-
PDF
- Split View
-
Views
-
Cite
Cite
Uwe Zeymer, Petr Widimsky, Nicolas Danchin, Maddalena Lettino, Alfredo Bardaji, Jose A. Barrabes, Angel Cequier, Marc J. Claeys, Leonardo De Luca, Jakob Dörler, David Erlinge, Paul Erne, Patrick Goldstein, Sasha M. Koul, Gilles Lemesle, Thomas F. Lüscher, Christian M. Matter, Gilles Montalescot, Dragana Radovanovic, Jose Lopez Sendón, Petr Tousek, Franz Weidinger, Clive F. M. Weston, Azfar Zaman, Pontus Andell, Jin Li, J. Wouter Jukema, on behalf of the PIRAEUS group, P2Y12 receptor inhibitors in patients with non-ST-elevation acute coronary syndrome in the real world: use, patient selection, and outcomes from contemporary European registries, European Heart Journal - Cardiovascular Pharmacotherapy, Volume 2, Issue 4, October 2016, Pages 229–243, https://doi.org/10.1093/ehjcvp/pvw005
Close - Share Icon Share
Abstract
Non-ST-elevation acute coronary syndrome (NSTE-ACS) is present in about 60–70% of patients admitted with acute coronary syndromes in clinical practice. This study provides a ‘real-life’ overview of NSTE-ACS patient characteristics, dual antiplatelet therapy clinical practice, and outcomes at both the time of discharge from hospital and up to 1-year post-discharge.
A total of 10 registries (documenting 84 054 NSTE-ACS patients) provided data in a systematic manner on patient characteristics and outcomes for NSTE-ACS in general, and 6 of these (with 52 173 NSTE-ACS patients) also provided more specific data according to P2Y12 receptor inhibitor used. Unadjusted analyses were performed at the study level, and no formal meta-analysis was performed due to large heterogeneity between studies in the settings, patient characteristics, and outcome definitions. All-cause death rates across registries ranged from 0.76 to 4.79% in-hospital, from 1.61 to 6.65% at 30 days, from 3.66 to 7.16% at 180 days, and from 3.14 to 9.73% at 1 year. Major bleeding events were reported in up to 2.77% of patients while in hospital (in seven registries), up to 1.08% at 30 days (data from one registry only), and 2.06% at 1 year (one registry).
There were substantial differences in the use of and patient selection for clopidogrel, prasugrel, and ticagrelor, which were associated with differences in short- and long-term ischaemic and bleeding events. In future registries, data collection should be performed in a more standardized way with respect to endpoints, definitions, and time points.
Background
Antiplatelet therapy is recommended in all patients with acute coronary syndrome (ACS) regardless of their revascularization strategy. The current guidelines of the European Society of Cardiology (ESC) on the management of patients with non-ST-segment elevation acute coronary syndromes (NSTE-ACS) recommend aspirin for all patients without contraindications at an initial oral loading dose of 150–300 mg, and at a daily oral maintenance dose of 75–100 mg, long-term, regardless of treatment strategy.1 Further, as part of dual antiplatelet therapy (DAPT), a P2Y12 receptor inhibitor should be added to aspirin and maintained over 12 months, unless there are contraindications such as excessive risk of bleeding (Class I level A recommendation). A 60 mg loading dose of prasugrel, followed by 10 mg/day maintenance doses, is recommended in patients who are proceeding to percutaneous coronary intervention (PCI) if there is no contraindication (Class I level B), but not in patients in whom coronary artery anatomy is not known (Class III level B). A 180 mg loading dose of ticagrelor, followed by 90 mg twice daily, is recommended in the absence of contraindications for all patients at moderate-to-high risk of ischaemic events, regardless of initial treatment strategy and including those pre-treated with clopidogrel (Class I level B). Finally, a 300–600 mg loading dose of clopidogrel, followed by 75 mg daily maintenance doses, is recommended for patients who cannot receive ticagrelor or prasugrel or who require oral anticoagulation (Class I level B).1 Because prasugrel and ticagrelor have higher antithrombotic potency and proven superiority in outcome trials [(as in ST-segment elevation myocardial infarction (STEMI)], these drugs are preferred over clopidogrel for patients presenting with NSTE-ACS.2
Registries and other observational studies are an important source of information on the efficacy and safety of medication under clinical practice conditions. The ‘Platelet Inhibition Registry in ACS EvalUation Study’ (PIRAEUS) group is composed of owners or principal investigators of national or multinational European ACS registries.3 The initiative aims to integrate the wide array of data generated by individual European ACS registries to gain a comprehensive overview on the efficacy and safety of the P2Y12 receptor inhibitors used for the treatment of this condition. We have described the participating registries in detail, in narrative and tabular form, in an earlier review,3 and also provided an overview on the outcomes of STEMI patients.4
This report presents data regarding NSTE-ACS patients in various European registries, with focus on the use of P2Y12 receptor inhibitor-based DAPT, patient selection, and outcomes.
Methods
In order to obtain a comprehensive contemporary overview of appropriate registries, the following selection criteria were applied: European multicentre or single-centre observational studies on real-life experience in the management of ACS within the last 5 years; large unselected patient cohorts; availability of data on PCI; availability of data on management during initial hospitalization for ACS; availability of follow-up data on outcomes (death, cardiac events, bleedings); previous publication of data in peer-reviewed journals and/or reporting of unpublished data, with information on outcomes of drug treatment of patients with P2Y12 receptor inhibitors at least until discharge from the hospital; and willingness of registry owners to take part in PIRAEUS and share data. Indeed, some of the registries started earlier than 5 years ago, but still provided data on clopidogrel-based DAPT therapy.
Of the registries fulfilling the above criteria and whose owners were contacted, a total of 17 registries were analysed. They are described in detail in a recent review paper including setting, aims and scope, and selected baseline characteristics of the included patients.3 Data on the completeness of values (i.e. considering rates of missing data) could not be obtained from the various registries.
Registry owners were asked to provide detailed current data on (i) the full ACS cohort, as well as for the STEMI and NSTE-ACS groups separately (irrespective of treatment) and (ii) subgroups of patients treated with the P2Y12 receptor inhibitors prasugrel, ticagrelor, or clopidogrel. Only aggregate data in tabular format were received, as the pooling of individual patient data was not covered by patients' informed consent and/or was not possible due to ownership of data issues. The data collection sheet specified time points at discharge from hospital, at 30 days, at 180 days, and at 1 year. Endpoints of interest were all-cause death, cardiovascular death, stroke, recurrent myocardial infarction (MI), and repeat PCI (for efficacy), as well as life-threatening/major and minor bleeding events (for safety). For bleeding events, the definition used by each registry was requested from the registry owners, but was not always available or sometimes had changed during the time of the registry data collection.
