-
PDF
- Split View
-
Views
-
Cite
Cite
Leo F Buckley, Michele M Viscusi, Benjamin W Van Tassell, Antonio Abbate, Interleukin-1 blockade for the treatment of pericarditis, European Heart Journal - Cardiovascular Pharmacotherapy, Volume 4, Issue 1, January 2018, Pages 46–53, https://doi.org/10.1093/ehjcvp/pvx018
Close - Share Icon Share
Abstract
Pericarditis is a debilitating condition that results from profound inflammation of the pericardial tissue. Between 10 and 15% of first episodes of acute pericarditis will be followed by several episodes refractory to conventional treatment. Current standard of care for pericarditis treatment includes high-dose non-steroidal anti-inflammatory drugs, colchicine, and systemic corticosteroids, each associated with potentially severe toxicities and nominal efficacy. Interleukin-1 (IL-1), an apical pro-inflammatory cytokine, plays an important role as an autocrine magnifier of systemic inflammation in pericarditis. Interruption of the IL-1 circuit has been shown to have a favourable risk profile in several disease states. In this review, we discuss the growing body of evidence which supports the use of IL-1 blockade in the treatment of recurrent pericarditis as well as provide practical considerations for the use of IL-1 blockade in clinical practice.
Introduction
Acute pericarditis is the most common form of pericardial disease and is characterized by severe, sharp, chest pain.1 The causes of acute pericarditis vary widely: in the USA and Europe, most cases are considered idiopathic (80–90%), without a clearly identifiable cause (although may often follow a viral illness), whereas tuberculosis is the leading cause (70%) in Africa.1
The pathophysiology of acute pericarditis is a stereotypical response to an acute injury to the mesothelial cells constituting the pericardial layers around the heart.2,3 This inflammation first is initiated by an irritant itself or the release of necrotic cellular debris and then amplified through the activation of the Nod-like receptor 3 (NLRP3) inflammasome, an intracellular macromolecular structure responsible for sensing danger or injury and intensifying the inflammatory response through the release of mature Interleukin-1β (IL-1β).4
Interleukin-1β is the predominant circulating isoform of IL-1, an apical pro-inflammatory cytokine that stimulates the synthesis of inflammatory mediators such as cyclo-oxygenase-2 (COX-2) and prostaglandins and is responsible for the hyperaemia, oedema, and hyperesthesia characteristic of the acute pericarditis syndrome (Figure 1).4 IL-1β exerts its actions through binding the cell-surface IL-1 receptor. The exact role of the other isoform, IL-1α, is autocrine in nature but remains under intense investigation. The naturally occurring receptor antagonist, named IL-1 receptor antagonist (IL-1ra), acts as a decoy to bind free IL-1 and prevent interaction with its receptor.
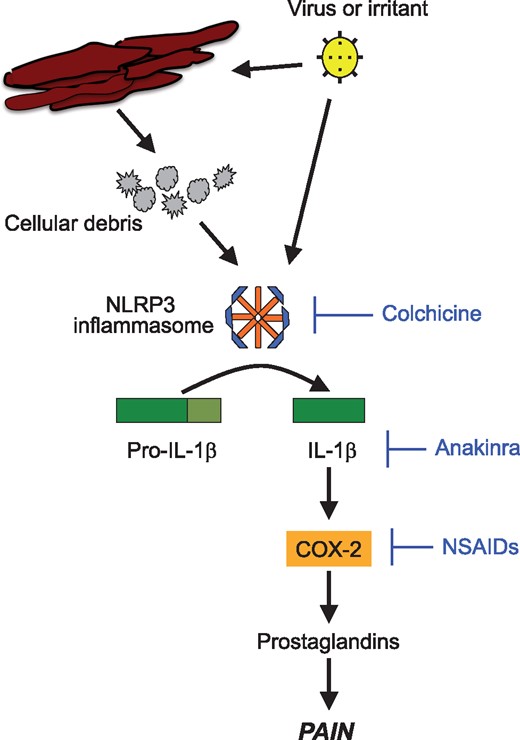
Interleukin-1 and the pathophysiology of pericarditis. An injury due to an infectious or non-infectious irritant triggers the formation of the NLRP3 inflammasome. The NLRP3 inflammasone subsequently initiates a local and systemic inflammatory response through activation and release of Interleukin-1.
Interleukin-1 blockade has been studied extensively in cryopyrin-associated periodic syndromes,4 rheumatoid arthritis,5 sepsis6–8 and, more recently, in cardiovascular disease.9 In comparison with alternative anti-inflammatory options in pericarditis, such as corticosteroids or azathioprine, IL-1 blockade does not impair the response of T and B lymphocytes to infectious antigens, and as such it is not considered immunosuppressive. IL-1 blockade is associated only with mild, self-limiting side effects, if any. In this review, we highlight emerging preliminary evidence to support the efficacy of IL-1 blockade in the treatment of pericarditis.
Search methodology
To identify studies on the use of anakinra in pericarditis, we identified English-language articles in PubMed and Google Scholar for articles published between 1994 and 2017 with the following keywords and their combinations: pericarditis, anakinra, Interleukin-1 receptor antagonist. Our initial search identified 19 articles, of which 9 were relevant. We then reviewed the references for these 9 papers and found 2 additional articles. We finally selected 11 publications which examined the use of anakinra in patients with pericarditis.
Background: diagnosis and treatment of pericarditis
Acute pericarditis is clinical condition characterized by inflammation of the pericardial sac that produces severe pain and can lead to life-threatening complications, such as cardiac tamponade.1 Although the pain is generally responsive to non-steroidal anti-inflammatory drugs (NSAIDs), pain tends to be suboptimally controlled and to recur upon cessation of treatment. The inflammation of the pericardial sac may indeed persist for days–weeks and may become chronic if not adequately treated.
