-
PDF
- Split View
-
Views
-
Cite
Cite
Cian P. McCarthy, Kieran V. Mullins, David M. Kerins, The role of trimetazidine in cardiovascular disease: beyond an anti-anginal agent, European Heart Journal - Cardiovascular Pharmacotherapy, Volume 2, Issue 4, October 2016, Pages 266–272, https://doi.org/10.1093/ehjcvp/pvv051
Close - Share Icon Share
Abstract
With evidence for efficacy in such diverse clinical settings such as stable coronary artery disease, reperfusion injury, and contrast-induced nephropathy, trimetazidine (TMZ) is novel among cardiovascular agents. In spite of this and almost half a century of clinical experience with the drug, it remains licensed only as an adjunct in the management of angina pectoris in patients who are inadequately controlled by or intolerant to first-line therapies. Although no single pharmacological mechanism has been hitherto universally accepted, TMZ is known to target deranged cellular energetics particularly in ischaemic myocardial tissue. Mechanistically, this separates the drug from conventional anti-anginal therapies, namely beta-adrenergic antagonists, calcium channel blockers, and nitrates. Moreover, a haemodynamically neutral side-effect profile should make TMZ a much more attractive therapeutic agent in this setting. Such ostensibly beneficial pharmacodynamics notwithstanding, the drug has a limited role in angina pectoris treatment algorithms. Concerns regarding a potential for new onset movement disorder further complicate its use and have led to a licensing revocation in some jurisdictions for the treatment of vestibular disorders. In this review article, we examine the pertinent literature and assess the evidence base for TMZ as a viable treatment option in a number of clinical settings.
Introduction
Despite being licensed as an anti-anginal medication in Europe for over 40 years,1 trimetazidine (TMZ) has played only a limited role in cardiovascular medicine and its therapeutic utility remains a source of significant debate among the cardiology community. Currently, the European Cardiology Society advocates this medication as a second-line therapy in patients with stable angina.2 However, the drug has not gained such acceptance in the USA and without FDA approval it remains outside the armamentarium of cardiovascular drugs available to clinicians there.
Recent evidence suggests that TMZ may play an important role in cardiovascular disease beyond the treatment of angina, in such diverse clinical situations such as in the management of heart failure, peripheral arterial disease, contrast-induced nephropathy, and reperfusion injury. However, concerns over an association with parkinsonism may limit its utility. In the current article, we will review the relevant pharmacology of TMZ, the evidence for its use in cardiovascular disease, and examine the evidence with regard to a concerning association with a movement disorder.
Mechanistic considerations
Inhibition of free fatty acid β-oxidation
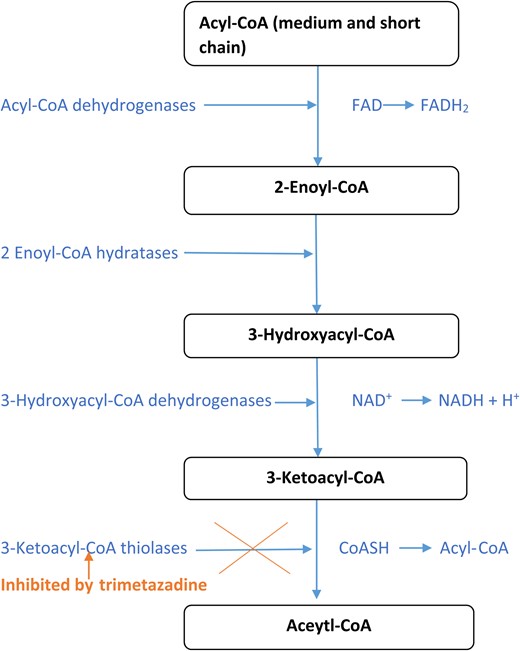
Free fatty acid oxidation is the primary energy source of cardiomyocytes, accounting for 60–90%, the remainder derived from glucose metabolism.3 Unfortunately, despite producing the same quantity of adenosine triphosphate, free fatty acid oxidation requires an additional 10–15% of oxygen, which represents a significant disadvantage under ischaemic conditions.4 Trimetazidine, widely considered to be a cytoprotective agent, acts as a metabolic regulator, promoting more efficient glucose oxidation in cardiomyocytes through inhibition of partial fatty acid oxidation (Table 1).5 It inhibits the enzymatic activity of long-chain 3-ketoacyl CoA thiolase, which is responsible for the last stage of fatty acyl beta-oxidation (Figure 1).4 In doing so, it causes a shift in cardiomyocyte energy derivation towards more efficient glycolytic biochemical pathways thereby maintaining energy production while conserving oxygen.4