Registry owners were asked to provide percentages for the various events together with event number and patient number at the various time points. Data were not adjusted or weighted.
Statistical analysis
For the current paper, patients with NSTE-ACS diagnosis at admission were selected for analysis. Patients from 10 registries were included for statistical analysis. The data at the study level (not individual patient level) were used by a statistician to calculate event rates for the total cohort and by DAPT regimen specifically for each registry separately, with two-sided 95% confidence intervals (CI) using the Clopper–Pearson interval. Cohorts comprising fewer than 100 patients were excluded from analyses because of the small number of events. Event rates were defined as cumulative incidence rates. Event rates and 95% CI for each cohort were shown using forest plots. Bubble plots were used to confirm the relationships between age and event rates whereby the size of the bubble depended on the number of patients in the respective subgroup. These analyses were sent to the individual registry holders for them to confirm the data, enter corrections, and, if indicated, provide additional data.
A description of the registries that provided NSTE-ACS data is given in the Supplementary material online.
Results
In total, 10 registries provided specific information about NSTE-ACS patients (Table 1). Of these, six provided specific data on P2Y12 DAPT (AAPCI/ADAPT, AMIS Plus, ATACS, DIOCLES, SCAAR, and SPUM). All reported data on clopidogrel, five reported data on prasugrel (all with the exception of DIOCLES), and three reported data on ticagrelor (AAPCI/ADAPT, AMIS Plus, and SCAAR). The remaining four registries (BLITZ-4, CZECH-2, FAST-MI, and Newcastle) had only data on NSTE-ACS patients overall, but no data on P2Y12 treatment groups.
Baseline characteristics in the non-ST-segment elevation acute coronary syndrome cohorts of the various registries
| Registry acronym . | AAPCI/ADAPT . | AMIS plus . | ATACS . | BLITZ-4 . | CZECH-2 . | DIOCLES . | FAST-MI 2010 . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient number (n) | 2181 | 5880 | 6777 | 5852 | 586 | 1769 | 1805 | |||||||
| Definition of (major) bleeding | TIMI | BARC (since 2012) | GUSTO | Fatal, intracranial or requiring surgery or blood transfusion | ||||||||||
| Characteristics of patients | ||||||||||||||
| Age, mean (SD) | 65 (13) | 66.6 (12.5) | 68.8 (12.0) | F 74 (11), M 68 (12) | 70 (11) | 69 (12) | 68 (13.6) | |||||||
| >75 years, % | 23 | 29.1 | 34.7 | 33.2 | 37.5 | |||||||||
| Gender, males/females, % | 68/32 | 75/25 | 70/30 | 66.6/33.4 | 65/35 | 73/27 | 70/30 | |||||||
| Diabetes mellitus, % | 22 | 22.4 | 33.7 | 30.6 | 40.5 | 34.8 | 26 | |||||||
| Chronic (congestive) heart failure, % | 2.6 | 0 | 7.9 | 7 | ||||||||||
| Atrial fibrillation, % | 8 | 4.4 | 19.6 | 14.5 | 8 | |||||||||
| Coronary artery disease (CAD, CHD), % | 38.8 | 100 | 35.1 | 36 | ||||||||||
| Previous stroke, % | 7 | 6.4 | 7.8 | 9.2 | 7.9 (stroke, TIA) | 4 | ||||||||
| Previous myocardial infarction (STEMI/NSTE-ACS), % | 17 | 20.7 | 28.2 | 17.3 | 29.1 | 27.3 | 22 | |||||||
| Previous PCI, % | 22 | 22.8 | 35.6 | 18.7 | 24.4 | 22.9 | 22 | |||||||
| Previous CABG, % | 8.5 | 14.2 | 9.1 | 12.1 | 6.5 | 7 | ||||||||
| Arterial hypertension, % | 67.6 | 85.9 | 67.2 | 76.5 | 71.2 | 62 | ||||||||
| Peripheral arterial disease (PAD), % | 6.2 | 11.5 | 10.6 | 12 | ||||||||||
| Current smoking, % | 33 | 35.3 | 28.9 | 24.7 | 25.9 | 22.8 | 26 | |||||||
| Chronic kidney disease/renal impairment, % | 7.2 | 23.3 | 11.9 | 6.4 (severe) | 6.5 | |||||||||
| Antithrombotic pre-treatment: | ||||||||||||||
| Patients on chronic aspirin (ASA), % | 47.1 | 52.8 | 46.5 | 48.9 | 30 | |||||||||
| Patients on chronic clopidogrel/prasugrel/ticagrelor, % | 13/0.9/1.2 | 21.5/3.4/ 0 | 9.6/0.25/0 | 18.3/1/0 | 20/0.5/0 | |||||||||
| Patients on oral anticoagulation (VKA or NOAC), % | 5 | 8.1 | 12.3 (any AC), 8.5 (VKA) | 6 | ||||||||||
| ACS characteristics—Killip classes: I/II/III/IV, % | 70/19/5/7 | 87.1/8.7/2.2/1.9 | 90.3/8.7 (II/III)/1.0 | 15.1(II) 7.5 (III-IV) | 70.9/16.3/9.1/3.6 | 86.5/7.7/5.1/0.7 | 80.5/11/7/1 | |||||||
| Time from first medical contact to PCI, mean±SQ or median (IQR) hours | 4.6 (2.2 to 11.5) | 6.8 (3.0 to 19.0) | 27.4 (14.7 to 55) | |||||||||||
| Intervention during initial hospitalization | ||||||||||||||
| Coronary angiography, % | 100 | 86 | 100 | 85.1 | 66 | 79.6 | 91.5 | |||||||
| PCI, % | 75 | 81.8 | 79.3 | 66.8 (of pts with angio) | 47 | 50.6 | 66 | |||||||
| CABG, % | 6 | 2.7 | 3.3 | 13.4 (of pts with angio) | 3.6 | 5 | ||||||||
