-
PDF
- Split View
-
Views
-
Cite
Cite
Bert-Jan H van den Born, Gregory Y H Lip, Jana Brguljan-Hitij, Antoine Cremer, Julian Segura, Enrique Morales, Felix Mahfoud, Fouad Amraoui, Alexandre Persu, Thomas Kahan, Enrico Agabiti Rosei, Giovanni de Simone, Philippe Gosse, Bryan Williams, ESC Council on hypertension position document on the management of hypertensive emergencies, European Heart Journal - Cardiovascular Pharmacotherapy, Volume 5, Issue 1, January 2019, Pages 37–46, https://doi.org/10.1093/ehjcvp/pvy032
Close - Share Icon Share
Abstract
Hypertensive emergencies are those situations where very high blood pressure (BP) values are associated with acute organ damage, and therefore, require immediate, but careful, BP reduction. The type of acute organ damage is the principal determinant of: (i) the drug of choice, (ii) the target BP, and (iii) the timeframe in which BP should be lowered. Key target organs are the heart, retina, brain, kidneys, and large arteries. Patients who lack acute hypertension-mediated end organ damage do not have a hypertensive emergency and can usually be treated with oral BP-lowering agents and usually discharged after a brief period of observation.
Introduction
Hypertensive emergencies are a heterogeneous group of acute hypertensive disorders that require rapid diagnostics and institution of appropriate therapy to avert or treat progressive organ dysfunction. The management of hypertensive emergencies is challenging because immediate treatment depends on the degree and extent of hypertension-mediated damage to key target organs. Although solid evidence regarding the optimal treatment strategy for most hypertensive emergencies is lacking, many studies have revealed important differences in the effect of different types of blood pressure (BP)-lowering medication and the magnitude of acute BP reduction for different hypertensive emergencies. At present, no formal international guidelines or recommendation exists to help guide clinical decision making. The aim of this position document is to offer a consensus of the most important definitions and provide an overview of the management of hypertensive emergencies. Based on available evidence, we have also defined areas where further research is needed.
Definitions
Patients with a hypertensive emergency should be admitted for close monitoring and, in most cases, treated with intravenous blood pressure (BP)-lowering agents to reach the recommended BP target in the designated time-frame.
Patients that lack acute hypertension-mediated end organ damage to the heart, retina, brain, kidneys, or large arteries do not have a hypertensive emergency and can be treated with oral BP-lowering agents and usually discharged after a brief period of observation.
An overview of current definitions used for hypertensive emergencies and associated conditions is given in Table 1. Hypertensive emergencies can be classified according to the presence of acute hypertension-mediated target organ damage.1 In patients with a hypertensive emergency immediate BP reduction is indicated to limit extension or promote regression of acute hypertension-mediated organ damage. The type of target organ damage is the principal determinant of the choice of treatment, target BP, and timeframe by which BP should be lowered. A typical example of a hypertensive emergency is the coexistence of very high BP values (often >200/120 mmHg) with advanced retinopathy, acute renal failure, and/or thrombotic microangiopathy (TMA). In general, the term ‘malignant hypertension’ has been used to describe this condition. This is based on studies showing limited survival in patients with very severe hypertension and retinal haemorrhages, cotton wools spots, and/or papilloedema at a time when treatment of hypertension was not yet possible. The Task Force recognizes that because of the significantly improved survival alternative terms to describe this condition may be more appropriate, although other definitions may need additional validation. Because systemic microcirculatory damage is the pathological hallmark of malignant hypertension,2 and retinal lesions can be absent in patients with acute microvascular damage to the kidney and brain,3,4,acute hypertensive microangiopathy could be considered as an alternative term. To recognize the high cardiovascular risk that is associated with the presence of acute and chronic hypertension-mediated organ damage in these patients it may be of additional clinical use to stratify patients according to the extent of hypertension-mediated organ damage.5
| Hypertensive emergencies are situations where very high BP values are associated with acute hypertension-mediated organ damage, and therefore, require immediate BP reduction to limit extension or promote regression of target organ damage |
| Key target organs of acute hypertension-mediated damage are the heart, retina, brain, kidneys, and large arteries |
| The type of target organ damage is the principal determinant of the choice of treatment, target BP, and timeframe by which BP should be lowered |
| Malignant hypertension is a hypertensive emergency characterized by the presence of a severe BP elevation (usually >200/120 mmHg) and advanced retinopathy, defined as the bilateral presence of flame-shaped haemorrhages, cotton wool spots, or papilloedema |
| Hypertensive encephalopathy is a hypertensive emergency characterized by severe hypertension and (one or more of the following): seizures, lethargy, cortical blindness and coma, and in the absence of an alternative explanation |
| Thrombotic microangiopathy: any situation where severe BP elevation coincides with a Coombs-negative haemolysis (elevated lactic dehydrogenase levels, unmeasurable haptoglobin, or schistocytes) and thrombocytopenia in the absence of another plausible cause and with improvement during BP-lowering therapy |
| Hypertensive emergencies are situations where very high BP values are associated with acute hypertension-mediated organ damage, and therefore, require immediate BP reduction to limit extension or promote regression of target organ damage |
| Key target organs of acute hypertension-mediated damage are the heart, retina, brain, kidneys, and large arteries |
| The type of target organ damage is the principal determinant of the choice of treatment, target BP, and timeframe by which BP should be lowered |
| Malignant hypertension is a hypertensive emergency characterized by the presence of a severe BP elevation (usually >200/120 mmHg) and advanced retinopathy, defined as the bilateral presence of flame-shaped haemorrhages, cotton wool spots, or papilloedema |
| Hypertensive encephalopathy is a hypertensive emergency characterized by severe hypertension and (one or more of the following): seizures, lethargy, cortical blindness and coma, and in the absence of an alternative explanation |
| Thrombotic microangiopathy: any situation where severe BP elevation coincides with a Coombs-negative haemolysis (elevated lactic dehydrogenase levels, unmeasurable haptoglobin, or schistocytes) and thrombocytopenia in the absence of another plausible cause and with improvement during BP-lowering therapy |
| Hypertensive emergencies are situations where very high BP values are associated with acute hypertension-mediated organ damage, and therefore, require immediate BP reduction to limit extension or promote regression of target organ damage |