| Mechanism . | References . |
|---|---|
| Inhibition of β-oxidation of free fatty acid | 3 |
| Increased pyruvate dehydrogenase activity | 3,5,6 |
| Increasing sarcolemma mechanical resistance | 8,9 |
| Inhibiting apoptosis by increased miR-21 expression | 10 |
| Reduced cardiac fibrosis by decreasing connective tissue growth factor (CTGF) expression in cardiac fibroblasts | 11 |
| Mechanism . | References . |
|---|---|
| Inhibition of β-oxidation of free fatty acid | 3 |
| Increased pyruvate dehydrogenase activity | 3,5,6 |
| Increasing sarcolemma mechanical resistance | 8,9 |
| Inhibiting apoptosis by increased miR-21 expression | 10 |
| Reduced cardiac fibrosis by decreasing connective tissue growth factor (CTGF) expression in cardiac fibroblasts | 11 |
| Mechanism . | References . |
|---|---|
| Inhibition of β-oxidation of free fatty acid | 3 |
| Increased pyruvate dehydrogenase activity | 3,5,6 |
| Increasing sarcolemma mechanical resistance | 8,9 |
| Inhibiting apoptosis by increased miR-21 expression | 10 |
| Reduced cardiac fibrosis by decreasing connective tissue growth factor (CTGF) expression in cardiac fibroblasts | 11 |
| Mechanism . | References . |
|---|---|
| Inhibition of β-oxidation of free fatty acid | 3 |
| Increased pyruvate dehydrogenase activity | 3,5,6 |
| Increasing sarcolemma mechanical resistance | 8,9 |
| Inhibiting apoptosis by increased miR-21 expression | 10 |
| Reduced cardiac fibrosis by decreasing connective tissue growth factor (CTGF) expression in cardiac fibroblasts | 11 |
Enhancement of pyruvate dehydrogenase activity
Trimetazidine is known to increase the activity of pyruvate dehydrogenase, a component of the pyruvate dehydrogenase complex.4,6,7 Through this mechanism, it facilitates the transformation of pyruvate to acetyl Co-A, thereby linking glycolysis with the citric acid cycle with the subsequent release of energy via nicotinamide adenine dinucleotide hydride. Other researchers posit an effect on cardiac mitochondria promoting adenosine triphospate synthesis via alterations in mitochondrial calcium metabolism to account for at least some of TMZ pharmacological effect.8
Inhibition of reperfusion injury
Recently, TMZ has been shown to increase microRNA-21.9 Increased microRNA-21 expression up-regulates the activity of Akt signalling resulting in a decrease in the ratio of bax/bcl-2 and also the expression of caspase-3, the ultimate effect being a reduction in hypoxia/reperfusion-induced apoptosis.9 Additionally, TMZ may protect cardiomyocytes from reperfusion injury following acute myocardial infarction (MI) via enhancement of the mechanical resistance of the sarcolemma.10,11 Following restoration of blood flow in acute MI, the sarcolemma is exposed to significant mechanical stress secondary to soft tissue oedema. By increasing sarcolemmal resistance, TMZ protects cells from potential apoptosis and the resultant ventricular dysfunction.10,11
Inhibition of cardiac fibrosis
Trimetazidine is thought to directly inhibit cardiac fibrosis. The drug reduces collagen accumulation, connective tissue growth factor expression in cardiac fibroblasts, nicotinamide adenine dinucleotide phosphate-oxidase levels, and the production of reactive oxygen species.12 Such mechanisms are thought to explain the beneficial effects of this agent in the setting of congestive cardiac failure.