| PCI access radial/ femoral, % | 46/54 | 34/66 | 26.5 | 77/23 | 66/22 | |||||||||
| Repeat revascularization during same hospital stay, % | 8 | 4.3 | 0.2 | 9 | ||||||||||
| Treatment | ||||||||||||||
| (I) Pre-hospital pre-treatment for ACS | ||||||||||||||
| Patients with available data at this time point, n | 2181 | - | ||||||||||||
| Clopidogrel, % overall | 29 | 14.7 | 20 | |||||||||||
| Loading dose was given in … % | 100 | 86 | ||||||||||||
| Prasugrel, % overall | 5 | 1 | ||||||||||||
| Loading dose was given in … % | 100 | 89.5 | ||||||||||||
| Ticagrelor, % overall | 24 | 0 | ||||||||||||
| Loading dose was given in … % | 100 | 0 | ||||||||||||
| Aspirin (ASA), % | 97 | 46 | 28 | |||||||||||
| GPIIb/IIIa inhibitors, % | 0 | 0.2 | ||||||||||||
| Unfractionated heparin (UFH), % | 52 | 15 | 9 | |||||||||||
| Low molecular weight heparin (LMWH), % | 31 | 8 | 13 | |||||||||||
| Fondaparinux, % | 2 | 1 | ||||||||||||
| (II) Treatment in hospital | ||||||||||||||
| Patients with available data at this time point, n | 2181 | 5880 | 6777 | 1760 | - | |||||||||
| Clopidogrel , % overall | 14 | 61.5 | 81.2 | 88.8 | 93 | |||||||||
| Loading dose was given in … % | 98 | 65.3 | 68.9 | 71.5 | ||||||||||
| Prasugrel, % overall | 4 | 15.7 | 20.9 | 3.3 | 18 | |||||||||
| Loading dose was given in … % | 95 | 16.6 | 46.3 | 28 | ||||||||||
| Ticagrelor, % overall | 13 | 43.4 | 0 | 0 | ||||||||||
| Loading dose was given in … % | 97 | 0 | 0 | |||||||||||
| Switching from clopidogrel to prasugrel, % | 0 | 7.5 | 1.9 | 11 | ||||||||||
| Switching from clopidogrel to ticagrelor, % | 1 | 8.1 | 0 | 0 | ||||||||||
| Switching from ticagrelor/prasugrel to clopidogrel, % | 0 | 7.3 | 0.7 | - | 13 | |||||||||
| Aspirin (ASA), % | – | 97.2 | 100 | 97.4 | 99 | |||||||||
| GPIIb/IIIa inhibitors, % | 0 | 9.2 | 11.4 | 4.5 | 25 | |||||||||
| Unfractionated heparin, % | 68.6 | 94.9 | 8.1 | 50.5 | ||||||||||
| Low molecular weight heparin, % | 25.6 | 6.4 | 82.2 | 63.5 | ||||||||||
| Fondaparinux, % | 5.3 | 7.2 | 8.4 | 19 | ||||||||||
| (III) Information on treatment at hospital discharge (D)/after hospital discharge (after) | D | After | D | After | D | After | D | After | D | After | D | After | ||
| Patients with available data at these 2 time points, n | 5291 | 6777 | 549 | 484 | 1716 | 1749 | ||||||||
| Clopidogrel treatment at discharge/after discharge, % | 55.1 | 73.6 | 70 | 27 | 67.2 | 68 | ||||||||
| Prasugrel treatment at discharge/after discharge, % | 17.7 | 17.8 | 0.7 | 0 | 4.3 | 16 | ||||||||
| Ticagrelor treatment at discharge/after discharge, % | 27.1 | 1.1 | 1.2 | 0 | 0 | |||||||||
| Newcastle 2010 | Newcastle 2011 | Newcastle 2012 | Newcastle 2013 | SCAAR | SPUM | |||||||||
| Patient number (n) | 1356 | 1578 | 1445 | 1575 | 52319 | 931 | ||||||||
| Definition of (major) bleeding | Study specific | |||||||||||||
| Characteristics of patients | ||||||||||||||
| Age, mean (SD) | 65.8 (12.7) | 65.6 (12.8) | 65.7 (13) | 65.9 (12.7) | 68 (11) | 65 (12.3) | ||||||||
| >75 years, % | 34.4 | 33.4 | 37.4 | 32.9 | 28.6 | 24.9 | ||||||||
| Gender, males/females, % | 68/32 | 68/32 | 66/34 | 67/33 | 68/32 | 77/23 | ||||||||
| Diabetes mellitus, % | 18.9 | 19.5 | 22.8 | 25.1 | 25.2 | 21.6 | ||||||||
| Chronic (congestive) heart failure, % | 10.8 | 2.6 | ||||||||||||
| Atrial fibrillation, % | 8.2 | |||||||||||||
| Coronary artery disease (CAD, CHD), % | 28.6 | |||||||||||||
| Previous stroke, % | 8 | 7.3 | 8.37 | 7.4 | 9.4 | 2.7 | ||||||||
| Previous myocardial infarction (STEMI/NSTE-ACS), % | 28.8 | 26.5 | 30.2 | 27.6 | 24.2 | 20.5 | ||||||||
| Previous PCI, % | 15.9 | 16 | 17.7 | 20.8 | 15.5 | 21.8 | ||||||||
| Previous CABG, % | 6.9 | 7 | 8.7 | 7.1 | 9.5 | 8.3 | ||||||||
| Arterial hypertension, % | 56.8 | 65.7 | ||||||||||||
| Peripheral arterial disease (PAD), % | 5.8 | 6.1 | 7.7 | 8.4 | 5.2 | 8.6 | ||||||||
| Current smoking, % | 24.3 | 21.5 | 21.5 | 20.3 | 21 | 36.6 | ||||||||
| Chronic kidney disease/renal impairment, % | 3.1 | 3.7 | 4 | 6.2 | 21.4 | 0.9 | ||||||||
| Antithrombotic pre-treatment: | ||||||||||||||
| Patients on chronic aspirin (ASA), % | 37.8 | 42 | ||||||||||||
| Patients on chronic clopidogrel/prasugrel/ticagrelor, % | 5.7/0/0.3 | 14.5/0.5/0.4 | ||||||||||||
| Patients on oral anticoagulation (VKA or NOAC), % | 5.3 | 3.9 | ||||||||||||
| ACS characteristics—Killip classes: I/II/III/IV, % | 58.2/1.8/0.5/0.3 | 87.8/8.4/2/0.8 | ||||||||||||
| Time from first medical contact to PCI, mean±SQ or median (IQR), hours | 10.5 (8.0) | 6.2 | ||||||||||||
| Intervention during initial hospitalization | ||||||||||||||
| Coronary angiography, % | 86.5 | 90.6 | 84.2 | 86.8 | 100 | 100 | ||||||||
| PCI, % | 71.5 | 63.6 | 63 | 66.3 | 67.5 | 98.4 | ||||||||