| Key target organs of acute hypertension-mediated damage are the heart, retina, brain, kidneys, and large arteries |
| The type of target organ damage is the principal determinant of the choice of treatment, target BP, and timeframe by which BP should be lowered |
| Malignant hypertension is a hypertensive emergency characterized by the presence of a severe BP elevation (usually >200/120 mmHg) and advanced retinopathy, defined as the bilateral presence of flame-shaped haemorrhages, cotton wool spots, or papilloedema |
| Hypertensive encephalopathy is a hypertensive emergency characterized by severe hypertension and (one or more of the following): seizures, lethargy, cortical blindness and coma, and in the absence of an alternative explanation |
| Thrombotic microangiopathy: any situation where severe BP elevation coincides with a Coombs-negative haemolysis (elevated lactic dehydrogenase levels, unmeasurable haptoglobin, or schistocytes) and thrombocytopenia in the absence of another plausible cause and with improvement during BP-lowering therapy |
| Hypertensive emergencies are situations where very high BP values are associated with acute hypertension-mediated organ damage, and therefore, require immediate BP reduction to limit extension or promote regression of target organ damage |
| Key target organs of acute hypertension-mediated damage are the heart, retina, brain, kidneys, and large arteries |
| The type of target organ damage is the principal determinant of the choice of treatment, target BP, and timeframe by which BP should be lowered |
| Malignant hypertension is a hypertensive emergency characterized by the presence of a severe BP elevation (usually >200/120 mmHg) and advanced retinopathy, defined as the bilateral presence of flame-shaped haemorrhages, cotton wool spots, or papilloedema |
| Hypertensive encephalopathy is a hypertensive emergency characterized by severe hypertension and (one or more of the following): seizures, lethargy, cortical blindness and coma, and in the absence of an alternative explanation |
| Thrombotic microangiopathy: any situation where severe BP elevation coincides with a Coombs-negative haemolysis (elevated lactic dehydrogenase levels, unmeasurable haptoglobin, or schistocytes) and thrombocytopenia in the absence of another plausible cause and with improvement during BP-lowering therapy |
Hypertensive encephalopathy occurs in 10–15% of patients presenting with malignant hypertension. However, advanced hypertensive retinopathy may be lacking in up to one-third of these patients.6 Therefore, the diagnosis principally relies on the presence of neurological symptoms supported by additional imaging. Seizures, lethargy, cortical blindness, and coma are among the most alarming symptoms, but more subtle neurological features can be present at an earlier stage.7 Other examples of hypertensive emergencies are severe hypertension in patients with intracranial haemorrhage and acute stroke, acute coronary syndrome, cardiogenic pulmonary oedema, acute aortic disease, and severe pre-eclampsia and eclampsia. A diagram showing the stratification of different hypertensive emergencies according to the condition or target organ involved is shown in Figure 1.
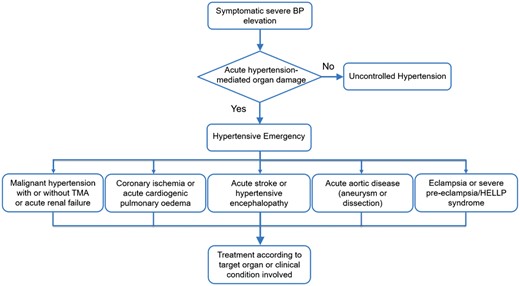
A diagram showing the stratification of hypertensive emergencies according to the condition or target organ involved. HELLP, haemolysis elevated liver enzymes low platelets; TMA, thrombotic microangiopathy.
The term hypertensive urgency has been previously used to refer to situations where very high BP values, usually >180/110 mmHg, prompt ED referral, but acute hypertension-mediated target organ damage is absent. This renders hypertensive urgencies not a separate entity, but a diagnosis of exclusion. Recent studies have also used a much broader definition for ‘hypertensive urgency’ to include all patients with a BP >180/110 mmHg presenting at the office. Cardiovascular risk was not particularly high in these patients and referral to the ED did not seem to improve cardiovascular outcome nor BP control after 6 months.8 Because, there is no evidence that treatment in patients who lack acute hypertension-mediated organ damage is different from patients with asymptomatic uncontrolled hypertension, the Task Force considers that it is preferable not to use the term ‘hypertensive urgency’ and only use hypertensive emergency to refer to those situations where immediate treatment is warranted. This also means that the term hypertensive crisis that was meant to discriminate between hypertensive urgencies and emergencies becomes obsolete.
Epidemiology
One in every 200 patients presents at the ED with a suspected hypertensive emergency,9 a proportion that has not changed in the past two decades,10 and seems comparable across continents.11–14 Based on current definitions, approximately one in every two to three patients have a hypertensive emergency.9,12,13 In a representative sample of ED visits in the US, heart failure, stroke, and myocardial infarction represented the largest proportion of all hypertensive emergencies, followed by intracranial haemorrhage and aortic dissection, whereas the incidence of hypertensive emergencies with advanced retinopathy was quite low.13 However, this may relate to differences in categorization as other studies using different coding systems have found much higher incidence rates.14
Despite improved treatment for hypertension in the past decades, the incidence of hypertensive emergencies has not declined.11,13–15 Limited access to healthcare and non-adherence to antihypertensive medications frequently contribute to the development of hypertensive emergencies and may, in part, explain the much higher prevalence among sub-Saharan African migrants and African Americans.15–17 This is further supported by data showing that many patients who present with a hypertensive emergency have not received anti-hypertensive medication.17,18
Pathophysiology
In patients presenting at the ED with malignant hypertension, secondary causes can be found in 20–40% and most often consist of renal parenchymal disease and renal artery stenosis, whereas endocrine causes appear to be rare.15,17 However, the majority of patients with malignant hypertension have unrecognized or uncontrolled essential hypertension. Many pathophysiological mechanisms are involved in the development and maintenance of malignant hypertension (Figure 2), but the initiating events for the sudden escalation in BP are not completely understood. Marked activation of the renin–angiotensin system is often present and associated with the degree of microvascular damage.3 In experimental models, the development of acute hypertensive microangiopathy is preceded by an increase in renal vasoconstriction and microvascular damage that leads to activation of the renin–angiotensin system.19,20 Pressure-induced natriuresis further contributes to contraction of blood volume and activation of the renin–angiotensin system.21,22 Microvascular damage and autoregulation failure are at the foundation of the target organ damage associated with malignant hypertension and result in hypertensive retinopathy, TMA, and encephalopathy, complications that are not observed in patients with chronic uncontrolled hypertension.