The diagnosis of pericarditis is based on the presence of at least two signs or symptoms (Table 1): pericardial chest pain, pericardial rub, new widespread ST elevation, and/or PR depression on electrocardiogram or pericardial effusion (new or worsening).10 Current guidelines recommend the inclusion of a careful history and physical exam, electrocardiography, transthoracic echocardiography, chest radiograph, and assessment of inflammatory and myocardial injury biomarkers as part of the initial diagnostic approach for suspected pericarditis (each Class I, Level of Evidence C). Furthermore, the initial workup should include testing for infectious causes of pericarditis, such as tuberculosis, if clinically suspected. Chest computed tomography may provide greater detail when the chest radiograph is abnormal.
| . | Acute pericarditis . | Recurrent pericarditis . | |
|---|---|---|---|
| Work-up |
| ||
| Diagnosis | 2 of 4 of the followinga:
| All of the following:
| |
| Treatment | First line |
|
|
| Second line | N/A |
| |
| Refractoryb | N/A |
| |
| . | Acute pericarditis . | Recurrent pericarditis . | |
|---|---|---|---|
| Work-up |
| ||
| Diagnosis | 2 of 4 of the followinga:
| All of the following:
| |
| Treatment | First line |
|
|
| Second line | N/A |
| |
| Refractoryb | N/A |
| |
This algorithm represents the opinion of the authors based on the available literature as reviewed above. This algorithm has not been endorsed by any societal guidelines.
NSAID, non-steroidal anti-inflammatory drug.
Additional supporting findings include: elevation of inflammation markers (i.e. C-reactive protein, erytrocyte sedimentation rate and white blood cell count) and evidence of pericardial inflammation by an imaging technique (computed tomography, magnetic resonance imaging).
Defined as proven infection-negative, corticosteroid-dependent, recurrent pericarditis not responsive to colchicine.
| . | Acute pericarditis . | Recurrent pericarditis . | |
|---|---|---|---|
| Work-up |
| ||
| Diagnosis | 2 of 4 of the followinga:
| All of the following:
| |
| Treatment | First line |
|
|
| Second line | N/A |
| |
| Refractoryb | N/A |
| |
| . | Acute pericarditis . | Recurrent pericarditis . | |
|---|---|---|---|
| Work-up |
| ||
| Diagnosis | 2 of 4 of the followinga:
| All of the following:
| |
| Treatment | First line |
|
|
| Second line | N/A |
| |
| Refractoryb | N/A |
| |
This algorithm represents the opinion of the authors based on the available literature as reviewed above. This algorithm has not been endorsed by any societal guidelines.
NSAID, non-steroidal anti-inflammatory drug.
Additional supporting findings include: elevation of inflammation markers (i.e. C-reactive protein, erytrocyte sedimentation rate and white blood cell count) and evidence of pericardial inflammation by an imaging technique (computed tomography, magnetic resonance imaging).
Defined as proven infection-negative, corticosteroid-dependent, recurrent pericarditis not responsive to colchicine.
The presence of infectious processes in the chest (i.e. pneumonia, abscess, cavitary lesions, empyema) or involving cardiac structures (i.e. endocarditis) or the presence of signs of systemic infection (sepsis) increases the possibility that the pericarditis is bacterial in origin. If bacterial pericarditis is suspected and an effusion is present, pericardiocentesis is indicated to drain the infection and identify the pathogen.
While echocardiography is not necessary for the diagnosis, since acute pericarditis often occurs without significant effusion or other notable abnormalities, a prompt evaluation by transthoracic echocardiography is recommended to exclude the presence of large effusions potentially impairing cardiac filling (tamponade) and to evaluate for concomitant pathologies (i.e. vegetations). Cardiac magnetic resonance imaging is reserved for those cases in which myocardial involvement in the process is suspected, based on a significant elevation of cardiac troponin I or T.
Non-steroidal anti-inflammatory drugs use is often precluded in patients with cardiovascular disease due to untoward effects on blood pressure, renal function, and an increased risk of myocardial infarction and heart failure.11 Moreover, NSAIDs are fraught with a significant risk of gastrointestinal bleeding that may occur even after a few days of treatment.12 Most important, NSAID efficacy in pericarditis has not been studied in randomized controlled trials and corticosteroids have been associated with an increased risk of recurrence.13
Colchicine is the only approved treatment for acute and recurrent pericarditis that has been studied in randomized, controlled clinical trials. Despite its efficacy for preventing recurrences of pericarditis,14 up to one-fifth of patients on colchicine are unable to complete the prescribed treatment course.15
Anakinra
Anakinra (Kineret®, Swedish Orphan Biovitrum) is recombinant human IL-1ra used for the treatment of rheumatoid arthritis and cyropyrin-associated periodic syndromes, which are characterized by overproduction of IL-1 (Table 2).4
| Description and mechanism of action . | Recombinant, non-glycosylated form of the human Interleukin-1 receptor antagonist (IL-1Ra) (single amino-acid substitution) . |
|---|---|
| Indications and Dosage |
|
| Contraindications | Patients with known hypersensitivity to E. coli-derived proteins, Kineret®, or any components of the product including latex. |
| Warning and precautions |
|
| Adverse reactions |
|
| Use in specific populations |
|
| Pharmacokinetics |
|
| Monitoring parameters |
|
| Description and mechanism of action . | Recombinant, non-glycosylated form of the human Interleukin-1 receptor antagonist (IL-1Ra) (single amino-acid substitution) . |
|---|---|
| Indications and Dosage |
|
| Contraindications | Patients with known hypersensitivity to E. coli-derived proteins, Kineret®, or any components of the product including latex. |
| Warning and precautions |
|
| Adverse reactions |
|
| Use in specific populations |
|
| Pharmacokinetics |
|
| Monitoring parameters |
|
US FDA, United States Food and Drug Administration.