| CABG, % | 6.9 | 1.6 | ||||||||||||
| PCI access radial/femoral, % | 82/18 | 83/17 | 82/18 | 81/19 | 66/34 | |||||||||
| Repeat revascularization during same hospital stay, % | 0.8 | 0.7 | 0.7 | 0.9 | 0.7 | 0 | ||||||||
| Treatment | ||||||||||||||
| (I) Pre-hospital treatment for ACS | ||||||||||||||
| Patients with available data at this time point, n | 52319 | 927 | ||||||||||||
| Clopidogrel, % overall | 48.9 | 14.5 | ||||||||||||
| Loading dose was given in … % | ||||||||||||||
| Prasugrel, % overall | 0.6 | 0.5 | ||||||||||||
| Loading dose was given in … % | ||||||||||||||
| Ticagrelor, % overall | 16.8 | 0.4 | ||||||||||||
| Loading dose was given in … % | ||||||||||||||
| Aspirin (ASA), % | 67 | 42 | ||||||||||||
| GPIIb/IIIa inhibitors, % | 0.5 | |||||||||||||
| Unfractionated heparin (UFH), % | 3 | |||||||||||||
| Low molecular weight heparin (LMWH), % | 4.7 | |||||||||||||
| Fondaparinux, % | 35.1 | |||||||||||||
| (II) Treatment in hospital | ||||||||||||||
| Patients with available data at this time point, n | 1827 | 1948 | 1945 | 1972 | 52319 | 926 | ||||||||
| Clopidogrel , % overall | 1.9 | 76.8 | ||||||||||||
| Loading dose was given in … % | 64.1 | 67.7 | 65.7 | 55.9 | 66 | |||||||||
| Prasugrel, % overall | 1.2 | 8.5 | ||||||||||||
| Loading dose was given in … % | 35.7 | 32.3 | 34.2 | 29.8 | 6 | |||||||||
| Ticagrelor, % overall | 1.5 | 9.5 | ||||||||||||
| Loading dose was given in … % | 0.05 | 0 | 0.05 | 14.3 | 9.2 | |||||||||
| Switching from clopidogrel to prasugrel, % | 1.7 | 0.5 | ||||||||||||
| Switching from clopidogrel to ticagrelor, % | 19.4 | 2.1 | ||||||||||||
| Switching from ticagrelor/prasugrel to clopidogrel, % | 3.3 | 0 | ||||||||||||
| Aspirin (ASA), % | 1.3 | 98.5 | ||||||||||||
| GPIIb/IIIa inhibitors, % | 5.4 | 19.5 | ||||||||||||
| Unfractionated heparin, % | 27 | 21.8 | 16.9 | 14.5 | 56.6 | 95.2 | ||||||||
| Low molecular weight heparin, % | 69.4 | 72.8 | 78.3 | 73.7 | 2 | 5.6 | ||||||||
| Fondaparinux, % | 7.1 | 7.8 | 12.4 | 11.4 | 0.3 | 5.2 | ||||||||
| (III) Information on treatment at hospital discharge (D)/after hospital discharge (after) | D | After | D | After | D | After | D | After | D | After | D | After | ||
| Patients with available data at these 2 time points, n | 1827 | 1948 | 1945 | 1972 | 52319 | 931 | ||||||||
| Clopidogrel treatment at discharge/after discharge, % | 63 | 64.1 | ||||||||||||
| Prasugrel treatment at discharge/after discharge, % | 64.1 | 67.7 | 65.7 | 55.9 | 0.9 | 14.5 | ||||||||
| Ticagrelor treatment at discharge/after discharge, % | 35.7 | 32.3 | 34.2 | 29.8 | 19.2 | 9.8 | ||||||||
| Registry acronym . | AAPCI/ADAPT . | AMIS plus . | ATACS . | BLITZ-4 . | CZECH-2 . | DIOCLES . | FAST-MI 2010 . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient number (n) | 2181 | 5880 | 6777 | 5852 | 586 | 1769 | 1805 | |||||||
| Definition of (major) bleeding | TIMI | BARC (since 2012) | GUSTO | Fatal, intracranial or requiring surgery or blood transfusion | ||||||||||
| Characteristics of patients | ||||||||||||||
| Age, mean (SD) | 65 (13) | 66.6 (12.5) | 68.8 (12.0) | F 74 (11), M 68 (12) | 70 (11) | 69 (12) | 68 (13.6) | |||||||
| >75 years, % | 23 | 29.1 | 34.7 | 33.2 | 37.5 | |||||||||
| Gender, males/females, % | 68/32 | 75/25 | 70/30 | 66.6/33.4 | 65/35 | 73/27 | 70/30 | |||||||
| Diabetes mellitus, % | 22 | 22.4 | 33.7 | 30.6 | 40.5 | 34.8 | 26 | |||||||
| Chronic (congestive) heart failure, % | 2.6 | 0 | 7.9 | 7 | ||||||||||
| Atrial fibrillation, % | 8 | 4.4 | 19.6 | 14.5 | 8 | |||||||||
| Coronary artery disease (CAD, CHD), % | 38.8 | 100 | 35.1 | 36 | ||||||||||
| Previous stroke, % | 7 | 6.4 | 7.8 | 9.2 | 7.9 (stroke, TIA) | 4 | ||||||||
| Previous myocardial infarction (STEMI/NSTE-ACS), % | 17 | 20.7 | 28.2 | 17.3 | 29.1 | 27.3 | 22 | |||||||
| Previous PCI, % | 22 | 22.8 | 35.6 | 18.7 | 24.4 | 22.9 | 22 | |||||||
| Previous CABG, % | 8.5 | 14.2 | 9.1 | 12.1 | 6.5 | 7 | ||||||||
| Arterial hypertension, % | 67.6 | 85.9 | 67.2 | 76.5 | 71.2 | 62 | ||||||||
| Peripheral arterial disease (PAD), % | 6.2 | 11.5 | 10.6 | 12 | ||||||||||
| Current smoking, % | 33 | 35.3 | 28.9 | 24.7 | 25.9 | 22.8 | 26 | |||||||
| Chronic kidney disease/renal impairment, % | 7.2 | 23.3 | 11.9 | 6.4 (severe) | 6.5 | |||||||||
| Antithrombotic pre-treatment: | ||||||||||||||
| Patients on chronic aspirin (ASA), % | 47.1 | 52.8 | 46.5 | 48.9 | 30 | |||||||||
| Patients on chronic clopidogrel/prasugrel/ticagrelor, % | 13/0.9/1.2 | 21.5/3.4/ 0 | 9.6/0.25/0 | 18.3/1/0 | 20/0.5/0 | |||||||||
| Patients on oral anticoagulation (VKA or NOAC), % | 5 | 8.1 | 12.3 (any AC), 8.5 (VKA) | 6 | ||||||||||