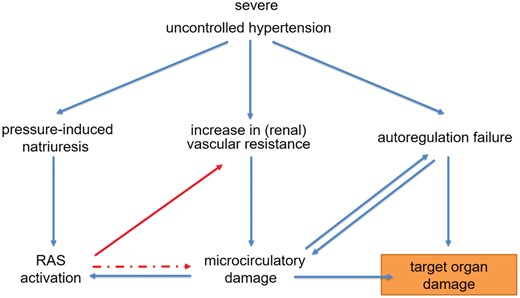
Acute hypertension-mediated end organ damage in malignant hypertension. In malignant hypertension, a sudden increase in vascular resistance and pressure-induced natriuresis trigger a vicious circle leading to renin–angiotensin system activation and a further increase in blood pressure and microcirculatory damage. Autoregulation failure and microcirculatory damage are at the foundation of the target organ damage associated with malignant hypertension. RAS, renin–angiotensin system.
Hypertensive retinopathy
The retinal abnormalities associated with malignant hypertension consist of flame shaped haemorrhages, cotton wool spots (Grade III) with or without the presence of papilloedema (Grade IV). These retinal abnormalities are rare in the normal population and, when they are bilaterally present, highly specific. Because Grade III and IV hypertensive retinopathy have the same pathophysiological background and carry the same prognosis,23,24 it is justified to stratify patients into those with and without advanced retinopathy. The presence of advanced hypertensive retinopathy in patients presenting at the ED with a suspected hypertensive emergency is associated with much higher renin–angiotensin system activation and more pronounced hypertension-mediated organ damage in other areas compared with patients without these retinal lesions, despite comparable BP values.3 A funduscopic image of advanced retinopathy is added as Supplementary material online.
Thrombotic microangiopathy
Both high BP and angiotensin II have been associated with activation of pro-inflammatory and pro-coagulant pathways.25,26 Endothelial detachment is one of the pathological hallmarks of hypertensive microangiopathy and is thought to result from high-shear forces. The subsequent exposure of blood to the subendothelium leads to coagulation activation, platelet activation, and the formation of a fibrin network. These result in: (i) the formation of platelet-rich thrombi with obliteration of the microcirculation and (ii) platelet consumption and intravascular haemolysis as a result of trapping and destruction of erythrocytes within the fibrin network. Discrimination from other causes of TMA such as thrombotic thrombocytopenic purpura (TTP) and haemolytic uraemic syndrome (HUS) can be difficult. TMA associated with malignant hypertension is usually less severe compared with patients with TTP and HUS with only moderate thrombocytopenia and few schistocytes present in a peripheral blood smear. Moreover, the coexistence of a severe BP elevation with advanced retinopathy is usually sufficient to discriminate TMA in patients with malignant hypertension from other causes of TMA. If needed, measurement of the activity of the von Willebrand factor cleaving protease, ADAMTS13, may further help to discriminate TTP from hypertension-induced TMA as very low activity levels are found in TTP and normal or slightly reduced activity levels are found in malignant hypertension.27 BP-lowering treatment will usually improve TMA associated with malignant hypertension within 24–48 h, whereas other treatment is required for TTP and HUS.
Hypertensive encephalopathy
When BP is markedly elevated and cerebral autoregulation cannot prevent a rise in intracranial pressure, cerebral oedema may develop, especially in the posterior areas of the brain where sympathetic innervation is less pronounced leading to less effective damping of BP oscillations.28 Histopathological changes in hypertensive encephalopathy include cerebral oedema, microscopic haemorrhages, and infarctions.2 Hypertensive encephalopathy is one of the causes of posterior reversible leukoencephalopathy syndrome (PRES), which is characterized by white matter lesions in the posterior regions of the brain that are fully reversible with timely recognition and management.7 Magnetic resonance imaging (MRI) demonstrating increased signal intensity on T2-weighted or fluid attenuated inversion recovery (FLAIR)-imaging may be of additional value to confirm the diagnosis, whereas computerized tomography (CT) is useful to exclude intracerebral haemorrhage. MRI with FLAIR imaging showing the typical lesions associated with hypertensive encephalopathy is added as Supplementary material online. Besides acute pressure-mediated damage to the microcirculation, PRES is also observed in other diseases characterized by acute microcirculatory damage including antiphospholipid antibody syndrome, TTP, HUS and the use of cytotoxic and anti-angiogenic drugs. Hypertension is often present in these cases, but to a variable degree.
Clinical presentation
There is no specific BP threshold to define hypertensive emergencies because at the same BP level hypertension-mediated organ damage can be present or absent.3,29 The rate of BP increase appears to be more important than the absolute BP value in the development of hypertensive emergencies. The medical history-taking should focus on emergency symptoms, possible causes (e.g. non-adherence, dietary habits), the use of drugs (e.g. steroids, NSAIDs, cyclosporin, sympathomimetics, cocaine, anti-angiogenic therapy), and secondary causes (e.g. kidney disease, renal artery stenosis). In patients with pre-existing hypertension, current antihypertensive treatment or treatment withdrawal, disease duration, and previous BP control should be recorded.
Emergency symptoms include headache, visual disturbances, chest pain, dyspnoea and focal, or general neurological symptoms. Other frequent, but less specific, symptoms include dizziness, resulting from impaired cerebral autoregulation, and gastrointestinal complaints (abdominal pain, nausea and anorexia). A proposed hierarchical strategy to diagnose patients with a hypertensive emergency based on the presence of emergency symptoms is added as Supplementary material online. In patients with hypertensive encephalopathy, the presence of somnolence, lethargy, tonic–clonic seizures, and cortical blindness may precede loss of consciousness. Focal neurological lesions are rare in hypertensive encephalopathy and should raise the suspicion of intracranial haemorrhage or ischaemic stroke. Physical examination should focus on cardiovascular and neurological assessment. BP should be measured according to current guidelines in both arms and at the lower limb to detect pressure differences caused by aortic dissection. Repeated measurements should be performed over time, since in a significant proportion of patients, the BP will fall considerably without antihypertensive medication.30 An overview of proposed laboratory analysis and diagnostic studies is listed in Table 2. Diagnostic work up is aimed at finding evidence of acute hypertension-mediated organ damage and should include an ECG for the detection of ischaemia. A funduscopic examination should be performed in case malignant hypertension is suspected. Chest X-ray or point of care ultrasonography can be used to discriminate cardiac from non-cardiac dyspnoea.31,32 A transthoracic echocardiogram can be considered to assess left ventricular structure and function. Further diagnostic work-up depends on the clinical presentation and may include brain CT scan or MRI, thoraco-abdominal CT scan and an abdominal and vascular ultrasound examination.