| Description and mechanism of action . | Recombinant, non-glycosylated form of the human Interleukin-1 receptor antagonist (IL-1Ra) (single amino-acid substitution) . |
|---|---|
| Indications and Dosage |
|
| Contraindications | Patients with known hypersensitivity to E. coli-derived proteins, Kineret®, or any components of the product including latex. |
| Warning and precautions |
|
| Adverse reactions |
|
| Use in specific populations |
|
| Pharmacokinetics |
|
| Monitoring parameters |
|
| Description and mechanism of action . | Recombinant, non-glycosylated form of the human Interleukin-1 receptor antagonist (IL-1Ra) (single amino-acid substitution) . |
|---|---|
| Indications and Dosage |
|
| Contraindications | Patients with known hypersensitivity to E. coli-derived proteins, Kineret®, or any components of the product including latex. |
| Warning and precautions |
|
| Adverse reactions |
|
| Use in specific populations |
|
| Pharmacokinetics |
|
| Monitoring parameters |
|
US FDA, United States Food and Drug Administration.
Evidence for the use of anakinra to treat recurrent pericarditis
Children
Pericarditis is more common is children and young adults, possibly due to the association with viral illnesses and/or due to the more intense inflammatory response with younger age. The use of anakinra in children with recurrent pericarditis has been described in several case reports and case series (Table 3).
| First author, publication year, journal, study design . | Diagnosis . | Patient characteristics . | Prior failed treatments . | Anakinra regimen and background therapy . | Outcomes . |
|---|---|---|---|---|---|
| Picco et al. 2009. Arthritis Rheumatism; Three case Reports | Idiopathic, recurrent pericarditis | Patient #1: female, age 14 years, 4 prior episodes | Patient #1: colchicine and prednisone | Patient #1: anakinra 1 mg/kg/day with prednisone for 6 weeks (course 1); 1 mg/kg/day with tapering of prednisone (course 2) | Patient #1: recurrence after discontinuation of course 1 but no on-treatment recurrences over 3 months |
| Patient #2: male, age 12 years, 3 prior episode | Patient #2: colchicine and prednisone | Patient #2: anakinra 1 mg/kg/day for 2 months with tapering of prednisone and colchicine (course 1); anakinra 1 mg/kg/day with tapering of prednisone | Patient #2: recurrence after discontinuation of course 1 but no on-treatment recurrences thereafter (4 months of follow-up) | ||
| Patient #3: female, age 13 years, 8 prior episodes | Patient #3: prednisone, NSAIDs, colchicine and methotrexate | Patient #3: anakinra 1.25 mg/kg/day monotherapy for 9 days (course 1) and then 4 months (course 2) | Patient #3: recurrence after discontinuation of course 1 but no on-treatment recurrences over 4 months | ||
| Scardapane et al. 2012. Pediatr Cardiol. Case report | Idiopathic, recurrent pericarditis | Male patient, 11 years old, 4 prior episodes | Ibuprofen, prednisone, colchicine, indomethacin |
| No recurrences after 12 months of follow-up |
| Camacho-Lovillo et al. 2013: Case report | Idiopathic recurrent pericarditis | Male, 12 years old, 1 prior episode | Oral corticosteroid, colchicine |
| Recurrence 4 weeks after discontinuation of course 1; no recurrences thereafter |
| Finetti et al. 2014. J Pediatr. Multicentre Retrospective Study | Idiopathic recurrent pericarditis; Myhre syndrome = 1 | Children (n = 12): Age (median, IQR) = 14 (13-15); prior episodes (median, IQR) = 5 (4-8) | Children: NSAID = 6; corticosteroids = 12; colchicine = 1; chloroquine = 2; methotrexate = 2 | Children: anakinra dose (median, IQR) = 1.2 (1-1.3) mg/kg/day; duration of therapy (median, IQR) = 11 (8-13) | Children: on-treatment recurrence = 0; 5/13 patients withdrawn from anakinra |
| First author, publication year, journal, study design . | Diagnosis . | Patient characteristics . | Prior failed treatments . | Anakinra regimen and background therapy . | Outcomes . |
|---|---|---|---|---|---|
| Picco et al. 2009. Arthritis Rheumatism; Three case Reports | Idiopathic, recurrent pericarditis | Patient #1: female, age 14 years, 4 prior episodes | Patient #1: colchicine and prednisone | Patient #1: anakinra 1 mg/kg/day with prednisone for 6 weeks (course 1); 1 mg/kg/day with tapering of prednisone (course 2) | Patient #1: recurrence after discontinuation of course 1 but no on-treatment recurrences over 3 months |
| Patient #2: male, age 12 years, 3 prior episode | Patient #2: colchicine and prednisone | Patient #2: anakinra 1 mg/kg/day for 2 months with tapering of prednisone and colchicine (course 1); anakinra 1 mg/kg/day with tapering of prednisone | Patient #2: recurrence after discontinuation of course 1 but no on-treatment recurrences thereafter (4 months of follow-up) | ||
| Patient #3: female, age 13 years, 8 prior episodes | Patient #3: prednisone, NSAIDs, colchicine and methotrexate | Patient #3: anakinra 1.25 mg/kg/day monotherapy for 9 days (course 1) and then 4 months (course 2) | Patient #3: recurrence after discontinuation of course 1 but no on-treatment recurrences over 4 months | ||
| Scardapane et al. 2012. Pediatr Cardiol. Case report | Idiopathic, recurrent pericarditis | Male patient, 11 years old, 4 prior episodes | Ibuprofen, prednisone, colchicine, indomethacin |
| No recurrences after 12 months of follow-up |
| Camacho-Lovillo et al. 2013: Case report | Idiopathic recurrent pericarditis | Male, 12 years old, 1 prior episode | Oral corticosteroid, colchicine |
| Recurrence 4 weeks after discontinuation of course 1; no recurrences thereafter |
| Finetti et al. 2014. J Pediatr. Multicentre Retrospective Study | Idiopathic recurrent pericarditis; Myhre syndrome = 1 | Children (n = 12): Age (median, IQR) = 14 (13-15); prior episodes (median, IQR) = 5 (4-8) | Children: NSAID = 6; corticosteroids = 12; colchicine = 1; chloroquine = 2; methotrexate = 2 | Children: anakinra dose (median, IQR) = 1.2 (1-1.3) mg/kg/day; duration of therapy (median, IQR) = 11 (8-13) | Children: on-treatment recurrence = 0; 5/13 patients withdrawn from anakinra |
IQR, interquartile range; NSAIDs, non-steroidal anti-inflammatory drugs; SD, standard deviation.