Trimetazidine as an anti-anginal agent
The most well recognized and established role of TMZ in cardiovascular medicine is as an anti-anginal agent. Conventional anti-ischaemic agents such as beta-blockers, calcium channel blockers, and nitrates play an important role in clinical practice in their relief of symptoms in patients with stable coronary artery disease. Unfortunately, these agents are not without side effects, impacting heart rate and blood pressure to varying degrees. Owing to its unique mechanism of action, TMZ has very limited haemodynamic effects13 and thus may be the ideal agent for patients susceptible to changes in haemodynamic variables. A plethora of clinical trials have assessed the efficacy of TMZ both a mono-therapeutic agent and as part of a combination regimen in the treatment of angina.
Trimetazidine monotherapy
In a double-blind, placebo-controlled study undertaken by Passeron14 involving 109 patients, TMZ caused a statistically significant reduction in angina attacks from 8.1 ± 0.3 to 2.9 ± 0.5 a week. Furthermore, nitroglycerin consumption among participants over a week decreased by over 50% from 9.1 ± 0.6 to 3.1 ± 0.5 tablets.14
In comparative trials with conventional anti-anginal agents, TMZ has shown equivalent results. In a multicentre double-blind comparative study conducted by Koylan et al.,15 the effects of TMZ were assessed in comparison with diltiazem on exercise performance in 116 patients with stable angina. Patients were randomized to receive TMZ (20 mg three times daily) or diltiazem (60 mg three times daily) for a 4-week treatment period after a placebo washout period of 2 weeks. Both medications produced similar and statistically significant decreases in anginal attacks and nitrate consumption per week, while also improving the recovery from anginal pain and maximal ST-segment depression on exercise treadmill testing.15 However, while diltiazem caused a slight prolongation of PR and QRS durations (P = 0.039) on ambulatory ECG, this effect was not seen in the TMZ subgroup.15
In another multicentre study (double-blind parallel group), involving 149 patients with stable angina, TMZ (20 mg three times daily), compared with propranolol (40 mg three times daily), produced similar anti-anginal efficacy in terms of anginal attack rate per week, exercise duration, and time to 1 mm ST-segment depression.16
Trimetazidine combination therapy
The use of TMZ as an adjunctive therapeutic agent has proved an attractive option in patients whose symptom control is suboptimal when managed with first-line agents or in those with significant haemodynamic disturbances. The efficacy of TMZ as add-on therapy has been investigated with both beta-blockers and calcium channel blockers.
Trimetazidine combined with beta-blockers
The Trimetazidine in Angina Combination Therapy (TACT) study was a randomized, multicentre, placebo-controlled study involving 177 patients with stable angina to assess the efficacy and acceptability of TMZ in combination with haemodynamic agents (beta-blockers or long-acting nitrates).17 Patients were randomly treated with TMZ (20 mg three times daily) or placebo orally for 12 weeks. After 12 weeks of therapy, exercise test duration increased by 89 s in the TMZ group vs. 23.6 s in the placebo group (P < 0.05). Time to 1 mm ST-segment depression increased by 90 s in the TMZ group vs. 16.7 s in the placebo group (P < 0.05). Time to onset of anginal pain increased by 100 s in the TMZ group vs. 21.3 s in the placebo group (P < 0.005). The mean number of anginal attacks per week decreased from 5.6 ± 0.6 to 2.7 ± 0.5 in the TMZ group vs. 6.8 ± 0.7 to 5.1 ± 0.7 in the placebo group (P < 0.05), and mean consumption short-acting nitrates per week decreased from 5.2 ± 0.9 to 2.8 ± 0.8 in the TMZ group vs. 5.5 ± 0.8 to 4.1 ± 0.9 in the placebo group.17
The TRIMetazidine in POLand (TRIMPOL) II study was a double-blind, placebo-controlled study, involving 426 patients with stable angina.18 It found that the addition of TMZ (60 mg/day) to metoprolol (50 mg/day), over a 12-week period, significantly improved total exercise time duration (+20.1 s, P = 0.023), time to 1 mm ST-segment depression (+33.4 s, P = 0.003), time to onset of angina (+33.9 s, P < 0.001) , and significantly decreased the mean number of angina attacks and consumption of short-acting nitrates per week with only minor side effects.18