Proposed diagnostic studies in patients with suspected hypertensive emergency
| Laboratory analysis |
|
| Diagnostic examination |
|
On indication
|
| Laboratory analysis |
|
| Diagnostic examination |
|
On indication
|
Proposed diagnostic studies in patients with suspected hypertensive emergency
| Laboratory analysis |
|
| Diagnostic examination |
|
On indication
|
| Laboratory analysis |
|
| Diagnostic examination |
|
On indication
|
Acute management of hypertensive emergencies
There are no randomized controlled trials that have examined different treatment strategies for most hypertensive emergencies, except for acute BP lowering in patients presenting with acute ischaemic or haemorrhagic stroke. Most strategies are based on consensus from clinical experience, observations and comparisons of intermediate outcomes, including the time needed to reach predefined BP targets and (surrogates of) tissue perfusion.
There is general agreement that patients without acute hypertension-mediated organ damage usually can be treated with oral BP-lowering medication or adaptation of their current BP-lowering medication. Rapid BP lowering is not recommended, as this can lead to cardiovascular complications.33 This means that a controlled BP reduction to safer levels without risk of hypotension should be the therapeutic goal. Hence, short acting nifedipine should not be used given the rapid BP falls. Among the different oral BP lowering drugs captopril, labetalol, and nifedipine retard have been proposed, but limited data are available regarding the optimal treatment in this situation.34 Once the decision to add medication is taken, an observation period of at least 2 h is suggested to evaluate BP lowering efficacy and safety.
Treatment in patients with a hypertensive emergency is driven by the type of hypertensive organ damage. Acute hypertension-mediated organ damage includes stroke (ischaemic or haemorrhagic), acute hypertensive microangiopathy and encephalopathy, cardiogenic pulmonary oedema, coronary ischaemia, and acute aortic disease. The treatment goal is to prevent or limit further hypertensive damage by a controlled BP reduction. In most cases, this can be best achieved by intravenous medication in a clinical area with facilities for close haemodynamic monitoring. The swiftness and magnitude of the BP reduction, as well as the type of BP-lowering medication, is strongly dependent on the clinical context. Rapid BP lowering is required in patients with pulmonary oedema and acute aortic dissection, whereas BP-lowering medication is generally withheld in patients with ischaemic stroke. Whether BP should be acutely lowered in patients presenting with severe hypertension and acute intracranial haemorrhage is still subject of debate. Although differences in preference and experience exist with regard to the use of intravenous BP-lowering medication, most hypertensive emergencies can be treated with either labetalol or nicardipine. These drugs are widely available throughout Europe and the Task Force feels that they should be included in the essential drug list of each hospital with an emergency room or intensive care unit. A summary of the treatment of hypertensive emergencies according to affected target organs is given in Table 3. An overview of recommended drugs that are used for the treatment of hypertensive emergencies is listed in Table 4.
| Clinical presentation . | Time line and target BP . | 1st line treatment . | Alternative . |
|---|---|---|---|
| Malignant hypertension with or without TMA or acute renal failure | Several hours, MAP −20% to −25% | Labetalol | Nitroprusside |
| Nicardipine | Urapidil | ||
| Hypertensive encephalopathy | Immediate, MAP −20% to −25% | Labetalol | Nitroprusside |
| Nicardipine | |||
| Acute ischaemic stroke and BP >220 mmHg systolic or >120 mmHg diastolic | 1 h, MAP −15% | Labetalol | Nitroprusside |
| Nicardipine | |||
| Acute ischaemic stroke with indication for thrombolytic therapy and BP >185 mmHg systolic or >110 mmHg diastolic | 1 h, MAP −15% | Labetalol | |
| Nicardipine | Nitroprusside | ||
| Acute haemorrhagic stroke and systolic BP >180 mmHg | Immediate, systolic 130<BP <180 mmHg | Labetalol | Urapidil |
| Nicardipine | |||
| Acute coronary event | Immediate, systolic BP <140 mmHg | Nitroglycerine | Urapidil |
| Labetalol | |||
| Acute cardiogenic pulmonary oedema | Immediate, systolic BP <140 mmHg | Nitroprusside or Nitroglycerine (with loop diuretic) | Urapidil (with loop diuretic) |
| Acute aortic disease | Immediate, systolic BP <120 mmHg and heart rate <60 b.p.m. | Esmolol and Nitroprusside or Nitroglycerine or Nicardipine | Labetalol or Metoprolol |
| Eclampsia and severe pre-eclampsia/HELLP | Immediate, systolic BP < 160 mmHg and diastolic BP <105 mmHg | Labetalol or Nicardipine and Magnesium sulphate |
| Clinical presentation . | Time line and target BP . | 1st line treatment . | Alternative . |
|---|---|---|---|
| Malignant hypertension with or without TMA or acute renal failure | Several hours, MAP −20% to −25% | Labetalol | Nitroprusside |
| Nicardipine | Urapidil | ||
| Hypertensive encephalopathy | Immediate, MAP −20% to −25% | Labetalol | Nitroprusside |
| Nicardipine | |||
| Acute ischaemic stroke and BP >220 mmHg systolic or >120 mmHg diastolic | 1 h, MAP −15% | Labetalol | Nitroprusside |
| Nicardipine | |||
| Acute ischaemic stroke with indication for thrombolytic therapy and BP >185 mmHg systolic or >110 mmHg diastolic | 1 h, MAP −15% | Labetalol | |
| Nicardipine | Nitroprusside | ||
| Acute haemorrhagic stroke and systolic BP >180 mmHg | Immediate, systolic 130<BP <180 mmHg | Labetalol | Urapidil |
| Nicardipine | |||
| Acute coronary event | Immediate, systolic BP <140 mmHg | Nitroglycerine | Urapidil |
| Labetalol | |||
| Acute cardiogenic pulmonary oedema | Immediate, systolic BP <140 mmHg | Nitroprusside or Nitroglycerine (with loop diuretic) | Urapidil (with loop diuretic) |
| Acute aortic disease | Immediate, systolic BP <120 mmHg and heart rate <60 b.p.m. | Esmolol and Nitroprusside or Nitroglycerine or Nicardipine | Labetalol or Metoprolol |
| Eclampsia and severe pre-eclampsia/HELLP | Immediate, systolic BP < 160 mmHg and diastolic BP <105 mmHg | Labetalol or Nicardipine and Magnesium sulphate |
BP, blood pressure; HELLP, haemolysis, elevated liver enzymes and low platelets; TMA, thrombotic microangiopathy.