| ACS characteristics—Killip classes: I/II/III/IV, % | 70/19/5/7 | 87.1/8.7/2.2/1.9 | 90.3/8.7 (II/III)/1.0 | 15.1(II) 7.5 (III-IV) | 70.9/16.3/9.1/3.6 | 86.5/7.7/5.1/0.7 | 80.5/11/7/1 | |||||||
| Time from first medical contact to PCI, mean±SQ or median (IQR) hours | 4.6 (2.2 to 11.5) | 6.8 (3.0 to 19.0) | 27.4 (14.7 to 55) | |||||||||||
| Intervention during initial hospitalization | ||||||||||||||
| Coronary angiography, % | 100 | 86 | 100 | 85.1 | 66 | 79.6 | 91.5 | |||||||
| PCI, % | 75 | 81.8 | 79.3 | 66.8 (of pts with angio) | 47 | 50.6 | 66 | |||||||
| CABG, % | 6 | 2.7 | 3.3 | 13.4 (of pts with angio) | 3.6 | 5 | ||||||||
| PCI access radial/ femoral, % | 46/54 | 34/66 | 26.5 | 77/23 | 66/22 | |||||||||
| Repeat revascularization during same hospital stay, % | 8 | 4.3 | 0.2 | 9 | ||||||||||
| Treatment | ||||||||||||||
| (I) Pre-hospital pre-treatment for ACS | ||||||||||||||
| Patients with available data at this time point, n | 2181 | - | ||||||||||||
| Clopidogrel, % overall | 29 | 14.7 | 20 | |||||||||||
| Loading dose was given in … % | 100 | 86 | ||||||||||||
| Prasugrel, % overall | 5 | 1 | ||||||||||||
| Loading dose was given in … % | 100 | 89.5 | ||||||||||||
| Ticagrelor, % overall | 24 | 0 | ||||||||||||
| Loading dose was given in … % | 100 | 0 | ||||||||||||
| Aspirin (ASA), % | 97 | 46 | 28 | |||||||||||
| GPIIb/IIIa inhibitors, % | 0 | 0.2 | ||||||||||||
| Unfractionated heparin (UFH), % | 52 | 15 | 9 | |||||||||||
| Low molecular weight heparin (LMWH), % | 31 | 8 | 13 | |||||||||||
| Fondaparinux, % | 2 | 1 | ||||||||||||
| (II) Treatment in hospital | ||||||||||||||
| Patients with available data at this time point, n | 2181 | 5880 | 6777 | 1760 | - | |||||||||
| Clopidogrel , % overall | 14 | 61.5 | 81.2 | 88.8 | 93 | |||||||||
| Loading dose was given in … % | 98 | 65.3 | 68.9 | 71.5 | ||||||||||
| Prasugrel, % overall | 4 | 15.7 | 20.9 | 3.3 | 18 | |||||||||
| Loading dose was given in … % | 95 | 16.6 | 46.3 | 28 | ||||||||||
| Ticagrelor, % overall | 13 | 43.4 | 0 | 0 | ||||||||||
| Loading dose was given in … % | 97 | 0 | 0 | |||||||||||
| Switching from clopidogrel to prasugrel, % | 0 | 7.5 | 1.9 | 11 | ||||||||||
| Switching from clopidogrel to ticagrelor, % | 1 | 8.1 | 0 | 0 | ||||||||||
| Switching from ticagrelor/prasugrel to clopidogrel, % | 0 | 7.3 | 0.7 | - | 13 | |||||||||
| Aspirin (ASA), % | – | 97.2 | 100 | 97.4 | 99 | |||||||||
| GPIIb/IIIa inhibitors, % | 0 | 9.2 | 11.4 | 4.5 | 25 | |||||||||
| Unfractionated heparin, % | 68.6 | 94.9 | 8.1 | 50.5 | ||||||||||
| Low molecular weight heparin, % | 25.6 | 6.4 | 82.2 | 63.5 | ||||||||||
| Fondaparinux, % | 5.3 | 7.2 | 8.4 | 19 | ||||||||||
| (III) Information on treatment at hospital discharge (D)/after hospital discharge (after) | D | After | D | After | D | After | D | After | D | After | D | After | ||
| Patients with available data at these 2 time points, n | 5291 | 6777 | 549 | 484 | 1716 | 1749 | ||||||||
| Clopidogrel treatment at discharge/after discharge, % | 55.1 | 73.6 | 70 | 27 | 67.2 | 68 | ||||||||
| Prasugrel treatment at discharge/after discharge, % | 17.7 | 17.8 | 0.7 | 0 | 4.3 | 16 | ||||||||
| Ticagrelor treatment at discharge/after discharge, % | 27.1 | 1.1 | 1.2 | 0 | 0 | |||||||||
| Newcastle 2010 | Newcastle 2011 | Newcastle 2012 | Newcastle 2013 | SCAAR | SPUM | |||||||||
| Patient number (n) | 1356 | 1578 | 1445 | 1575 | 52319 | 931 | ||||||||
| Definition of (major) bleeding | Study specific | |||||||||||||
| Characteristics of patients | ||||||||||||||
| Age, mean (SD) | 65.8 (12.7) | 65.6 (12.8) | 65.7 (13) | 65.9 (12.7) | 68 (11) | 65 (12.3) | ||||||||
| >75 years, % | 34.4 | 33.4 | 37.4 | 32.9 | 28.6 | 24.9 | ||||||||
| Gender, males/females, % | 68/32 | 68/32 | 66/34 | 67/33 | 68/32 | 77/23 | ||||||||
| Diabetes mellitus, % | 18.9 | 19.5 | 22.8 | 25.1 | 25.2 | 21.6 | ||||||||
| Chronic (congestive) heart failure, % | 10.8 | 2.6 | ||||||||||||
| Atrial fibrillation, % | 8.2 | |||||||||||||
| Coronary artery disease (CAD, CHD), % | 28.6 | |||||||||||||
| Previous stroke, % | 8 | 7.3 | 8.37 | 7.4 | 9.4 | 2.7 | ||||||||
| Previous myocardial infarction (STEMI/NSTE-ACS), % | 28.8 | 26.5 | 30.2 | 27.6 | 24.2 | 20.5 | ||||||||
| Previous PCI, % | 15.9 | 16 | 17.7 | 20.8 | 15.5 | 21.8 | ||||||||
| Previous CABG, % | 6.9 | 7 | 8.7 | 7.1 | 9.5 | 8.3 | ||||||||
| Arterial hypertension, % | 56.8 | 65.7 | ||||||||||||
| Peripheral arterial disease (PAD), % | 5.8 | 6.1 | 7.7 | 8.4 | 5.2 | 8.6 | ||||||||
| Current smoking, % | 24.3 | 21.5 | 21.5 | 20.3 | 21 | 36.6 | ||||||||
| Chronic kidney disease/renal impairment, % | 3.1 | 3.7 | 4 | 6.2 | 21.4 | 0.9 | ||||||||
| Antithrombotic pre-treatment: | ||||||||||||||