The largest trial, the VASCO-angina study, was a 12-week randomized double-blind, placebo-controlled trial aimed at assessing the anti-anginal efficacy and safety of two doses of TMZ MR (TMZ 70 mg/day, TMZ 140 mg/day) in 645 symptomatic patients with chronic stable angina receiving background atenolol treatment.19 Compared with baseline, in the overall cohort of patients with chronic stable angina, TMZ significantly improved total exercise duration (TMZ: 6 ± 23%; placebo: 0.7 ± 5%; t-value: −2.689; P-value: 0.0074) with similar improvements in total exercise duration seen in both TMZ treatment doses (TMZ 70 mg/day: 5.3 ± 20%, P = 0.0338; TMZ 140 mg/day: 6.8 ± 20%, P = 0.0044).19 While a greater total exercise duration improvement was observed in TMZ 140 mg/day than in TMZ 70 mg/day, this was not significant.19
Trimetazidine in combination with calcium channel blockers
In a double-blind, randomized, placebo-controlled trial of 4 weeks duration, conducted by Manchanda et al.20 involving 64 patients with stable angina uncontrolled with diltiazem, compared with placebo, there was net improvement with TMZ in mean anginal attacks of 4.8/week (P < 0.002); in mean exercise times to 1 mm ST-segment depression by 94.2 s (P < 0.05), in time to onset of angina by 113.1 s (P < 0.02); and in mean maximum work at peak exercise of 1.4 metabolic equivalents (P < 0.05). In a subsequent study by the same investigators, using a lower dose of diltiazem (90 mg/day), similar benefits were demonstrated.21 Compared with placebo, there was an improvement with TMZ in mean exercise time to 1 mm ST-segment depression of 128 s (P < 0.01); in the mean duke treadmill score of 57.4% (P < 0.02); and in mean anginal attacks of 5.1 per week (P < 0.01).21
Meta-analyses
The results from monotherapy and combination therapy trials is supported by meta-analyses. In a meta-analysis of 23 trials with 1378 patients with stable angina conducted by Ciapponi et al.,22 TMZ reduced the number of weekly angina attacks (P < 0.0001) and weekly nitroglycerin tablet consumption (P < 0.0001) and improved exercise time to 1 mm ST-segment depression on the exercise test (P = 0.0002), compared with placebo. In the largest meta-analysis, conducted by Danchin et al.23 evaluating 218 trials with a total 19 028 patients, TMZ significantly improved exercise tolerance, weekly angina episodes, and use of short-acting nitrates when compared with placebo.
Trimetazidine and reperfusion injury
Over the last 30 years, thrombolytic therapy and percutaneous coronary intervention have revolutionized the management of acute MI, with resultant reduction in infarct sizes.24 Although overwhelmingly beneficial, the abrupt restoration of blood flow unfortunately has been found to cause additional and accelerated myocardial injury beyond the region of ischaemia alone, in a process known as ‘reperfusion injury’.25,26 With evidence to suggest that reperfusion injury accounts for 50% of the final infarct size,26 several drugs aimed at tackling this phenomenon have been proposed. Following consistent evidence from elegantly performed animal studies demonstrating the ability of TMZ to limit ischaemia–reperfusion injury,27–31 clinical trials on human subjects were undertaken. In one such randomized prospective trial by Bonello et al.32 on 582 patients with stable angina undergoing percutaneous intervention, patients were randomized to receive an acute loading dose of 60 mg of TMZ or no treatment prior to intervention. The group found that post-procedural cardiac troponin I levels were significantly reduced in the TMZ group, whereas the total amount of cardiac troponin I released after percutaneous intervention, as assessed by the area under the curve of serial measurements, was also significantly reduced (P = 0.05).32
The benefit of TMZ during percutaneous intervention has also been investigated in the setting of acute MI. In a multicentre, randomized, double-blind study by Steg et al., the investigators detected an earlier and more complete return of the ST-segment toward baseline in patients who were pretreated with TMZ for 48 h.33 This result was supported by another randomized trial, this time by Labrou et al.,34 which found that TMZ minimized myocardial reperfusion injury during percutaneous intervention and improved global and regional wall motion at 1 and 3 months after percutaneous intervention. However, both trials were limited by small patient numbers.