| First author, publication year, journal, study design . | Diagnosis . | Patient characteristics . | Prior failed treatments . | Anakinra regimen and background therapy . | Outcomes . |
|---|---|---|---|---|---|
| Picco et al. 2009. Arthritis Rheumatism; Three case Reports | Idiopathic, recurrent pericarditis | Patient #1: female, age 14 years, 4 prior episodes | Patient #1: colchicine and prednisone | Patient #1: anakinra 1 mg/kg/day with prednisone for 6 weeks (course 1); 1 mg/kg/day with tapering of prednisone (course 2) | Patient #1: recurrence after discontinuation of course 1 but no on-treatment recurrences over 3 months |
| Patient #2: male, age 12 years, 3 prior episode | Patient #2: colchicine and prednisone | Patient #2: anakinra 1 mg/kg/day for 2 months with tapering of prednisone and colchicine (course 1); anakinra 1 mg/kg/day with tapering of prednisone | Patient #2: recurrence after discontinuation of course 1 but no on-treatment recurrences thereafter (4 months of follow-up) | ||
| Patient #3: female, age 13 years, 8 prior episodes | Patient #3: prednisone, NSAIDs, colchicine and methotrexate | Patient #3: anakinra 1.25 mg/kg/day monotherapy for 9 days (course 1) and then 4 months (course 2) | Patient #3: recurrence after discontinuation of course 1 but no on-treatment recurrences over 4 months | ||
| Scardapane et al. 2012. Pediatr Cardiol. Case report | Idiopathic, recurrent pericarditis | Male patient, 11 years old, 4 prior episodes | Ibuprofen, prednisone, colchicine, indomethacin |
| No recurrences after 12 months of follow-up |
| Camacho-Lovillo et al. 2013: Case report | Idiopathic recurrent pericarditis | Male, 12 years old, 1 prior episode | Oral corticosteroid, colchicine |
| Recurrence 4 weeks after discontinuation of course 1; no recurrences thereafter |
| Finetti et al. 2014. J Pediatr. Multicentre Retrospective Study | Idiopathic recurrent pericarditis; Myhre syndrome = 1 | Children (n = 12): Age (median, IQR) = 14 (13-15); prior episodes (median, IQR) = 5 (4-8) | Children: NSAID = 6; corticosteroids = 12; colchicine = 1; chloroquine = 2; methotrexate = 2 | Children: anakinra dose (median, IQR) = 1.2 (1-1.3) mg/kg/day; duration of therapy (median, IQR) = 11 (8-13) | Children: on-treatment recurrence = 0; 5/13 patients withdrawn from anakinra |
| First author, publication year, journal, study design . | Diagnosis . | Patient characteristics . | Prior failed treatments . | Anakinra regimen and background therapy . | Outcomes . |
|---|---|---|---|---|---|
| Picco et al. 2009. Arthritis Rheumatism; Three case Reports | Idiopathic, recurrent pericarditis | Patient #1: female, age 14 years, 4 prior episodes | Patient #1: colchicine and prednisone | Patient #1: anakinra 1 mg/kg/day with prednisone for 6 weeks (course 1); 1 mg/kg/day with tapering of prednisone (course 2) | Patient #1: recurrence after discontinuation of course 1 but no on-treatment recurrences over 3 months |
| Patient #2: male, age 12 years, 3 prior episode | Patient #2: colchicine and prednisone | Patient #2: anakinra 1 mg/kg/day for 2 months with tapering of prednisone and colchicine (course 1); anakinra 1 mg/kg/day with tapering of prednisone | Patient #2: recurrence after discontinuation of course 1 but no on-treatment recurrences thereafter (4 months of follow-up) | ||
| Patient #3: female, age 13 years, 8 prior episodes | Patient #3: prednisone, NSAIDs, colchicine and methotrexate | Patient #3: anakinra 1.25 mg/kg/day monotherapy for 9 days (course 1) and then 4 months (course 2) | Patient #3: recurrence after discontinuation of course 1 but no on-treatment recurrences over 4 months | ||
| Scardapane et al. 2012. Pediatr Cardiol. Case report | Idiopathic, recurrent pericarditis | Male patient, 11 years old, 4 prior episodes | Ibuprofen, prednisone, colchicine, indomethacin |
| No recurrences after 12 months of follow-up |
| Camacho-Lovillo et al. 2013: Case report | Idiopathic recurrent pericarditis | Male, 12 years old, 1 prior episode | Oral corticosteroid, colchicine |
| Recurrence 4 weeks after discontinuation of course 1; no recurrences thereafter |
| Finetti et al. 2014. J Pediatr. Multicentre Retrospective Study | Idiopathic recurrent pericarditis; Myhre syndrome = 1 | Children (n = 12): Age (median, IQR) = 14 (13-15); prior episodes (median, IQR) = 5 (4-8) | Children: NSAID = 6; corticosteroids = 12; colchicine = 1; chloroquine = 2; methotrexate = 2 | Children: anakinra dose (median, IQR) = 1.2 (1-1.3) mg/kg/day; duration of therapy (median, IQR) = 11 (8-13) | Children: on-treatment recurrence = 0; 5/13 patients withdrawn from anakinra |
IQR, interquartile range; NSAIDs, non-steroidal anti-inflammatory drugs; SD, standard deviation.