| Patients on chronic aspirin (ASA), % | 37.8 | 42 | ||||||||||||
| Patients on chronic clopidogrel/prasugrel/ticagrelor, % | 5.7/0/0.3 | 14.5/0.5/0.4 | ||||||||||||
| Patients on oral anticoagulation (VKA or NOAC), % | 5.3 | 3.9 | ||||||||||||
| ACS characteristics—Killip classes: I/II/III/IV, % | 58.2/1.8/0.5/0.3 | 87.8/8.4/2/0.8 | ||||||||||||
| Time from first medical contact to PCI, mean±SQ or median (IQR), hours | 10.5 (8.0) | 6.2 | ||||||||||||
| Intervention during initial hospitalization | ||||||||||||||
| Coronary angiography, % | 86.5 | 90.6 | 84.2 | 86.8 | 100 | 100 | ||||||||
| PCI, % | 71.5 | 63.6 | 63 | 66.3 | 67.5 | 98.4 | ||||||||
| CABG, % | 6.9 | 1.6 | ||||||||||||
| PCI access radial/femoral, % | 82/18 | 83/17 | 82/18 | 81/19 | 66/34 | |||||||||
| Repeat revascularization during same hospital stay, % | 0.8 | 0.7 | 0.7 | 0.9 | 0.7 | 0 | ||||||||
| Treatment | ||||||||||||||
| (I) Pre-hospital treatment for ACS | ||||||||||||||
| Patients with available data at this time point, n | 52319 | 927 | ||||||||||||
| Clopidogrel, % overall | 48.9 | 14.5 | ||||||||||||
| Loading dose was given in … % | ||||||||||||||
| Prasugrel, % overall | 0.6 | 0.5 | ||||||||||||
| Loading dose was given in … % | ||||||||||||||
| Ticagrelor, % overall | 16.8 | 0.4 | ||||||||||||
| Loading dose was given in … % | ||||||||||||||
| Aspirin (ASA), % | 67 | 42 | ||||||||||||
| GPIIb/IIIa inhibitors, % | 0.5 | |||||||||||||
| Unfractionated heparin (UFH), % | 3 | |||||||||||||
| Low molecular weight heparin (LMWH), % | 4.7 | |||||||||||||
| Fondaparinux, % | 35.1 | |||||||||||||
| (II) Treatment in hospital | ||||||||||||||
| Patients with available data at this time point, n | 1827 | 1948 | 1945 | 1972 | 52319 | 926 | ||||||||
| Clopidogrel , % overall | 1.9 | 76.8 | ||||||||||||
| Loading dose was given in … % | 64.1 | 67.7 | 65.7 | 55.9 | 66 | |||||||||
| Prasugrel, % overall | 1.2 | 8.5 | ||||||||||||
| Loading dose was given in … % | 35.7 | 32.3 | 34.2 | 29.8 | 6 | |||||||||
| Ticagrelor, % overall | 1.5 | 9.5 | ||||||||||||
| Loading dose was given in … % | 0.05 | 0 | 0.05 | 14.3 | 9.2 | |||||||||
| Switching from clopidogrel to prasugrel, % | 1.7 | 0.5 | ||||||||||||
| Switching from clopidogrel to ticagrelor, % | 19.4 | 2.1 | ||||||||||||
| Switching from ticagrelor/prasugrel to clopidogrel, % | 3.3 | 0 | ||||||||||||
| Aspirin (ASA), % | 1.3 | 98.5 | ||||||||||||
| GPIIb/IIIa inhibitors, % | 5.4 | 19.5 | ||||||||||||
| Unfractionated heparin, % | 27 | 21.8 | 16.9 | 14.5 | 56.6 | 95.2 | ||||||||
| Low molecular weight heparin, % | 69.4 | 72.8 | 78.3 | 73.7 | 2 | 5.6 | ||||||||
| Fondaparinux, % | 7.1 | 7.8 | 12.4 | 11.4 | 0.3 | 5.2 | ||||||||
| (III) Information on treatment at hospital discharge (D)/after hospital discharge (after) | D | After | D | After | D | After | D | After | D | After | D | After | ||
| Patients with available data at these 2 time points, n | 1827 | 1948 | 1945 | 1972 | 52319 | 931 | ||||||||
| Clopidogrel treatment at discharge/after discharge, % | 63 | 64.1 | ||||||||||||
| Prasugrel treatment at discharge/after discharge, % | 64.1 | 67.7 | 65.7 | 55.9 | 0.9 | 14.5 | ||||||||
| Ticagrelor treatment at discharge/after discharge, % | 35.7 | 32.3 | 34.2 | 29.8 | 19.2 | 9.8 | ||||||||
F, female; M, male.
Baseline characteristics in the non-ST-segment elevation acute coronary syndrome cohorts of the various registries
| Registry acronym . | AAPCI/ADAPT . | AMIS plus . | ATACS . | BLITZ-4 . | CZECH-2 . | DIOCLES . | FAST-MI 2010 . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient number (n) | 2181 | 5880 | 6777 | 5852 | 586 | 1769 | 1805 | |||||||
| Definition of (major) bleeding | TIMI | BARC (since 2012) | GUSTO | Fatal, intracranial or requiring surgery or blood transfusion | ||||||||||
| Characteristics of patients | ||||||||||||||
| Age, mean (SD) | 65 (13) | 66.6 (12.5) | 68.8 (12.0) | F 74 (11), M 68 (12) | 70 (11) | 69 (12) | 68 (13.6) | |||||||
| >75 years, % | 23 | 29.1 | 34.7 | 33.2 | 37.5 | |||||||||
| Gender, males/females, % | 68/32 | 75/25 | 70/30 | 66.6/33.4 | 65/35 | 73/27 | 70/30 | |||||||
| Diabetes mellitus, % | 22 | 22.4 | 33.7 | 30.6 | 40.5 | 34.8 | 26 | |||||||
| Chronic (congestive) heart failure, % | 2.6 | 0 | 7.9 | 7 | ||||||||||
| Atrial fibrillation, % | 8 | 4.4 | 19.6 | 14.5 | 8 | |||||||||
| Coronary artery disease (CAD, CHD), % | 38.8 | 100 | 35.1 | 36 | ||||||||||
| Previous stroke, % | 7 | 6.4 | 7.8 | 9.2 | 7.9 (stroke, TIA) | 4 | ||||||||
| Previous myocardial infarction (STEMI/NSTE-ACS), % | 17 | 20.7 | 28.2 | 17.3 | 29.1 | 27.3 | 22 | |||||||
| Previous PCI, % | 22 | 22.8 | 35.6 | 18.7 | 24.4 | 22.9 | 22 | |||||||
