-
PDF
- Split View
-
Views
-
Cite
Cite
Leonardo De Luca, Stefano De Servi, Giuseppe Musumeci, Leonardo Bolognese, Is ticagrelor safe in octogenarian patients with non-ST elevation acute coronary syndromes?, European Heart Journal - Cardiovascular Pharmacotherapy, Volume 4, Issue 1, January 2018, Pages 12–14, https://doi.org/10.1093/ehjcvp/pvx034
Close - Share Icon Share
Elderly patients with acute coronary syndromes (ACSs) are at higher risk of recurrent ischaemic events and death compared with younger patients.1 Antiplatelet agents reduce the risk of ischaemic events in ACS but are associated with increased bleeding risk, especially among patients with advanced age.1,2 The significant clinical benefit and overall safety of ticagrelor compared with clopidogrel in ACS patients enrolled in the PLATO (PLATelet inhibition and clinical Outcome) trial were not found to depend on age.3 However, specific real-world data on safety profile of ticagrelor in very elderly ACS patients are still lacking.
Using data from the multicentre, prospective, SCOPE (Switching from Clopidogrel to New Oral Antiplatelet Agents during PErcutaneous Coronary Intervention) registry,4 we sought to assess the clinical benefit of ticagrelor compared with clopidogrel in octogenarian patients with non-ST elevation ACS (NSTE-ACS).
The SCOPE registry has been described elsewhere.4 Briefly, the registry was aimed to evaluate the incidence and short-term outcome of switching of oral P2Y12 receptor inhibitors in consecutive ACS patients undergoing percutaneous coronary intervention (PCI) during a 3-month period.4 A follow-up was planned 1 month after hospital discharge.
Bleeding events were classified according to the Bleeding Academic Research Consortium (BARC) criteria by an independent Central Adjudication Committee.
Major adverse cardiac events (MACE) were defined as the combination of death, myocardial infarction (MI), stent thrombosis and stroke/transient ischaemic attacks. Net adverse cardiac events (NACE) were defined as the combination of MACE and bleeding events.
Continuous variables were reported as mean and standard deviation and were compared using the Student’s t-test or Wilcoxon rank sum test as appropriate. Categorical variables were reported as percentages and compared using the χ2 or Fisher’s exact test as appropriate. To account for differences in baseline characteristics between patients treated with ticagrelor and clopidogrel, a propensity analysis approach was used for data analysis. A parsimonious model of risk factors for selection of the two antiplatelet agents was created using 11 baseline patient characteristics (see Figure 1). A stepwise logistic regression approach (backward, remove P ≤0.20) was used for variable selection to create the final model. From this model, propensity scores were generated for each patient. Propensity scores were then nearest neighbour matched to create a matched cohort for analysis. For all analyses, the conventional P value of 0.05 or less was used to determine level of statistical significance. All reported P values are two sided.
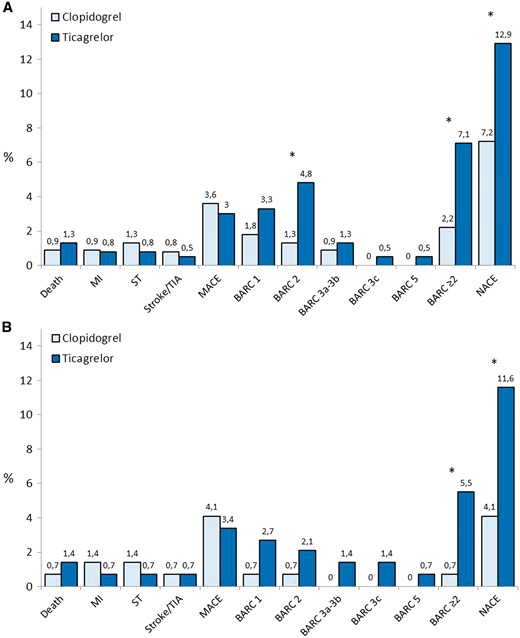
Cumulative incidence of MACE, NACE and single adverse events in patients treated with ticagrelor or clopidogrel in the unmatched (Panel A) and matched cohort (Panel B) at 1-month follow-up. The matched cohort was created considering the following characteristics: gender, diabetes mellitus, renal dysfunction, hypertension, previous cerebrovascular events, previous bleeding events, severe chronic obstructive pulmonary disease, presence of Killip class III–IV at admission, 1 vs. 2 coronary artery disease, ejection fraction ≥ 40% vs. <40% and concomitant use of glycoprotein IIb/IIIa inhibitors. *P < 0.05.