While the majority of studies of anakinra for recurrent pericarditis have included idiopathic cases,16–19 there is one report of anakinra for the treatment of recurrent pericarditis associated with Myhre syndrome.19 All children in the series had recurrences of pericarditis despite treatment of NSAIDs, colchicine, systemic steroids and, in several cases, immunosuppressants such as methotrexate16,19 and chloroquine.19
Systemic steroids were often tapered until discontinuation16–18 while the use of other background therapies was continued. In one instance, anakinra allowed discontinuation of both prednisone and colchicine without recurrence.16 The dose of anakinra in these cases was 1.0–1.3 mg/kg/day, although one 12 year-old male received anakinra 2 mg/kg/day for 1 year and then 100 mg every other day thereafter without adverse effects.19 For comparison, the starting dose of anakinra for children with cryopyrin-associated periodic syndrome (CAPS) is 1–2 mg/kg/day and this dose may be increased up to 8 mg/kg/day as needed (Table 2).20
Anakinra induced remission and prevented recurrences in all reported cases. While cases of anakinra discontinuation without recurrence have been reported,19 many cases reported recurrence after cessation of anakinra therapy. In recurrent idiopathic pericarditis, the optimal treatment duration is likely 6 months or longer followed by a long taper over several months. Reduced dosing frequency (every other day) has been reported to effective in preventing pericarditis recurrence.17,18
Adults
The evidence base for anakinra as a treatment for recurrent pericarditis in adults derives from several case series and a randomized controlled trial (Anakinra—Treatment of Recurrent Idiopathic Pericarditis or AIRTRIP)21 (Table 4).
| First author, publication year, journal, study design . | Diagnosis . | Patient characteristics . | Prior failed treatments . | Anakinra regimen and background therapy . | Outcomes . |
|---|---|---|---|---|---|
| Vassilopoulos et al. 2012. Int J Cardiol. Case report. | Idiopathic, recurrent pericarditis | Patient: male, 26 years old, 8 prior episodes | NSAIDs, colchicine, steroids and azathioprine | Anakinra 150 mg daily for 3 months then 150 mg every other day for 3 months with steroid tapering (course 1); 150 mg daily monotherapy for 6 months then every other day thereafter (course 2) | 1 recurrence during alternate day treatment of course 1; disease-free thereafter |
| Lazaros et al. 2014. Ann Rheum Dis. Case series. | Idiopathic recurrent pericarditis |
|
|
|
|
| Finetti et al. 2014. J Pediatr. Multicentre retrospective study | Idiopathic recurrent pericarditis; Myhre syndrome = 1 | Adults: ages 24, 37, 49; prior episodes = 18, 11, 28, respectively | Adults: NSAIDs, colchicine, corticosteroids (n = 3) | Adults: anakinra doses = 1.2, 1.2, 1.5 mg/kg/day for 12, 6, 17 months, respectively | Adults: on-treatment recurrence = 0; 0 patients withdrawn from anakinra |
| D’Elia et al. 2015. Clin Exp Rheumatol. Case Report | Idiopathic recurrent pericarditis | Female, age 47 years, 1 prior episode | Ibuprofen, methylprednisolone, colchicine |
| No recurrences over 11 months of follow-up |
| Lazaros et al. 2015. Clin Exp Rheumatol. Case Report | Effusive-constrictive pericarditis attributed to Coxsackie B3 virus | Male, 46 years old, 1 prior episode | NSAIDs, prednisone, colchicine | Anakinra 100 mg daily for 12 months then every other day thereafter (18 months total follow-up) | No recurrences after 18 months of follow-up |
| Idiopathic recurrent pericarditis | 21 patients (20 adults, 1 child): | Corticosteroids: 21 (100%) | All patients received anakinra for 60 days then were randomized 1:1 to continue anakinra or switch to placebo for an additional 6 months
| Primary endpoint of recurrence at 8 months:
|
Age (mean ± SD): 45.4 ± 14.3 years
| NSAIDs: 15 (71%)
| ||||
| Jain et al. 2015. Am J Cardiol. Case series. | Idiopathic recurrent pericarditis (n = 12); post-infarction pericarditis (n = 1) | 13 adults:
|
|
|
|
| Schatz et al. 2016: Case Report | Pericarditis associated with rheumatoid arthritis | Female, 39 years old, 1 prior episode |
| Anakinra 100 mg daily for 3 months | No recurrences after 15 months of follow-up |
| First author, publication year, journal, study design . | Diagnosis . | Patient characteristics . | Prior failed treatments . | Anakinra regimen and background therapy . | Outcomes . |
|---|---|---|---|---|---|
| Vassilopoulos et al. 2012. Int J Cardiol. Case report. | Idiopathic, recurrent pericarditis | Patient: male, 26 years old, 8 prior episodes | NSAIDs, colchicine, steroids and azathioprine | Anakinra 150 mg daily for 3 months then 150 mg every other day for 3 months with steroid tapering (course 1); 150 mg daily monotherapy for 6 months then every other day thereafter (course 2) | 1 recurrence during alternate day treatment of course 1; disease-free thereafter |
| Lazaros et al. 2014. Ann Rheum Dis. Case series. | Idiopathic recurrent pericarditis |
|
|
|
|
| Finetti et al. 2014. J Pediatr. Multicentre retrospective study | Idiopathic recurrent pericarditis; Myhre syndrome = 1 | Adults: ages 24, 37, 49; prior episodes = 18, 11, 28, respectively | Adults: NSAIDs, colchicine, corticosteroids (n = 3) | Adults: anakinra doses = 1.2, 1.2, 1.5 mg/kg/day for 12, 6, 17 months, respectively | Adults: on-treatment recurrence = 0; 0 patients withdrawn from anakinra |