| Clinical presentation . | Time line and target BP . | 1st line treatment . | Alternative . |
|---|---|---|---|
| Malignant hypertension with or without TMA or acute renal failure | Several hours, MAP −20% to −25% | Labetalol | Nitroprusside |
| Nicardipine | Urapidil | ||
| Hypertensive encephalopathy | Immediate, MAP −20% to −25% | Labetalol | Nitroprusside |
| Nicardipine | |||
| Acute ischaemic stroke and BP >220 mmHg systolic or >120 mmHg diastolic | 1 h, MAP −15% | Labetalol | Nitroprusside |
| Nicardipine | |||
| Acute ischaemic stroke with indication for thrombolytic therapy and BP >185 mmHg systolic or >110 mmHg diastolic | 1 h, MAP −15% | Labetalol | |
| Nicardipine | Nitroprusside | ||
| Acute haemorrhagic stroke and systolic BP >180 mmHg | Immediate, systolic 130<BP <180 mmHg | Labetalol | Urapidil |
| Nicardipine | |||
| Acute coronary event | Immediate, systolic BP <140 mmHg | Nitroglycerine | Urapidil |
| Labetalol | |||
| Acute cardiogenic pulmonary oedema | Immediate, systolic BP <140 mmHg | Nitroprusside or Nitroglycerine (with loop diuretic) | Urapidil (with loop diuretic) |
| Acute aortic disease | Immediate, systolic BP <120 mmHg and heart rate <60 b.p.m. | Esmolol and Nitroprusside or Nitroglycerine or Nicardipine | Labetalol or Metoprolol |
| Eclampsia and severe pre-eclampsia/HELLP | Immediate, systolic BP < 160 mmHg and diastolic BP <105 mmHg | Labetalol or Nicardipine and Magnesium sulphate |
| Clinical presentation . | Time line and target BP . | 1st line treatment . | Alternative . |
|---|---|---|---|
| Malignant hypertension with or without TMA or acute renal failure | Several hours, MAP −20% to −25% | Labetalol | Nitroprusside |
| Nicardipine | Urapidil | ||
| Hypertensive encephalopathy | Immediate, MAP −20% to −25% | Labetalol | Nitroprusside |
| Nicardipine | |||
| Acute ischaemic stroke and BP >220 mmHg systolic or >120 mmHg diastolic | 1 h, MAP −15% | Labetalol | Nitroprusside |
| Nicardipine | |||
| Acute ischaemic stroke with indication for thrombolytic therapy and BP >185 mmHg systolic or >110 mmHg diastolic | 1 h, MAP −15% | Labetalol | |
| Nicardipine | Nitroprusside | ||
| Acute haemorrhagic stroke and systolic BP >180 mmHg | Immediate, systolic 130<BP <180 mmHg | Labetalol | Urapidil |
| Nicardipine | |||
| Acute coronary event | Immediate, systolic BP <140 mmHg | Nitroglycerine | Urapidil |
| Labetalol | |||
| Acute cardiogenic pulmonary oedema | Immediate, systolic BP <140 mmHg | Nitroprusside or Nitroglycerine (with loop diuretic) | Urapidil (with loop diuretic) |
| Acute aortic disease | Immediate, systolic BP <120 mmHg and heart rate <60 b.p.m. | Esmolol and Nitroprusside or Nitroglycerine or Nicardipine | Labetalol or Metoprolol |
| Eclampsia and severe pre-eclampsia/HELLP | Immediate, systolic BP < 160 mmHg and diastolic BP <105 mmHg | Labetalol or Nicardipine and Magnesium sulphate |
BP, blood pressure; HELLP, haemolysis, elevated liver enzymes and low platelets; TMA, thrombotic microangiopathy.
| Drug . | Onset of action . | Duration of action . | Dose . | Contraindications . | Adverse effects . |
|---|---|---|---|---|---|
| Esmolol | 1–2 min | 10–30 min | 0.5–1 mg/kg i.v. bolus; 50–300 µg/kg/min as continuous i.v. infusion | History of 2nd or 3rd degree AV block (and in the absence of rhythm support), systolic heart failure, asthma, and bradycardia | Bradycardia |
| Metoprolol | 1–2 min | 5–8 h | 2.5-5 mg i.v. bolus over 2 minutes; may repeat every 5 minutes to a maximum dose of 15 mg | History of 2nd or 3rd degree AV block, systolic heart failure, asthma, and bradycardia | Bradycardia |
| Labetalol | 5–10 min | 3–6 h | 0.25–0.5 mg/kg i.v. bolus; 2–4 mg/min continuous infusion until goal BP is reached, thereafter 5–20 mg/h | History of 2nd or 3rd degree AV block, systolic heart failure, asthma, and bradycardia | Bronchoconstriction and foetal bradycardia |
| Fenoldopam | 5–15 min | 30–60 min | 0.1 µg/kg/min i.v. infusion, increase every 15 min until goal BP is reached with 0.05 to 0.1 µg/kg/min increments | ||
| Clevidipine | 2–3 min | 5–15 min | 2 mg/h i.v. infusion, increase every 2 min with 2 mg/h until goal BP | Headache and reflex-tachycardia | |
| Nicardipine | 5–15 min | 30–40 min | 5–15 mg/h as continuous i.v. infusion, starting dose 5 mg/h, increase every 15–30 min with 2.5 mg until goal BP, thereafter decrease to 3 mg/h | Liver failure | Headache and reflex-tachycardia |
| Nitroglycerine | 1–5 min | 3–5 min | 5–200 µg/min, 5 µg/min increase every 5 min | Headache and reflex tachycardia | |
| Nitroprusside | Immediate | 1–2 min | 0.3–10 µg/kg/min, increase by 0.5 µg/kg/min every 5 min until goal BP | Liver/kidney failure (relative) | Cyanide intoxication |
| Enalaprilat | 5–15 min | 4–6 h | 0.625–1.25 mg i.v. | History of angioedema | |
| Urapidil | 3–5 min | 4–6 h | 12.5–25 mg i.v. bolus, 5–40 mg/h as continuous infusion | ||
| Clonidine | 30 min | 4–6 h | 150–300 µg i.v. bolus in 5–10 min | Sedation and rebound hypertension | |
| Phentolamine | 1–2 min | 10–30 min | 0.5–1 mg/kg i.v. bolus OR 50–300 µg/kg/min as continuous i.v. infusion | Tachyarrhythmias and chest pain |
| Drug . | Onset of action . | Duration of action . | Dose . | Contraindications . | Adverse effects . |
|---|---|---|---|---|---|
| Esmolol | 1–2 min | 10–30 min | 0.5–1 mg/kg i.v. bolus; 50–300 µg/kg/min as continuous i.v. infusion | History of 2nd or 3rd degree AV block (and in the absence of rhythm support), systolic heart failure, asthma, and bradycardia | Bradycardia |