From 15 February to 15 May 2016, a total of 1363 ACS patients naïve from P2Y12 receptor inhibitors and treated with a successful PCI have been enrolled in 39 centres. Out of 1032 NSTE-ACS patients, 640 (62.0%) were older than 80 years. Among these patients, 223 (34.8%) were treated with clopidogrel (without switching to other oral P2Y12 inhibitors during hospitalization) and 396 (61.9%) with ticagrelor, in addition to aspirin. We excluded from the present analysis 4 (0.6%) patients treated with prasugrel and the remaining 17 (2.7%) patients aged ≥80 years discharged with aspirin alone.
Baseline clinical characteristics and procedural variables of the two groups are summarized in Table 1. After nearest neighbour matching on derived propensity score, a total of 292 matched cases (146 per group) were generated, 47.2% of the original cohort. Matched pairs were created across all the range of propensity score. Once matched, there were no longer significant differences among major baseline characteristics between groups. At follow-up, the cumulative incidence of MACE and single ischaemic endpoints was comparable between the two groups, but ticagrelor compared with clopidogrel, significantly increased the rate of BARC ≥2 bleeding events (2.2% vs. 7.1%; P = 0.009 and 0.7% vs. 5.5%; P = 0.04) and the rate of NACE (12.9% vs. 7.2%; P = 0.02 and 11.6% vs. 4.1%; P = 0.03) in both the unmatched and matched populations, respectively (Figure 1).
| . | Overall, n = 619 . | Clopidogrel, n = 223 . | Ticagrelor, n = 396 . | P value . |
|---|---|---|---|---|
| Age (years) | 83 ± 4 | 84 ± 3 | 82 ± 2 | |
| Final diagnosis, n (%) | ||||
| NSTEMI | 489 (79.0) | 172 (77.1) | 317 (80.1) | 0.41 |
| Unstable angina | 130 (21.0) | 51 (22.9) | 79 (19.9) | 0.42 |
| Female gender, n (%) | 183 (29.6) | 72 (32.3) | 111 (28.0) | 0.27 |
| Hypertension, n (%) | 482 (77.9) | 197 (88.3) | 285 (72.0) | 0.0001 |
| Hyperlipidaemia, n (%) | 316 (51.1) | 112 (50.2) | 204 (51.5) | 0.80 |
| Previous smoker, n (%) | 177 (28.6) | 71 (31.8) | 106 (26.8) | 0.20 |
| Diabetes mellitus, n (%) | 212 (34.2) | 98 (43.9) | 114 (28.8) | 0.0002 |
| Previous stroke/TIA, n (%) | 78 (12.6) | 56 (25.1) | 22 (5.6) | 0.0001 |
| PAD, n (%) | 133 (21.5) | 52 (23.3) | 81 (20.5) | 0.42 |
| Previous bleeding, n (%) | 47 (7.6) | 31 (13.9) | 16 (4.0) | 0.0001 |
| Renal dysfunction, n (%) | 101 (16.3) | 78 (35.0) | 23 (5.8) | 0.0001 |
| Dialysis, n (%) | 26 (4.2) | 21 (9.4) | 5 (1.3) | 0.0001 |
| Malignancy, n (%) | 19 (3.1) | 10 (4.5) | 9 (2.3) | 0.15 |
| Severe COPD, n (%) | 46 (7.4) | 32 (14.3) | 14 (3.5) | 0.0001 |
| Previous ACS, n (%) | 189 (30.5) | 79 (35.4) | 110 (27.8) | 0.06 |
| Previous PCI, n (%) | 234 (37.8) | 88 (39.5) | 146 (36.9) | 0.55 |
| Previous CABG, n (%) | 36 (5.8) | 19 (8.5) | 17 (4.3) | 0.05 |