Trimetazidine may also be useful in preventing reperfusion following coronary artery bypass grafting. In a recent meta-analysis of six randomized controlled trials investigating the efficacy of preoperative TMZ therapy on myocardial preservation in coronary artery bypass grafting patients, Zhang et al. found significantly lower postoperative levels of creatine kinase, creatine kinase-MB, Troponin T, and Troponin I in the TMZ-treated coronary artery bypass grafting patients relative to control coronary artery bypass grafting patients. These results were consistent in both the ≤12 and >12 h post-coronary artery bypass grafting subgroup analyses.35
Trimetazidine and contrast-induced nephropathy
The incidence of contrast-induced nephropathy in patients undergoing angiography is <2% of the general population. However, this figure can increase to up to 50% in patients with advanced kidney disease.36 Identification of renoprotective agents that can prevent contrast-induced nephropathy has proved a challenging area of research. Trimetazidine has shown some promise in tackling this issue in several clinical trials.
In one such trial undertaken by Onbasili et al.37 on 82 patients undergoing coronary angiography, the investigators assessed whether pretreatment of patients with TMZ (20 mg three times daily) in addition to isotonic saline would reduce the incidence of contrast-induced nephropathy. Their results found contrast-induced nephropathy developed in 2.5% (1/40) of patients in the TMZ group and in 16.6% (7/42) of patients in the control group (P < 0.05), suggesting a clear benefit. These encouraging results were supported by a prospective, randomized, controlled trial undertaken by Rahman et al.38 involving 400 patients, evaluating the efficacy of TMZ in the prevention of contrast-induced nephropathy in patients with raised serum creatinine levels undergoing coronary angiogram. The investigators found that the incidence of contrast-induced nephropathy was significantly (P < 0.05) reduced by TMZ administration with saline in comparison with saline alone in patients undergoing coronary angiography (4 vs. 14%).38
Another study by Shehata et al.39 evaluated the effect of peri-procedural administration of TMZ on the incidence of percutaneous intervention-induced myocardial injury and contrast-induced nephropathy in the high-risk population of diabetic patients with mild–moderate renal dysfunction. The trial found that peri-procedural treatment with TMZ (70 mg/day) for 72 h resulted in an incidence of contrast-induced nephropathy of 12% in comparison with 28% in the control group (P < 0.05).39 Cardiac troponin I levels were significantly reduced in the TMZ group at 6 h post-percutaneous intervention (8 ± 0.3 vs. 16 ± 0.2 pg/mL) 12 h post-percutaneous intervention (13 ± 0.9 vs. 24 ± 0.8 pg/mL) and 24 h post-percutaneous intervention (7 ± 0.7 vs. 14 ± 0.3 pg/mL P < 0.001).39 The evidence from this trial further endorses results from previous trials highlighting the benefit of TMZ in preventing both myocardial injury and contrast-induced nephropathy in patients undergoing percutaneous intervention.
In a meta-analysis of randomized controlled trials, Nadkarni et al.,40 investigated whether the addition of TMZ to normal saline plus/minus N-acetylcysteine could reduce the incidence of contrast-induced nephropathy. The group found that TMZ significantly reduced the incidence of contrast-induced nephropathy by 11% (P < 0.01).40
Trimetazidine and heart failure
Over the past 30 years, much research interest has centred on an association between heart failure and derangements in cellular energy derivation.41 Trimetazidine via metabolic modulation4 and through inhibition of cardiac fibrosis12 has emerged as an attractive agent. There is now a substantial evidence base to suggest that TMZ can not only improve symptoms of chronic heart failure but also effect an improvement in cardiac ejection fraction (EF) in this patient cohort. As far back as 1990, a small double-blind, placebo-controlled study carried out on 20 patients, comparing the addition of TMZ vs. placebo to standard therapy for patients with ischaemic cardiomyopathy and NYHA class III/IV found that at 6 months, all TMZ-treated patients were free from angina.42 Moreover, dyspnoea was improved in all those in the TMZ-treated group in comparison with only one placebo-treated patient, while EF increased by 9.3% in the TMZ group and decreased by 15.6% in the placebo group (P < 0.018).42 More recently, a trial by Fragasso et al., though again with small study numbers, demonstrated that the addition of TMZ to conventional therapy in heart failure patients led not only to improvement in the NYHA HF class (P < 0.0001) and left ventricular end-diastolic volume (from 98 ± 36 to 81 ± 27 mL; P = 0.04) and but also to a significantly improved left ventricular EF (from 36 ± 7 to 43 ± 10%; P = 0.002) while the same parameters deteriorated among the placebo-treated group.43