| Metoprolol | 1–2 min | 5–8 h | 2.5-5 mg i.v. bolus over 2 minutes; may repeat every 5 minutes to a maximum dose of 15 mg | History of 2nd or 3rd degree AV block, systolic heart failure, asthma, and bradycardia | Bradycardia |
| Labetalol | 5–10 min | 3–6 h | 0.25–0.5 mg/kg i.v. bolus; 2–4 mg/min continuous infusion until goal BP is reached, thereafter 5–20 mg/h | History of 2nd or 3rd degree AV block, systolic heart failure, asthma, and bradycardia | Bronchoconstriction and foetal bradycardia |
| Fenoldopam | 5–15 min | 30–60 min | 0.1 µg/kg/min i.v. infusion, increase every 15 min until goal BP is reached with 0.05 to 0.1 µg/kg/min increments | ||
| Clevidipine | 2–3 min | 5–15 min | 2 mg/h i.v. infusion, increase every 2 min with 2 mg/h until goal BP | Headache and reflex-tachycardia | |
| Nicardipine | 5–15 min | 30–40 min | 5–15 mg/h as continuous i.v. infusion, starting dose 5 mg/h, increase every 15–30 min with 2.5 mg until goal BP, thereafter decrease to 3 mg/h | Liver failure | Headache and reflex-tachycardia |
| Nitroglycerine | 1–5 min | 3–5 min | 5–200 µg/min, 5 µg/min increase every 5 min | Headache and reflex tachycardia | |
| Nitroprusside | Immediate | 1–2 min | 0.3–10 µg/kg/min, increase by 0.5 µg/kg/min every 5 min until goal BP | Liver/kidney failure (relative) | Cyanide intoxication |
| Enalaprilat | 5–15 min | 4–6 h | 0.625–1.25 mg i.v. | History of angioedema | |
| Urapidil | 3–5 min | 4–6 h | 12.5–25 mg i.v. bolus, 5–40 mg/h as continuous infusion | ||
| Clonidine | 30 min | 4–6 h | 150–300 µg i.v. bolus in 5–10 min | Sedation and rebound hypertension | |
| Phentolamine | 1–2 min | 10–30 min | 0.5–1 mg/kg i.v. bolus OR 50–300 µg/kg/min as continuous i.v. infusion | Tachyarrhythmias and chest pain |
| Drug . | Onset of action . | Duration of action . | Dose . | Contraindications . | Adverse effects . |
|---|---|---|---|---|---|
| Esmolol | 1–2 min | 10–30 min | 0.5–1 mg/kg i.v. bolus; 50–300 µg/kg/min as continuous i.v. infusion | History of 2nd or 3rd degree AV block (and in the absence of rhythm support), systolic heart failure, asthma, and bradycardia | Bradycardia |
| Metoprolol | 1–2 min | 5–8 h | 2.5-5 mg i.v. bolus over 2 minutes; may repeat every 5 minutes to a maximum dose of 15 mg | History of 2nd or 3rd degree AV block, systolic heart failure, asthma, and bradycardia | Bradycardia |
| Labetalol | 5–10 min | 3–6 h | 0.25–0.5 mg/kg i.v. bolus; 2–4 mg/min continuous infusion until goal BP is reached, thereafter 5–20 mg/h | History of 2nd or 3rd degree AV block, systolic heart failure, asthma, and bradycardia | Bronchoconstriction and foetal bradycardia |
| Fenoldopam | 5–15 min | 30–60 min | 0.1 µg/kg/min i.v. infusion, increase every 15 min until goal BP is reached with 0.05 to 0.1 µg/kg/min increments | ||
| Clevidipine | 2–3 min | 5–15 min | 2 mg/h i.v. infusion, increase every 2 min with 2 mg/h until goal BP | Headache and reflex-tachycardia | |
| Nicardipine | 5–15 min | 30–40 min | 5–15 mg/h as continuous i.v. infusion, starting dose 5 mg/h, increase every 15–30 min with 2.5 mg until goal BP, thereafter decrease to 3 mg/h | Liver failure | Headache and reflex-tachycardia |
| Nitroglycerine | 1–5 min | 3–5 min | 5–200 µg/min, 5 µg/min increase every 5 min | Headache and reflex tachycardia | |
| Nitroprusside | Immediate | 1–2 min | 0.3–10 µg/kg/min, increase by 0.5 µg/kg/min every 5 min until goal BP | Liver/kidney failure (relative) | Cyanide intoxication |
| Enalaprilat | 5–15 min | 4–6 h | 0.625–1.25 mg i.v. | History of angioedema | |
| Urapidil | 3–5 min | 4–6 h | 12.5–25 mg i.v. bolus, 5–40 mg/h as continuous infusion | ||
| Clonidine | 30 min | 4–6 h | 150–300 µg i.v. bolus in 5–10 min | Sedation and rebound hypertension | |
| Phentolamine | 1–2 min | 10–30 min | 0.5–1 mg/kg i.v. bolus OR 50–300 µg/kg/min as continuous i.v. infusion | Tachyarrhythmias and chest pain |
| Drug . | Onset of action . | Duration of action . | Dose . | Contraindications . | Adverse effects . |
|---|---|---|---|---|---|
| Esmolol | 1–2 min | 10–30 min | 0.5–1 mg/kg i.v. bolus; 50–300 µg/kg/min as continuous i.v. infusion | History of 2nd or 3rd degree AV block (and in the absence of rhythm support), systolic heart failure, asthma, and bradycardia | Bradycardia |
| Metoprolol | 1–2 min | 5–8 h | 2.5-5 mg i.v. bolus over 2 minutes; may repeat every 5 minutes to a maximum dose of 15 mg | History of 2nd or 3rd degree AV block, systolic heart failure, asthma, and bradycardia | Bradycardia |
| Labetalol | 5–10 min | 3–6 h | 0.25–0.5 mg/kg i.v. bolus; 2–4 mg/min continuous infusion until goal BP is reached, thereafter 5–20 mg/h | History of 2nd or 3rd degree AV block, systolic heart failure, asthma, and bradycardia | Bronchoconstriction and foetal bradycardia |
| Fenoldopam | 5–15 min | 30–60 min | 0.1 µg/kg/min i.v. infusion, increase every 15 min until goal BP is reached with 0.05 to 0.1 µg/kg/min increments | ||
| Clevidipine | 2–3 min | 5–15 min | 2 mg/h i.v. infusion, increase every 2 min with 2 mg/h until goal BP | Headache and reflex-tachycardia | |
| Nicardipine | 5–15 min | 30–40 min | 5–15 mg/h as continuous i.v. infusion, starting dose 5 mg/h, increase every 15–30 min with 2.5 mg until goal BP, thereafter decrease to 3 mg/h | Liver failure | Headache and reflex-tachycardia |
| Nitroglycerine | 1–5 min | 3–5 min | 5–200 µg/min, 5 µg/min increase every 5 min | Headache and reflex tachycardia | |