| Killip class III–IV, n (%) | 61 (9.9) | 41 (18.4) | 20 (5.1) | 0.0001 |
| SBP, mmHg (mean ± SD) | 139 ± 21 | 140 ± 18 | 139 ± 10 | 0.37 |
| HR, bpm (mean ± SD) | 78 ± 16 | 79 ± 12 | 77 ± 23 | 0.23 |
| Ejection fraction, % (mean ± SD) | 41 ± 3 | 40 ± 5 | 41 ± 1 | 0.0001 |
| Hb, gr/dl (mean ± SD) | 12 ± 1 | 12 ± 5 | 12 ± 1 | 1.0 |
| Platelet count, x1000 (mean ± SD) | 261 ± 63 | 262 ± 59 | 261 ± 22 | 0.76 |
| Vascular access (%) | ||||
| Radial | 428 (69.1) | 158 (70.9) | 270 (68.2) | 0.53 |
| Femoral | 191 (30.9) | 65 (29.1) | 126 (31.8) | 0.53 |
| Number of stent, mean ± SD | ||||
| DES | 1.7 ± 1.0 | 1.7 ± 0.8 | 1.7 ± 1.1 | 1.0 |
| BMS | 1.4 ± 0.6 | 1.5 ± 0.8 | 1.4 ± 0.4 | 0.04 |
| Extension of CAD (%) | ||||
| Single | 254 (41.0) | 81 (36.3) | 173 (43.7) | 0.07 |
| Dual | 257 (41.5) | 78 (35.0) | 179 (45.2) | 0.01 |
| Three | 108 (17.5) | 64 (28.7) | 44 (11.1) | 0.0001 |
| Glycoprotein IIb/IIIa inhibitors | 51 (8.2) | 36 (16.1) | 15 (3.8) | 0.0001 |
| . | Overall, n = 619 . | Clopidogrel, n = 223 . | Ticagrelor, n = 396 . | P value . |
|---|---|---|---|---|
| Age (years) | 83 ± 4 | 84 ± 3 | 82 ± 2 | |
| Final diagnosis, n (%) | ||||
| NSTEMI | 489 (79.0) | 172 (77.1) | 317 (80.1) | 0.41 |
| Unstable angina | 130 (21.0) | 51 (22.9) | 79 (19.9) | 0.42 |
| Female gender, n (%) | 183 (29.6) | 72 (32.3) | 111 (28.0) | 0.27 |
| Hypertension, n (%) | 482 (77.9) | 197 (88.3) | 285 (72.0) | 0.0001 |
| Hyperlipidaemia, n (%) | 316 (51.1) | 112 (50.2) | 204 (51.5) | 0.80 |
| Previous smoker, n (%) | 177 (28.6) | 71 (31.8) | 106 (26.8) | 0.20 |
| Diabetes mellitus, n (%) | 212 (34.2) | 98 (43.9) | 114 (28.8) | 0.0002 |
| Previous stroke/TIA, n (%) | 78 (12.6) | 56 (25.1) | 22 (5.6) | 0.0001 |
| PAD, n (%) | 133 (21.5) | 52 (23.3) | 81 (20.5) | 0.42 |
| Previous bleeding, n (%) | 47 (7.6) | 31 (13.9) | 16 (4.0) | 0.0001 |
| Renal dysfunction, n (%) | 101 (16.3) | 78 (35.0) | 23 (5.8) | 0.0001 |
| Dialysis, n (%) | 26 (4.2) | 21 (9.4) | 5 (1.3) | 0.0001 |
| Malignancy, n (%) | 19 (3.1) | 10 (4.5) | 9 (2.3) | 0.15 |
| Severe COPD, n (%) | 46 (7.4) | 32 (14.3) | 14 (3.5) | 0.0001 |
| Previous ACS, n (%) | 189 (30.5) | 79 (35.4) | 110 (27.8) | 0.06 |
| Previous PCI, n (%) | 234 (37.8) | 88 (39.5) | 146 (36.9) | 0.55 |
| Previous CABG, n (%) | 36 (5.8) | 19 (8.5) | 17 (4.3) | 0.05 |
| Killip class III–IV, n (%) | 61 (9.9) | 41 (18.4) | 20 (5.1) | 0.0001 |
| SBP, mmHg (mean ± SD) | 139 ± 21 | 140 ± 18 | 139 ± 10 | 0.37 |
| HR, bpm (mean ± SD) | 78 ± 16 | 79 ± 12 | 77 ± 23 | 0.23 |
| Ejection fraction, % (mean ± SD) | 41 ± 3 | 40 ± 5 | 41 ± 1 | 0.0001 |
| Hb, gr/dl (mean ± SD) | 12 ± 1 | 12 ± 5 | 12 ± 1 | 1.0 |
| Platelet count, x1000 (mean ± SD) | 261 ± 63 | 262 ± 59 | 261 ± 22 | 0.76 |
| Vascular access (%) | ||||
