10.1093/ehjcvp/pvz023
Close - Share Icon Share
This editorial refers to ‘Efficacy and safety with ticagrelor in patients with prior myocardial infarction in the approved European label: insights from PEGASUS-TIMI 54’, by M. Dellborg et al., on page 200.
Current European guidelines recommend dual antiplatelet therapy (DAPT), consisting of the combination of aspirin and a P2Y12 inhibitor, for 1 year as the default strategy in patients with acute coronary syndromes (ACS), irrespective of whether an invasive or non-invasive approach is chosen.1 In this setting, ticagrelor is clearly preferred over clopidogrel and, in addition, it has advantages over prasugrel, which has a more restricted usage profile (e.g. pre-treatment or non-invasive management).1 The optimal duration of DAPT, however, has not yet been fully elucidated as there is a trade-off between the benefit obtained in terms of reducing ischaemic events and the harm due to an augmented risk of bleeding, which is directly associated with DAPT duration.2
The Prevention of Cardiovascular Events in Patients with Prior Heart Attack Using Ticagrelor Compared to Placebo on a Background of Aspirin–Thrombolysis in Myocardial Infarction 54 (PEGASUS-TIMI 54) trial evaluated the efficacy and safety of DAPT (ticagrelor plus aspirin) in secondary prevention in 21 162 patients with prior history of myocardial infarction (MI) 1–3 years before enrolment and an additional high-risk feature.3 Patients were randomized to receive ticagrelor 90 mg b.i.d., ticagrelor 60 mg b.i.d., or placebo, always on a background of low-dose aspirin. Briefly, the two ticagrelor doses significantly reduced the primary efficacy endpoint (a composite of cardiovascular death, MI, or stroke) at 3 years, with a trend for reduction in cardiovascular mortality. Of note, the 60 mg dose showed a slightly better profile in terms of safety, with a numerically lower rate of major bleeding than the 90 mg dose.3 These results led to the approval of ticagrelor 60 mg in this setting, although its net clinical benefit in the overall trial was relatively small. As per guidelines, ticagrelor 60 mg is the agent of choice for prolonging DAPT in MI patients and high ischaemic risk who have tolerated DAPT without a bleeding complication.1
In this issue of the journal, Dellborg and colleagues investigated in an interesting post-hoc analysis of the PEGASUS-TIMI 54 trial the efficacy and safety of ticagrelor 60 mg compared with placebo in the population recommended for treatment in the European label (n = 10 779), i.e. initiated up to 2 years after the MI, or within 1 year after stopping previous treatment with a P2Y12 antagonist.4 In brief, ticagrelor reduced the relative risk of the composite efficacy endpoint by 20% and cardiovascular death by 29%, whereas TIMI major bleeding was more frequent with ticagrelor (2.5 vs. 1.1%, P < 0.001), without significant differences in fatal or intracranial bleeding. It is noteworthy that the benefit of ticagrelor was greater among patients fulfilling the two requirements of the European label, which may help in selecting those subjects that may benefit the most from this therapy. Nevertheless, the recommendation is to start ticagrelor 60 mg without interruption as continuation therapy after the initial 1-year treatment with ticagrelor 90 mg or another P2Y12 inhibitor, since the benefit was larger in those patients continuing on or interrupting P2Y12 blockade for shorter periods of time.1,5
The benefit of ticagrelor in MI patients appears, therefore, to be well established. However, a ticagrelor-based strategy of antithrombotic therapy in this scenario may not be as simple and straightforward as one may think since different alternatives to conventional DAPT are currently being explored. In fact, several antiplatelet regimens including ticagrelor have been studied in recent investigations or are currently being tested. These strategies can be categorized as follows: (i) prolonged DAPT; (ii) potent P2Y12 monotherapy; and (iii) de-escalation of P2Y12 inhibition (Figure 1).
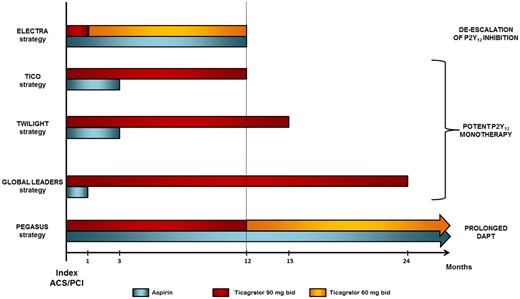
Ticagrelor-based strategies in patients with acute coronary syndromes or undergoing percutaneous coronary intervention. Strategies are named after the trial in which they were or are being explored.
First, as described above, the PEGASUS-TIMI 54 trial was specifically designed to assess the potential benefit of prolonging DAPT with ticagrelor on top of aspirin beyond 1 year after an MI. Since both reducing atherothrombotic events and increasing major haemorrhages have an impact on mortality, the risk–benefit ratio of this strategy prevents its universal use in clinical practice and strongly suggests an individualized approach based on a careful evaluation of ischaemic and bleeding risk. Secondly, it has been hypothesized that a potent P2Y12 inhibition with ticagrelor monotherapy after a short course of DAPT may reduce haemorrhagic outcomes without increasing the ischaemic risk compared with standard DAPT in patients with ACS or undergoing percutaneous coronary intervention (PCI). The GLOBAL LEADERS trial failed to show superiority of 23 months of ticagrelor monotherapy after 1 month DAPT of (aspirin plus ticagrelor) over 12 months of standard DAPT followed by 12 months of aspirin monotherapy in 15 968 patients undergoing drug-eluting stent implantation.6 Two ongoing trials are also assessing the efficacy and safety of ticagrelor monotherapy after a 3-month DAPT period compared with standard DAPT (aspirin plus ticagrelor): the TWILIGHT (NCT02270242) and the TICO (NCT02494895) trials, which are evaluating 12 months of monotherapy in high-risk PCI patients and 9 months of monotherapy in ACS patients undergoing PCI, respectively. Finally, de-escalation of P2Y12 inhibition by reducing the maintenance dose of ticagrelor from 90 mg b.i.d. to 60 mg b.i.d. at 1 month after an MI could also contribute to diminishing bleeding outcomes. The platelet inhibition with standard vs. lower maintenance dose of ticagrelor early after myocardial infarction (ELECTRA) trial did not find differences in the pharmacodynamic efficacy of standard (90 mg b.i.d.) vs. reduced (60 mg b.i.d.) dosage of ticagrelor, which suggests that de-escalating ticagrelor maintenance dose 1 month after an acute MI would not impair its antiplatelet effect.7 Anyhow, no study with clinical endpoints has evaluated this strategy to date.
In conclusion, the available evidence confirms the benefit of ticagrelor in patients with MI. However, the proliferation of different ticagrelor-based treatment strategies may be somewhat confusing for physicians and, subsequently, could add a layer of complexity to the choice of the antiplatelet regimen. The results of ongoing studies will provide relevant insights into the matter, and further investigation is warranted in order to help in determining and personalizing the optimal antiplatelet strategy in this scenario.
Conflict of interest: J.L.F. reports honoraria for lectures from Eli Lilly Co, Daiichi Sankyo, Inc., AstraZeneca, Roche Diagnostics, Pfizer, Abbott, Boehringer Ingelheim, and Bristol-Myers Squibb; consulting fees from AstraZeneca, Eli Lilly Co., Ferrer, Boston Scientific, and Pfizer; and research grants from AstraZeneca. L.M.L. reports no conflict of interest.
The opinions expressed in this article are not necessarily those of the Editors of the European Heart Journal – Cardiovascular Pharmacotherapy or of the European Society of Cardiology.