| D’Elia et al. 2015. Clin Exp Rheumatol. Case Report | Idiopathic recurrent pericarditis | Female, age 47 years, 1 prior episode | Ibuprofen, methylprednisolone, colchicine |
| No recurrences over 11 months of follow-up |
| Lazaros et al. 2015. Clin Exp Rheumatol. Case Report | Effusive-constrictive pericarditis attributed to Coxsackie B3 virus | Male, 46 years old, 1 prior episode | NSAIDs, prednisone, colchicine | Anakinra 100 mg daily for 12 months then every other day thereafter (18 months total follow-up) | No recurrences after 18 months of follow-up |
| Idiopathic recurrent pericarditis | 21 patients (20 adults, 1 child): | Corticosteroids: 21 (100%) | All patients received anakinra for 60 days then were randomized 1:1 to continue anakinra or switch to placebo for an additional 6 months
| Primary endpoint of recurrence at 8 months:
|
Age (mean ± SD): 45.4 ± 14.3 years
| NSAIDs: 15 (71%)
| ||||
| Jain et al. 2015. Am J Cardiol. Case series. | Idiopathic recurrent pericarditis (n = 12); post-infarction pericarditis (n = 1) | 13 adults:
|
|
|
|
| Schatz et al. 2016: Case Report | Pericarditis associated with rheumatoid arthritis | Female, 39 years old, 1 prior episode |
| Anakinra 100 mg daily for 3 months | No recurrences after 15 months of follow-up |
IQR, interquartile range; NSAIDs, non-steroidal anti-inflammatory drugs; SD, standard deviation.
| First author, publication year, journal, study design . | Diagnosis . | Patient characteristics . | Prior failed treatments . | Anakinra regimen and background therapy . | Outcomes . |
|---|---|---|---|---|---|
| Vassilopoulos et al. 2012. Int J Cardiol. Case report. | Idiopathic, recurrent pericarditis | Patient: male, 26 years old, 8 prior episodes | NSAIDs, colchicine, steroids and azathioprine | Anakinra 150 mg daily for 3 months then 150 mg every other day for 3 months with steroid tapering (course 1); 150 mg daily monotherapy for 6 months then every other day thereafter (course 2) | 1 recurrence during alternate day treatment of course 1; disease-free thereafter |
| Lazaros et al. 2014. Ann Rheum Dis. Case series. | Idiopathic recurrent pericarditis |
|
|
|
|
| Finetti et al. 2014. J Pediatr. Multicentre retrospective study | Idiopathic recurrent pericarditis; Myhre syndrome = 1 | Adults: ages 24, 37, 49; prior episodes = 18, 11, 28, respectively | Adults: NSAIDs, colchicine, corticosteroids (n = 3) | Adults: anakinra doses = 1.2, 1.2, 1.5 mg/kg/day for 12, 6, 17 months, respectively | Adults: on-treatment recurrence = 0; 0 patients withdrawn from anakinra |
| D’Elia et al. 2015. Clin Exp Rheumatol. Case Report | Idiopathic recurrent pericarditis | Female, age 47 years, 1 prior episode | Ibuprofen, methylprednisolone, colchicine |
| No recurrences over 11 months of follow-up |
| Lazaros et al. 2015. Clin Exp Rheumatol. Case Report | Effusive-constrictive pericarditis attributed to Coxsackie B3 virus | Male, 46 years old, 1 prior episode | NSAIDs, prednisone, colchicine | Anakinra 100 mg daily for 12 months then every other day thereafter (18 months total follow-up) | No recurrences after 18 months of follow-up |
| Idiopathic recurrent pericarditis | 21 patients (20 adults, 1 child): | Corticosteroids: 21 (100%) | All patients received anakinra for 60 days then were randomized 1:1 to continue anakinra or switch to placebo for an additional 6 months
| Primary endpoint of recurrence at 8 months:
|
Age (mean ± SD): 45.4 ± 14.3 years
| NSAIDs: 15 (71%)
| ||||
| Jain et al. 2015. Am J Cardiol. Case series. | Idiopathic recurrent pericarditis (n = 12); post-infarction pericarditis (n = 1) | 13 adults:
|
|
|
|
| Schatz et al. 2016: Case Report | Pericarditis associated with rheumatoid arthritis | Female, 39 years old, 1 prior episode |
| Anakinra 100 mg daily for 3 months | No recurrences after 15 months of follow-up |
| First author, publication year, journal, study design . | Diagnosis . | Patient characteristics . | Prior failed treatments . | Anakinra regimen and background therapy . | Outcomes . |
|---|---|---|---|---|---|
| Vassilopoulos et al. 2012. Int J Cardiol. Case report. | Idiopathic, recurrent pericarditis | Patient: male, 26 years old, 8 prior episodes | NSAIDs, colchicine, steroids and azathioprine | Anakinra 150 mg daily for 3 months then 150 mg every other day for 3 months with steroid tapering (course 1); 150 mg daily monotherapy for 6 months then every other day thereafter (course 2) | 1 recurrence during alternate day treatment of course 1; disease-free thereafter |
| Lazaros et al. 2014. Ann Rheum Dis. Case series. | Idiopathic recurrent pericarditis |
|
|
|
|
| Finetti et al. 2014. J Pediatr. Multicentre retrospective study | Idiopathic recurrent pericarditis; Myhre syndrome = 1 | Adults: ages 24, 37, 49; prior episodes = 18, 11, 28, respectively | Adults: NSAIDs, colchicine, corticosteroids (n = 3) | Adults: anakinra doses = 1.2, 1.2, 1.5 mg/kg/day for 12, 6, 17 months, respectively | Adults: on-treatment recurrence = 0; 0 patients withdrawn from anakinra |
| D’Elia et al. 2015. Clin Exp Rheumatol. Case Report | Idiopathic recurrent pericarditis | Female, age 47 years, 1 prior episode | Ibuprofen, methylprednisolone, colchicine |