| Radial | 428 (69.1) | 158 (70.9) | 270 (68.2) | 0.53 |
| Femoral | 191 (30.9) | 65 (29.1) | 126 (31.8) | 0.53 |
| Number of stent, mean ± SD | ||||
| DES | 1.7 ± 1.0 | 1.7 ± 0.8 | 1.7 ± 1.1 | 1.0 |
| BMS | 1.4 ± 0.6 | 1.5 ± 0.8 | 1.4 ± 0.4 | 0.04 |
| Extension of CAD (%) | ||||
| Single | 254 (41.0) | 81 (36.3) | 173 (43.7) | 0.07 |
| Dual | 257 (41.5) | 78 (35.0) | 179 (45.2) | 0.01 |
| Three | 108 (17.5) | 64 (28.7) | 44 (11.1) | 0.0001 |
| Glycoprotein IIb/IIIa inhibitors | 51 (8.2) | 36 (16.1) | 15 (3.8) | 0.0001 |
ACS, acute coronary syndrome; BMS, bare metal stent, CABG, coronary artery bypass grafting; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; DES, drug eluting stent; Hb, haemoglobin; HR, heart rate; NSTEMI, non-ST elevation myocardial infarction; PAD, peripheral artery disease; PCI, percutaneous coronary intervention; TIA, transient ischemic attack.
| . | Overall, n = 619 . | Clopidogrel, n = 223 . | Ticagrelor, n = 396 . | P value . |
|---|---|---|---|---|
| Age (years) | 83 ± 4 | 84 ± 3 | 82 ± 2 | |
| Final diagnosis, n (%) | ||||
| NSTEMI | 489 (79.0) | 172 (77.1) | 317 (80.1) | 0.41 |
| Unstable angina | 130 (21.0) | 51 (22.9) | 79 (19.9) | 0.42 |
| Female gender, n (%) | 183 (29.6) | 72 (32.3) | 111 (28.0) | 0.27 |
| Hypertension, n (%) | 482 (77.9) | 197 (88.3) | 285 (72.0) | 0.0001 |
| Hyperlipidaemia, n (%) | 316 (51.1) | 112 (50.2) | 204 (51.5) | 0.80 |
| Previous smoker, n (%) | 177 (28.6) | 71 (31.8) | 106 (26.8) | 0.20 |
| Diabetes mellitus, n (%) | 212 (34.2) | 98 (43.9) | 114 (28.8) | 0.0002 |
| Previous stroke/TIA, n (%) | 78 (12.6) | 56 (25.1) | 22 (5.6) | 0.0001 |
| PAD, n (%) | 133 (21.5) | 52 (23.3) | 81 (20.5) | 0.42 |
| Previous bleeding, n (%) | 47 (7.6) | 31 (13.9) | 16 (4.0) | 0.0001 |
| Renal dysfunction, n (%) | 101 (16.3) | 78 (35.0) | 23 (5.8) | 0.0001 |
| Dialysis, n (%) | 26 (4.2) | 21 (9.4) | 5 (1.3) | 0.0001 |
| Malignancy, n (%) | 19 (3.1) | 10 (4.5) | 9 (2.3) | 0.15 |
| Severe COPD, n (%) | 46 (7.4) | 32 (14.3) | 14 (3.5) | 0.0001 |
| Previous ACS, n (%) | 189 (30.5) | 79 (35.4) | 110 (27.8) | 0.06 |
| Previous PCI, n (%) | 234 (37.8) | 88 (39.5) | 146 (36.9) | 0.55 |
| Previous CABG, n (%) | 36 (5.8) | 19 (8.5) | 17 (4.3) | 0.05 |
| Killip class III–IV, n (%) | 61 (9.9) | 41 (18.4) | 20 (5.1) | 0.0001 |
| SBP, mmHg (mean ± SD) | 139 ± 21 | 140 ± 18 | 139 ± 10 | 0.37 |
| HR, bpm (mean ± SD) | 78 ± 16 | 79 ± 12 | 77 ± 23 | 0.23 |
| Ejection fraction, % (mean ± SD) | 41 ± 3 | 40 ± 5 | 41 ± 1 | 0.0001 |
| Hb, gr/dl (mean ± SD) | 12 ± 1 | 12 ± 5 | 12 ± 1 | 1.0 |
| Platelet count, x1000 (mean ± SD) | 261 ± 63 | 262 ± 59 | 261 ± 22 | 0.76 |
| Vascular access (%) | ||||
| Radial | 428 (69.1) | 158 (70.9) | 270 (68.2) | 0.53 |
| Femoral | 191 (30.9) | 65 (29.1) | 126 (31.8) | 0.53 |