The clinical utility of any drug is ultimately judged on its overall impact on disease mortality. Crucially, we now have data demonstrating a mortality benefit among patients with chronic heart failure treated with TMZ. In a meta-analysis by Gao et al.,44 involving 17 randomized studies from the period between 1966 and May 2010 and including a total of 955 patients with chronic heart failure, in comparison with placebo, TMZ treatment was associated with increased exercise tolerance (weighted mean difference 30.26 s; P < 0.01), NYHA class reduction (weighted mean difference 0.41; P < 0.01), improved left ventricular EF in ischaemic heart failure (weighted mean difference 7.37%; P < 0.01), and non-ischaemic heart failure patients (weighted mean difference 8.72%; P < 0.01). Moreover, their analysis showed that TMZ reduced hospitalizations (RR 0.42, P < 0.00001) and overall mortality (RR 0.29, P < 0.00001).44 These findings were supported by a multicentre retrospective trial by Fragasso et al.45 in 2013 involving 669 chronic heart failure patients. This trial showed that the addition of TMZ to conventional therapy vs. conventional therapy alone was associated with reduced cardiovascular hospitalization rate (adjusted hazard ratio [HR] 0.524, 95% CI 0.352–0.781, P = 0.001), cardiovascular mortality (HR 0.072, 95% CI 0.019–0.268, P = 0.0001), as well as overall mortality (HR 0.102, 95% CI 0.046–0.227, P = 0.0001).
Trimetazidine and peripheral arterial disease
A few studies have assessed the value of TMZ in the treatment of peripheral arterial disease. In a study by Syrkin et al.,46 the authors demonstrated the ability of TMZ to extend the intermittent claudication distance. More recently, in 2011, a double-blind study by Vitale et al. randomized 100 patients with symptomatic peripheral arterial disease to TMZ or placebo. While the ankle brachial index values did not differ at a 3-month follow-up, the maximal walking distance increased by 23% in the TMZ vs. 14% in the placebo.47 It is important to note that these were small and underpowered studies. Thus, larger trials are necessary before any judgements concerning the true efficacy of this agent in peripheral arterial disease can be made.
Trimetazidine adverse effects
Unlike other conventional anti-anginal agents, TMZ has a contrasting side-effect profile. Generally, treatment-related side effects are mild and well tolerated mostly comprising gastrointestinal disturbances such as nausea and vomiting and minor headaches.48 However, much more concerning is an association with a parkinsonism, which led to the de-licensing of this agent as a treatment option in the management of vertigo and tinnitus by the European Medicines Agency.49 In a study by Marti Masso et al.,48 56 of the 130 patients who were treated with TMZ (43%) reported adverse motor function. Drug-induced parkinsonism was detected in 20 patients, gait disturbances in 15 subjects, and 9 experienced tremor, while 12 of the patients who had a pre-existing diagnosis of Parkinson's disease exhibited a worsening of symptoms.48 However, and perhaps more telling, examination of post-marketing analysis reveals the incidence of this side effect to be very low, with an incidence of 0.36/100 000 person-years. In the majority of patients, symptoms resolve within 4 months of discontinuation of the medication. The European Medicines Agency recommends referral to a neurologist only if the symptoms persist beyond 4 months.49 Nonetheless, patients must be advised of the potential risk and of the early signs of the condition while in those with pre-existing Parkinson's disease, the drug should be considered contraindicated.
Conclusion
Trimetazidine, a unique and undervalued anti-anginal medication, is an attractive and potentially advantageous alternative to conventional agents, especially among those patients with disturbed haemodynamic profiles. The drug has shown additional promise in the treatment of reperfusion injury, contrast-induced nephropathy, and peripheral arterial disease, but further investigation is warranted. With meta-analysis of retrospective trials suggesting a mortality benefit with this agent in the setting of chronic heart failure, further large prospective randomized controlled trials are indicated to assess the true utility of TMZ in this cohort and should be seen as a necessity by the cardiovascular research community. Although rare, physicians must be aware of the potential risk of movement disorders with this medication and refer their patients to a neurologist if symptoms persist beyond 4 months.
Conflict of interest: none declared.