| No recurrences over 11 months of follow-up |
| Lazaros et al. 2015. Clin Exp Rheumatol. Case Report | Effusive-constrictive pericarditis attributed to Coxsackie B3 virus | Male, 46 years old, 1 prior episode | NSAIDs, prednisone, colchicine | Anakinra 100 mg daily for 12 months then every other day thereafter (18 months total follow-up) | No recurrences after 18 months of follow-up |
| Idiopathic recurrent pericarditis | 21 patients (20 adults, 1 child): | Corticosteroids: 21 (100%) | All patients received anakinra for 60 days then were randomized 1:1 to continue anakinra or switch to placebo for an additional 6 months
| Primary endpoint of recurrence at 8 months:
|
Age (mean ± SD): 45.4 ± 14.3 years
| NSAIDs: 15 (71%)
| ||||
| Jain et al. 2015. Am J Cardiol. Case series. | Idiopathic recurrent pericarditis (n = 12); post-infarction pericarditis (n = 1) | 13 adults:
|
|
|
|
| Schatz et al. 2016: Case Report | Pericarditis associated with rheumatoid arthritis | Female, 39 years old, 1 prior episode |
| Anakinra 100 mg daily for 3 months | No recurrences after 15 months of follow-up |
IQR, interquartile range; NSAIDs, non-steroidal anti-inflammatory drugs; SD, standard deviation.
Lazaros et al. reported the outcomes of 10 patients treated with anakinra 100 mg daily for 12 months.22 This case series included five males (mean age 51 years). Five patients were receiving NSAIDs, nine patients were receiving colchicine and eight were receiving corticosteroids as concomitant therapies during anakinra treatment. C-reactive protein (CRP) levels exceeded 18 mg/dL in all patients (range 18–165 mg/dL) and were reduced significantly, usually within 1 week of treatment. Steroids were successfully tapered to discontinuation in 8 (80%) patients after initiation of anakinra. Over 24 months of follow-up, five patients remained free-from-recurrence. Of these five patients with recurrences, two completed only 6 months of anakinra treatment. Minor injection site reactions occurred in six patients.
In a series of 13 adult patients19 who failed ≥6 months of treatment with NSAIDs, colchicine and prednisone (49 years of age, 77% females), 12 (92%) had a treatment response, defined according to the treating physician, as ‘complete’ symptom relief (n = 11) and ‘partial’ symptom relief in the remaining 1 (8%) patient. Response to anakinra treatment was rapid, occurring between 2 and 5 days after treatment initiation. Other pericarditis treatments were discontinued in 11 (84%) of patients. Four (31%) patients reported mild injection site reactions that resolved within 3–4 weeks with treatment with oral antihistamine and topical corticosteroid therapy. Anakinra was reduced to a lower dose and an every-other-day schedule prior to stopping if symptoms did not recur. Anakinra was stopped without symptom recurrence in 8 (61%) patients, whereas reduced doses (50 mg/day or 50 mg every other day) were utilized without pericarditis recurrence in 4 (31%) patients, with 1 (8%) patient remaining on 100 mg daily dose.
Several case reports support the use of anakinra in patients with recurrent pericarditis in order to reduce recurrences and taper corticosteroids and other medication therapies.19,23–25 Anakinra has been studied in non-idiopathic pericarditis cases, such as one related to rheumatoid arthritis,26 with similar results as idiopathic cases.
In the AIRTRIP randomized controlled trial, patients were eligible if they had ≥3 recurrences of idiopathic pericarditis, documented CRP ≥1 mg/dL for each prior recurrence and corticosteroid dependence.21 All patients received anakinra treatment for 60 days followed by randomization to either anakinra 100 mg daily or placebo for an additional 6 months. During the 60-day open-label period, the study protocol mandated tapered withdrawal of all medications except corticosteroids and colchicine between days 8 and 15 and tapered withdrawal of corticosteroids between days 8 and 42 in patients who had a response to anakinra at day 4, defined as ≥30% reduction in pericardial pain, ≥30% reduction in CRP and no evidence of pericardial effusion on echocardiography. Non-responder patients were to be withdrawn from the study and treated with rescue therapy at the discretion of their physician.
There was one adolescent (15 years old, male) in the study and the remaining adult patients had a mean age 45 years (range 15–69). There were 14 (67%) female patients. The average number of prior idiopathic pericarditis recurrences was 7 (range 3–16) over a period of 28 months (range 4–100).
All 21 patients met criteria for response at day 4 and continued into the randomized treatment phase. At 8 months, there were 9 (90%) pericarditis recurrences in the placebo group compared with 2 (18%) in the anakinra group (P = 0.001) (Figure 2). During the open-label period, 20 (95%) patients reported injection site reactions. Injection site reactions resolved within 1 month and did not lead to permanent study drug discontinuation in any patients. During the double-blind phase, there were no reported injection site reactions. Pericardial effusion resolved by day 60 in 17 of 18 (94%) patients with effusion at baseline and new effusion did not develop in any patient.