| Number of stent, mean ± SD | ||||
| DES | 1.7 ± 1.0 | 1.7 ± 0.8 | 1.7 ± 1.1 | 1.0 |
| BMS | 1.4 ± 0.6 | 1.5 ± 0.8 | 1.4 ± 0.4 | 0.04 |
| Extension of CAD (%) | ||||
| Single | 254 (41.0) | 81 (36.3) | 173 (43.7) | 0.07 |
| Dual | 257 (41.5) | 78 (35.0) | 179 (45.2) | 0.01 |
| Three | 108 (17.5) | 64 (28.7) | 44 (11.1) | 0.0001 |
| Glycoprotein IIb/IIIa inhibitors | 51 (8.2) | 36 (16.1) | 15 (3.8) | 0.0001 |
| . | Overall, n = 619 . | Clopidogrel, n = 223 . | Ticagrelor, n = 396 . | P value . |
|---|---|---|---|---|
| Age (years) | 83 ± 4 | 84 ± 3 | 82 ± 2 | |
| Final diagnosis, n (%) | ||||
| NSTEMI | 489 (79.0) | 172 (77.1) | 317 (80.1) | 0.41 |
| Unstable angina | 130 (21.0) | 51 (22.9) | 79 (19.9) | 0.42 |
| Female gender, n (%) | 183 (29.6) | 72 (32.3) | 111 (28.0) | 0.27 |
| Hypertension, n (%) | 482 (77.9) | 197 (88.3) | 285 (72.0) | 0.0001 |
| Hyperlipidaemia, n (%) | 316 (51.1) | 112 (50.2) | 204 (51.5) | 0.80 |
| Previous smoker, n (%) | 177 (28.6) | 71 (31.8) | 106 (26.8) | 0.20 |
| Diabetes mellitus, n (%) | 212 (34.2) | 98 (43.9) | 114 (28.8) | 0.0002 |
| Previous stroke/TIA, n (%) | 78 (12.6) | 56 (25.1) | 22 (5.6) | 0.0001 |
| PAD, n (%) | 133 (21.5) | 52 (23.3) | 81 (20.5) | 0.42 |
| Previous bleeding, n (%) | 47 (7.6) | 31 (13.9) | 16 (4.0) | 0.0001 |
| Renal dysfunction, n (%) | 101 (16.3) | 78 (35.0) | 23 (5.8) | 0.0001 |
| Dialysis, n (%) | 26 (4.2) | 21 (9.4) | 5 (1.3) | 0.0001 |
| Malignancy, n (%) | 19 (3.1) | 10 (4.5) | 9 (2.3) | 0.15 |
| Severe COPD, n (%) | 46 (7.4) | 32 (14.3) | 14 (3.5) | 0.0001 |
| Previous ACS, n (%) | 189 (30.5) | 79 (35.4) | 110 (27.8) | 0.06 |
| Previous PCI, n (%) | 234 (37.8) | 88 (39.5) | 146 (36.9) | 0.55 |
| Previous CABG, n (%) | 36 (5.8) | 19 (8.5) | 17 (4.3) | 0.05 |
| Killip class III–IV, n (%) | 61 (9.9) | 41 (18.4) | 20 (5.1) | 0.0001 |
| SBP, mmHg (mean ± SD) | 139 ± 21 | 140 ± 18 | 139 ± 10 | 0.37 |
| HR, bpm (mean ± SD) | 78 ± 16 | 79 ± 12 | 77 ± 23 | 0.23 |
| Ejection fraction, % (mean ± SD) | 41 ± 3 | 40 ± 5 | 41 ± 1 | 0.0001 |
| Hb, gr/dl (mean ± SD) | 12 ± 1 | 12 ± 5 | 12 ± 1 | 1.0 |
| Platelet count, x1000 (mean ± SD) | 261 ± 63 | 262 ± 59 | 261 ± 22 | 0.76 |
| Vascular access (%) | ||||
| Radial | 428 (69.1) | 158 (70.9) | 270 (68.2) | 0.53 |
| Femoral | 191 (30.9) | 65 (29.1) | 126 (31.8) | 0.53 |
| Number of stent, mean ± SD | ||||
| DES | 1.7 ± 1.0 | 1.7 ± 0.8 | 1.7 ± 1.1 | 1.0 |
| BMS | 1.4 ± 0.6 | 1.5 ± 0.8 | 1.4 ± 0.4 | 0.04 |
| Extension of CAD (%) | ||||
| Single | 254 (41.0) | 81 (36.3) | 173 (43.7) | 0.07 |
| Dual | 257 (41.5) | 78 (35.0) | 179 (45.2) | 0.01 |
| Three | 108 (17.5) | 64 (28.7) | 44 (11.1) | 0.0001 |
| Glycoprotein IIb/IIIa inhibitors | 51 (8.2) | 36 (16.1) | 15 (3.8) | 0.0001 |