| Previous CABG, % | 8.5 | 14.2 | 9.1 | 12.1 | 6.5 | 7 | ||||||||
| Arterial hypertension, % | 67.6 | 85.9 | 67.2 | 76.5 | 71.2 | 62 | ||||||||
| Peripheral arterial disease (PAD), % | 6.2 | 11.5 | 10.6 | 12 | ||||||||||
| Current smoking, % | 33 | 35.3 | 28.9 | 24.7 | 25.9 | 22.8 | 26 | |||||||
| Chronic kidney disease/renal impairment, % | 7.2 | 23.3 | 11.9 | 6.4 (severe) | 6.5 | |||||||||
| Antithrombotic pre-treatment: | ||||||||||||||
| Patients on chronic aspirin (ASA), % | 47.1 | 52.8 | 46.5 | 48.9 | 30 | |||||||||
| Patients on chronic clopidogrel/prasugrel/ticagrelor, % | 13/0.9/1.2 | 21.5/3.4/ 0 | 9.6/0.25/0 | 18.3/1/0 | 20/0.5/0 | |||||||||
| Patients on oral anticoagulation (VKA or NOAC), % | 5 | 8.1 | 12.3 (any AC), 8.5 (VKA) | 6 | ||||||||||
| ACS characteristics—Killip classes: I/II/III/IV, % | 70/19/5/7 | 87.1/8.7/2.2/1.9 | 90.3/8.7 (II/III)/1.0 | 15.1(II) 7.5 (III-IV) | 70.9/16.3/9.1/3.6 | 86.5/7.7/5.1/0.7 | 80.5/11/7/1 | |||||||
| Time from first medical contact to PCI, mean±SQ or median (IQR) hours | 4.6 (2.2 to 11.5) | 6.8 (3.0 to 19.0) | 27.4 (14.7 to 55) | |||||||||||
| Intervention during initial hospitalization | ||||||||||||||
| Coronary angiography, % | 100 | 86 | 100 | 85.1 | 66 | 79.6 | 91.5 | |||||||
| PCI, % | 75 | 81.8 | 79.3 | 66.8 (of pts with angio) | 47 | 50.6 | 66 | |||||||
| CABG, % | 6 | 2.7 | 3.3 | 13.4 (of pts with angio) | 3.6 | 5 | ||||||||
| PCI access radial/ femoral, % | 46/54 | 34/66 | 26.5 | 77/23 | 66/22 | |||||||||
| Repeat revascularization during same hospital stay, % | 8 | 4.3 | 0.2 | 9 | ||||||||||
| Treatment | ||||||||||||||
| (I) Pre-hospital pre-treatment for ACS | ||||||||||||||
| Patients with available data at this time point, n | 2181 | - | ||||||||||||
| Clopidogrel, % overall | 29 | 14.7 | 20 | |||||||||||
| Loading dose was given in … % | 100 | 86 | ||||||||||||
| Prasugrel, % overall | 5 | 1 | ||||||||||||
| Loading dose was given in … % | 100 | 89.5 | ||||||||||||
| Ticagrelor, % overall | 24 | 0 | ||||||||||||
| Loading dose was given in … % | 100 | 0 | ||||||||||||
| Aspirin (ASA), % | 97 | 46 | 28 | |||||||||||
| GPIIb/IIIa inhibitors, % | 0 | 0.2 | ||||||||||||
| Unfractionated heparin (UFH), % | 52 | 15 | 9 | |||||||||||
| Low molecular weight heparin (LMWH), % | 31 | 8 | 13 | |||||||||||
| Fondaparinux, % | 2 | 1 | ||||||||||||
| (II) Treatment in hospital | ||||||||||||||
| Patients with available data at this time point, n | 2181 | 5880 | 6777 | 1760 | - | |||||||||
| Clopidogrel , % overall | 14 | 61.5 | 81.2 | 88.8 | 93 | |||||||||
| Loading dose was given in … % | 98 | 65.3 | 68.9 | 71.5 | ||||||||||
| Prasugrel, % overall | 4 | 15.7 | 20.9 | 3.3 | 18 | |||||||||
| Loading dose was given in … % | 95 | 16.6 | 46.3 | 28 | ||||||||||
| Ticagrelor, % overall | 13 | 43.4 | 0 | 0 | ||||||||||
| Loading dose was given in … % | 97 | 0 | 0 | |||||||||||
| Switching from clopidogrel to prasugrel, % | 0 | 7.5 | 1.9 | 11 | ||||||||||
| Switching from clopidogrel to ticagrelor, % | 1 | 8.1 | 0 | 0 | ||||||||||
| Switching from ticagrelor/prasugrel to clopidogrel, % | 0 | 7.3 | 0.7 | - | 13 | |||||||||
| Aspirin (ASA), % | – | 97.2 | 100 | 97.4 | 99 | |||||||||
| GPIIb/IIIa inhibitors, % | 0 | 9.2 | 11.4 | 4.5 | 25 | |||||||||
| Unfractionated heparin, % | 68.6 | 94.9 | 8.1 | 50.5 | ||||||||||
| Low molecular weight heparin, % | 25.6 | 6.4 | 82.2 | 63.5 | ||||||||||
| Fondaparinux, % | 5.3 | 7.2 | 8.4 | 19 | ||||||||||
| (III) Information on treatment at hospital discharge (D)/after hospital discharge (after) | D | After | D | After | D | After | D | After | D | After | D | After | ||
| Patients with available data at these 2 time points, n | 5291 | 6777 | 549 | 484 | 1716 | 1749 | ||||||||
| Clopidogrel treatment at discharge/after discharge, % | 55.1 | 73.6 | 70 | 27 | 67.2 | 68 | ||||||||
| Prasugrel treatment at discharge/after discharge, % | 17.7 | 17.8 | 0.7 | 0 | 4.3 | 16 | ||||||||
| Ticagrelor treatment at discharge/after discharge, % | 27.1 | 1.1 | 1.2 | 0 | 0 | |||||||||
| Newcastle 2010 | Newcastle 2011 | Newcastle 2012 | Newcastle 2013 | SCAAR | SPUM | |||||||||
| Patient number (n) | 1356 | 1578 | 1445 | 1575 | 52319 | 931 | ||||||||
| Definition of (major) bleeding | Study specific | |||||||||||||
| Characteristics of patients | ||||||||||||||
| Age, mean (SD) | 65.8 (12.7) | 65.6 (12.8) | 65.7 (13) | 65.9 (12.7) | 68 (11) | 65 (12.3) | ||||||||
| >75 years, % | 34.4 | 33.4 | 37.4 | 32.9 | 28.6 | 24.9 | ||||||||
| Gender, males/females, % | 68/32 | 68/32 | 66/34 | 67/33 | 68/32 | 77/23 | ||||||||
| Diabetes mellitus, % | 18.9 | 19.5 | 22.8 | 25.1 | 25.2 | 21.6 | ||||||||
| Chronic (congestive) heart failure, % | 10.8 | 2.6 | ||||||||||||