| Nitroprusside | Immediate | 1–2 min | 0.3–10 µg/kg/min, increase by 0.5 µg/kg/min every 5 min until goal BP | Liver/kidney failure (relative) | Cyanide intoxication |
| Enalaprilat | 5–15 min | 4–6 h | 0.625–1.25 mg i.v. | History of angioedema | |
| Urapidil | 3–5 min | 4–6 h | 12.5–25 mg i.v. bolus, 5–40 mg/h as continuous infusion | ||
| Clonidine | 30 min | 4–6 h | 150–300 µg i.v. bolus in 5–10 min | Sedation and rebound hypertension | |
| Phentolamine | 1–2 min | 10–30 min | 0.5–1 mg/kg i.v. bolus OR 50–300 µg/kg/min as continuous i.v. infusion | Tachyarrhythmias and chest pain |
Malignant hypertension and hypertensive encephalopathy
Activation of the renin–angiotensin system in patients with malignant hypertension is highly variable,3 making the BP-lowering response to renin–angiotensin system blockers unpredictable. Large reductions in BP (exceeding a >50% decrease in mean arterial pressure) have been associated with ischaemic stroke and death.35,36 Sodium nitroprusside, labetalol, nicardipine, and urapidil all appear to be safe and effective for the treatment of malignant hypertension.37–39 Fenoldopam, a short acting selective dopamine-1 agonist, and clevidipine, an ultra-short acting calcium-channel blocker for intravenous use, have been used for the treatment of patients with severe hypertension40,41 but are not widely available. Alternatively, oral administration of ACE-inhibitors is currently used by some teams,42 but must be started at a very low dose to prevent sudden decreases in BP. Because patients are often volume depleted as a result of pressure natriuresis, intravenous saline infusion can be used to correct precipitous BP falls if necessary. In patients with hypertensive encephalopathy labetalol may be preferred as it leaves cerebral blood flow relatively intact for a given BP reduction compared with nitroprusside,43 and does not increase intracranial pressure.39 Nitroprusside and nicardipine can alternatively be used for this type of emergency.
Acute ischaemic and haemorrhagic stroke
In ischaemic stroke, acute BP reduction within the first 5–7 days is associated with adverse neurological outcome.44,45 If BP is very high (>220/120 mmHg) or BP-lowering therapy is indicated for another reason (e.g. acute coronary event, acute heart failure, aortic dissection), it is probably safe to lower mean arterial pressure by 15% in the first 24 h or faster if deemed necessary by the presence of a concomitant condition.46 For acute ischaemic stroke and an indication for thrombolytic therapy, lowering BP to <185 mmHg systolic and 110 mmHg diastolic is recommended before thrombolysis is given.46 The same BP criteria have been used in recent trials that examined the effect of thrombectomy with or without prior thrombolysis.47–49 Acute BP-lowering treatment to systolic BP <140 mmHg in patients with intracerebral haemorhage reduces intracranial haematoma volume and had a (borderline) significant effect in improving functional outcome in the Intensive BP Reduction in Acute Cerebral Haemorrhage Trial (INTERACT)-2,50,51 while the Antihypertensive Treatment of Acute Cerebral Haemorrhage II (ATACH-2) failed to show any benefit of acute BP-lowering treatment.52 Although both trials had the same BP targets, there were large discrepancies in the desired BP target and achieved BP level, which may have mitigated any positive effect. In case acute reduction in BP is desired, labetalol is the drug of choice with nicardipine and sodium nitroprusside being useful alternatives.
Acute coronary artery events
For patients presenting with an acute coronary event we also refer to the 2015 European Society of Cardiology (ESC) guideline for the management of acute coronary syndromes.53 In case severe hypertension is associated with acute coronary syndrome (cardiac ischaemia or myocardial infarction), afterload needs to be reduced without an increase in heart rate in order to decrease myocardial oxygen demand without jeopardizing diastolic filling time. Both nitroglycerine and labetalol have been used to lower BP in patients with an acute coronary event.54–56 In comparison with nitroglycerine, sodium nitroprusside decreases regional blood flow in patients with coronary abnormalities and increases myocardial damage after acute myocardial infarction.57,58 Additional beta-blockade may be indicated for patients receiving nitroglycerine, especially if tachycardia is present. Urapidil may be a good alternative for the management of hypertension in patients with myocardial ischaemia.59,60
Acute cardiogenic pulmonary oedema
In patients with acute pulmonary oedema caused by hypertensive heart failure, both nitroglycerine and sodium nitroprusside can be used as they will optimize preload and decrease afterload.61 Nitroprusside is the drug of choice as it will acutely lower ventricular pre- and afterload. Nitroglycerine may be a good alternative, although high doses (>200 mg/min) may be required to achieve the desired BP-lowering effect. Compared with nitroglycerine, urapidil gives a better BP reduction and improvement of arterial oxygen content without reflex tachycardia.62 Non-invasive continuous positive airway pressure may be of additional benefit as it acutely reduces pulmonary oedema and venous return. Concomitant administration of loop diuretics decreases volume overload and helps to further lower BP.