ACS, acute coronary syndrome; BMS, bare metal stent, CABG, coronary artery bypass grafting; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; DES, drug eluting stent; Hb, haemoglobin; HR, heart rate; NSTEMI, non-ST elevation myocardial infarction; PAD, peripheral artery disease; PCI, percutaneous coronary intervention; TIA, transient ischemic attack.
This is the first ‘real-world’ study assessing the safety of ticagrelor in octogenarian patients with NSTE-ACS undergoing successful PCI, suggesting that this novel oral P2Y12 inhibitor is associated with similar rates of ischaemic events but significantly higher clinically relevant bleeding complications, compared with clopidogrel. Recent registries suggest that novel P2Y12 inhibitors are usually employed in younger ACS patients compared with clopidogrel.5–9 Indeed, several studies have suggested that in ACS patients, the risk of recurrent ischaemic events increased with age, but the relative excess of ischaemic over major bleeding events diminished over time.1,2 Moreover, in older patients, major bleeding events are often disabling or fatal, therefore antithrombotic therapies should be used with caution.1,2 Accordingly, in the TRITON-TIMI 38 (Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel-Thrombolysis in MI) trial, patients aged ≥75 years who were treated with prasugrel had an increased risk of developing major and fatal bleeding vs. clopidogrel, resulting into a neutral net clinical benefit.10 In the PLATO trial, the clinical benefit of ticagrelor over clopidogrel was not significantly different between patients aged ≥75 and <75 years of age with respect to the composite of cardiovascular death, MI or stroke. In addition, no increase in PLATO-defined overall major bleeding with ticagrelor vs. clopidogrel was observed in elderly compared with younger patients (hazard ratio, 1.02; 95% confidence interval, 0.82–1.27), even if the rate of non–CABG-related bleeding was slightly lower with clopidogrel compared with ticagrelor treatment throughout the evaluated age range.3 Notably, the population evaluated in this pre-specified analysis of the PLATO trial accounted for all the spectrum of ACS (<80% presented a final diagnosis of NSTE-ACS and about 70% received a PCI during the index admission), and their risk profile, as in the majority of randomized clinical trials, was more favourable compared with our real-world scenario. These findings, together with a different classification of bleeding events, might explain the different safety outcome observed in our series. Further studies specifically assessing the safety of ticagrelor in elderly patients with ACS are warranted, especially considering the recently proposed long-term use of this drug in the post-MI setting.11
Conflict of interest: all authors received speaker fees from AstraZeneca.