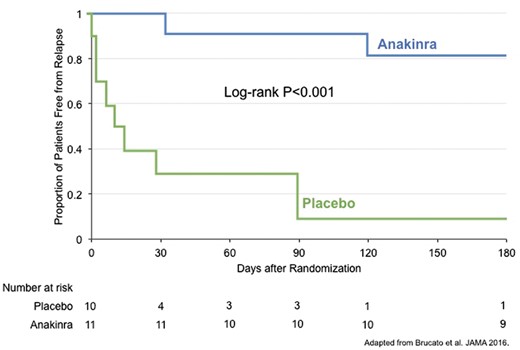
Efficacy of anakinra for the prevention of relapse in idiopathic recurrent pericarditis: results from the AIRTRIP Clinical Trial.
There have also been reports of successful management of autoimmune myocarditis with anakinra.27–30 Considering that many cases of myocarditis present concurrently with pericarditis and the similarities in pathophysiology between these two syndromes, such reports lend additional support to the use of anakinra for the treatment of pericarditis.39 Importantly, anakinra has not been studied in cases of pericarditis due to a bacterial infectious cause.
Practical considerations and place in therapy for anakinra
Despite the strong rationale for the use of anakinra in idiopathic pericarditis, there is a lack of evidence to support anakinra as first-line therapy in place of NSAIDs and colchicine. However, anakinra has notable advantages over alternate second line therapies, such as tolerability and the possibility of reduced recurrence risk. Thus, we consider anakinra as an option in patients with non-autoimmune or non-infectious pericarditis refractory to NSAIDs and colchicine in place of corticosteroids. Alternatively, corticosteroids would be preferred in confirmed cases of autoimmune pericarditis.
Prior to initiation of anakinra, an infectious aetiology of pericarditis must be ruled out. In adults, we recommend initiation of anakinra at 100 mg subcutaneously once daily. Symptomatic improvement should occur within hours–days, after which a corticosteroid taper can be initiated over several weeks, if applicable. After 6–8 months of treatment, consideration should be given to a slow taper which decreases the total weekly dose of anakinra 100 mg over 7 weeks.
In children, we recommend 1 mg/kg/day, although doses up to 2 mg/kg/day have been reported to be tolerable. The full-dose treatment duration should be at least 6 months, and perhaps longer given the reports of recurrence upon treatment cessation in children. Tapering can be accomplished by decreasing the total weekly dose by 100 mg over 7 weeks.
Although rare, anakinra has been associated with a risk of neutropenia. We recommend checking absolute neutrophil count after 2–4 weeks of treatment. Patients must be educated on anakinra’s antipyretic properties and the need for awareness of alternate signs and symptoms of infection to enable timely treatment initiation.
Injection site reactions are typically mild and rarely require permanent treatment cessation. It is important to rotate injection sites in a clockwise manner. Prior to dose administration, allowing anakinra to warm to room temperature improves local tolerability. If necessary, topical steroids and systemic antihistamines can alleviate injection site reactions.
Future directions
There is growing recognition of the central role of IL-1 in cardiovascular disease and ongoing research is investigating upstream mediators of IL-1 production.9,32,33 The NLRP3 inflammasome is an intracellular, macromolecular complex activated by cellular injury to produce IL-1. The anti-inflammatory properties of colchicine in pericarditis and gout are indeed mediated through inhibition of the NLRP3 inflammasome (Figure 1). Several NLRP3 inflammasome inhibitors gave been tested in preclinical studies.34–37 In a preclinical murine model of pericarditis, NLRP3 inhibition reduced pericardial thickening, pericardial effusion size and inflammatory markers.38 NLRP3 inflammasome inhibition may provide very potent yet targeted anti-inflammatory effects.
Conclusions
A growing body of evidence implicates IL-1 as the proinflammatory mediator responsible for amplification of the inflammatory response in acute pericarditis. Similarly, a growing body of evidence, including the results of the randomized AIRTRIP trial, indicates that IL-1 blockade with anakinra interrupts the autoinflammatory processes involved in recurrent pericarditis. Anakinra has been studied extensively in patients with rheumatologic diseases5 and is being developed for several cardiovascular indications, including myocardial infarction39,–41 and heart failure.42–44 Anakinra is well-tolerated by the majority of patients. The most commonly occurring adverse effect is injection site reaction, which usually is mild, transient and resolves spontaneously or with topical steroids or oral antihistamines. Anakinra does not increase the risk of serious infection in patients not receiving background immunosuppressants.9 Given the favourable safety profile of anakinra, and the promising therapeutic efficacy for the treatment of recurrent idiopathic pericarditis, anakinra may be considered for patients with recurrent pericarditis who have either failed to achieve complete remission colchicine or could not tolerate colchicine. It is important to note, however, that anakinra has yet to be studied as first-line monotherapy in place of NSAIDs and colchicine, and randomized studies in patients with a first episode of acute pericarditis are warranted. Anakinra however should be avoided in cases of bacterial pericarditis due to the possibility of promoting the spread of infection.
Conflict of interest: Drs Van Tassell and Abbate have received research grant support from Swedish Orphan Biovitrum (Stockholm, Sweden) for the completion of clinical trials sponsored by the National Heart, Lung, and Blood Institute (National Institutes of Health, USA) (NCT02173548, NCT01950299, and NCT01936909). Drs Buckley and Viscusi have nothing to disclose.
References
Author notes
Leo F. Buckley and Michele M. Viscusi contributed equally to the realization of this manuscript.