Acute aortic disease
In patients with acute aortic disease (dissection or rupture) systolic BP and heart rate need to be immediately reduced to 120 mmHg or lower and 60 b.p.m. or less to reduce aortic wall stress and disease progression. Beta-blockers are therefore considered first line treatment. Esmolol can be used together with ultra-short acting vasodilating agents such as nitroprusside or clevidipine.63 Alternatively, bolus injections of metoprolol or labetalol can be used with the possible disadvantage that its long half-life prohibits immediate correction of BP in case of hypotension. For further management of acute aortic dissection, we refer to the US and European Society of Cardiology guidelines.64,65
Eclampsia and severe pre-eclampsia
In patients with eclampsia or severe pre-eclampsia (with or without haemolysis, elevated liver enzymes and low platelets syndrome), BP-lowering therapy is given next to intravenous magnesium Sulfate and delivery needs to be considered after the maternal condition has stabilized.66 The consensus is to lower systolic and diastolic BP <160/105 mmHg to prevent acute hypertensive complications in the mother. Both labetalol and nicardipine have shown to be safe and effective for the treatment of severe pre-eclampsia if intravenous BP-lowering therapy is necessary.67 In both cases monitoring of foetal heart rate is necessary. To prevent foetal bradycardia the cumulative dose of labetalol should not exceed 800 mg/24 h. Timely institution of oral BP-lowering therapy (e.g. methyldopa or long-acting nifedipine) or dose adaptation may help to improve BP control and reduces the risk of foetal bradycardia in case labetalol is used. Treatment with hydralazine is not widely available anymore and not recommended as it has been associated with adverse perinatal outcomes,68 whereas treatment with nitroprusside is contraindicated because it carries the risk of foetal cyanide toxicity.
Specific situations
In patients with autonomic hyper-reactivity because of suspected (meth)amphetamine or cocaine intoxication, treatment with benzodiazepines should be initiated first. In case additional BP-lowering treatment is needed phentolamine, a competitive alpha blocking agent for intravenous use, nicardipine or nitroprusside can be considered. Alternatively, clonidine can be used, which apart from its sympathicolytic action also has sedative effects. In case of coronary ischaemia, treatment with nitroglycerine and aspirin is recommended next to benzodiazepines. In high-risk patients with (non-) ST-segment elevation myocardial infarction a percutaneous coronary intervention should be considered.69 Non-dihydropyridine calcium channel blocking agents such as diltiazem and verapamil can be used to treat patients with tachyarrhythmias under close ECG monitoring. Beta-blocking agents (including labetalol) are relatively contraindicated because they do not seem to be effective in reducing coronary vasoconstriction.70,71 In patients with adrenergic overstimulation because of pheochromocytoma, treatment with labetalol has been associated with acceleration of hypertension in individual cases.72,73 Phentolamine, nitroprusside, and urapidil have been successfully used in the perioperative management of pheochromocytoma.74,75 Nicardipine may also be a good alternative. In patients with a hypertensive emergency elicited by cytotoxic or anti-angiogenic drugs, the offending agent should be withheld until BP control with oral medication is achieved. Alternative agents, dose adjustments and follow-up according to the recommendations by the Cardiovascular Toxicities Panel of the National Cancer Institute can be considered.76
Prognosis and follow-up
Survival has improved drastically over the past decades,77 but patients admitted for a hypertensive emergency remain at increased risk of cardiovascular and renal disease compared with hypertensive patients who did not experience an emergency.6,78 In patients admitted to the coronary care unit with a hypertensive emergency, the mortality was significantly higher (4.6%), when compared with hypertensive patients without a hypertensive emergency (0.8%).78 Prognostic factors for major adverse cardiac or cerebrovascular events in patients presenting with a hypertensive emergency are elevated cardiac troponin-I levels5 and renal impairment at presentation, whereas BP control and the amount of proteinuria during follow-up are the main risk factors for renal survival during follow-up.79,80
Few studies have addressed the optimal follow-up in patients with previous hypertensive emergency. In the absence of specific data, it appears reasonable to extrapolate guidelines for severe hypertension in general.80 In treated patients, improving adherence and persistence are pivotal in reducing the risk of complications and recurrent hospitalization for hypertensive emergencies. Simplification of therapy, whenever possible, should be considered. Increasing the dose of existing antihypertensive medications, adding diuretics, or other antihypertensive agents, preferably as fixed combination therapy, and reinforcing dietary sodium restriction are all worth considering. In parallel with treatment intensification and optimization of adherence, in-depth aetiological work-up and careful assessment of hypertension-mediated organ damage are recommended.
We suggest frequent, at least monthly, visits in a specialized setting until target BP is reached, and a protracted follow-up until hypertension-mediated organ damage (renal function, proteinuria, left ventricular mass) has regressed. In patients with suboptimally treated hypertension, suspected non-adherence and hypertension-mediated organ damage regular visits should be scheduled to tackle existing burdens by counselling and motivational interviewing with the aim to improve compliance with the treatment regimen.
Perspectives
The management of hypertensive emergencies is mainly based on consensus from clinical experience, observations, and comparisons on intermediate outcomes. The Task Force recognizes that evidence-based treatment decisions for hypertensive emergencies can be improved in different areas, including the safety and efficacy of oral medication when compared with intravenous treatment for the treatment of malignant hypertension and the optimal BP target for acute BP-lowering treatment in patients with stroke and acute intracerebral haemorrhage. Further research is needed to assess the impact of acute hypertension-mediated organ damage on future cardiovascular risk and its therapeutic consequences in these patients. Finally, hypertensive emergencies as an extreme phenotype of hypertension and hypertension-mediated complications may help us to understand more about the complex pathophysiology and aetiology of hypertension in general.
Conflict of interest: none declared.