| Atrial fibrillation, % | 8.2 | |||||||||||||
| Coronary artery disease (CAD, CHD), % | 28.6 | |||||||||||||
| Previous stroke, % | 8 | 7.3 | 8.37 | 7.4 | 9.4 | 2.7 | ||||||||
| Previous myocardial infarction (STEMI/NSTE-ACS), % | 28.8 | 26.5 | 30.2 | 27.6 | 24.2 | 20.5 | ||||||||
| Previous PCI, % | 15.9 | 16 | 17.7 | 20.8 | 15.5 | 21.8 | ||||||||
| Previous CABG, % | 6.9 | 7 | 8.7 | 7.1 | 9.5 | 8.3 | ||||||||
| Arterial hypertension, % | 56.8 | 65.7 | ||||||||||||
| Peripheral arterial disease (PAD), % | 5.8 | 6.1 | 7.7 | 8.4 | 5.2 | 8.6 | ||||||||
| Current smoking, % | 24.3 | 21.5 | 21.5 | 20.3 | 21 | 36.6 | ||||||||
| Chronic kidney disease/renal impairment, % | 3.1 | 3.7 | 4 | 6.2 | 21.4 | 0.9 | ||||||||
| Antithrombotic pre-treatment: | ||||||||||||||
| Patients on chronic aspirin (ASA), % | 37.8 | 42 | ||||||||||||
| Patients on chronic clopidogrel/prasugrel/ticagrelor, % | 5.7/0/0.3 | 14.5/0.5/0.4 | ||||||||||||
| Patients on oral anticoagulation (VKA or NOAC), % | 5.3 | 3.9 | ||||||||||||
| ACS characteristics—Killip classes: I/II/III/IV, % | 58.2/1.8/0.5/0.3 | 87.8/8.4/2/0.8 | ||||||||||||
| Time from first medical contact to PCI, mean±SQ or median (IQR), hours | 10.5 (8.0) | 6.2 | ||||||||||||
| Intervention during initial hospitalization | ||||||||||||||
| Coronary angiography, % | 86.5 | 90.6 | 84.2 | 86.8 | 100 | 100 | ||||||||
| PCI, % | 71.5 | 63.6 | 63 | 66.3 | 67.5 | 98.4 | ||||||||
| CABG, % | 6.9 | 1.6 | ||||||||||||
| PCI access radial/femoral, % | 82/18 | 83/17 | 82/18 | 81/19 | 66/34 | |||||||||
| Repeat revascularization during same hospital stay, % | 0.8 | 0.7 | 0.7 | 0.9 | 0.7 | 0 | ||||||||
| Treatment | ||||||||||||||
| (I) Pre-hospital treatment for ACS | ||||||||||||||
| Patients with available data at this time point, n | 52319 | 927 | ||||||||||||
| Clopidogrel, % overall | 48.9 | 14.5 | ||||||||||||
| Loading dose was given in … % | ||||||||||||||
| Prasugrel, % overall | 0.6 | 0.5 | ||||||||||||
| Loading dose was given in … % | ||||||||||||||
| Ticagrelor, % overall | 16.8 | 0.4 | ||||||||||||
| Loading dose was given in … % | ||||||||||||||
| Aspirin (ASA), % | 67 | 42 | ||||||||||||
| GPIIb/IIIa inhibitors, % | 0.5 | |||||||||||||
| Unfractionated heparin (UFH), % | 3 | |||||||||||||
| Low molecular weight heparin (LMWH), % | 4.7 | |||||||||||||
| Fondaparinux, % | 35.1 | |||||||||||||
| (II) Treatment in hospital | ||||||||||||||
| Patients with available data at this time point, n | 1827 | 1948 | 1945 | 1972 | 52319 | 926 | ||||||||
| Clopidogrel , % overall | 1.9 | 76.8 | ||||||||||||
| Loading dose was given in … % | 64.1 | 67.7 | 65.7 | 55.9 | 66 | |||||||||
| Prasugrel, % overall | 1.2 | 8.5 | ||||||||||||
| Loading dose was given in … % | 35.7 | 32.3 | 34.2 | 29.8 | 6 | |||||||||
| Ticagrelor, % overall | 1.5 | 9.5 | ||||||||||||
| Loading dose was given in … % | 0.05 | 0 | 0.05 | 14.3 | 9.2 | |||||||||
| Switching from clopidogrel to prasugrel, % | 1.7 | 0.5 | ||||||||||||
| Switching from clopidogrel to ticagrelor, % | 19.4 | 2.1 | ||||||||||||
| Switching from ticagrelor/prasugrel to clopidogrel, % | 3.3 | 0 | ||||||||||||
| Aspirin (ASA), % | 1.3 | 98.5 | ||||||||||||
| GPIIb/IIIa inhibitors, % | 5.4 | 19.5 | ||||||||||||
| Unfractionated heparin, % | 27 | 21.8 | 16.9 | 14.5 | 56.6 | 95.2 | ||||||||
| Low molecular weight heparin, % | 69.4 | 72.8 | 78.3 | 73.7 | 2 | 5.6 | ||||||||
| Fondaparinux, % | 7.1 | 7.8 | 12.4 | 11.4 | 0.3 | 5.2 | ||||||||
| (III) Information on treatment at hospital discharge (D)/after hospital discharge (after) | D | After | D | After | D | After | D | After | D | After | D | After | ||
| Patients with available data at these 2 time points, n | 1827 | 1948 | 1945 | 1972 | 52319 | 931 | ||||||||
| Clopidogrel treatment at discharge/after discharge, % | 63 | 64.1 | ||||||||||||
| Prasugrel treatment at discharge/after discharge, % | 64.1 | 67.7 | 65.7 | 55.9 | 0.9 | 14.5 | ||||||||
| Ticagrelor treatment at discharge/after discharge, % | 35.7 | 32.3 | 34.2 | 29.8 | 19.2 | 9.8 | ||||||||
| Registry acronym . | AAPCI/ADAPT . | AMIS plus . | ATACS . | BLITZ-4 . | CZECH-2 . | DIOCLES . | FAST-MI 2010 . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient number (n) | 2181 | 5880 | 6777 | 5852 | 586 | 1769 | 1805 | |||||||
| Definition of (major) bleeding | TIMI | BARC (since 2012) | GUSTO | Fatal, intracranial or requiring surgery or blood transfusion | ||||||||||
| Characteristics of patients | ||||||||||||||
| Age, mean (SD) | 65 (13) | 66.6 (12.5) | 68.8 (12.0) | F 74 (11), M 68 (12) | 70 (11) | 69 (12) | 68 (13.6) | |||||||
| >75 years, % | 23 | 29.1 | 34.7 | 33.2 | 37.5 | |||||||||
| Gender, males/females, % | 68/32 | 75/25 | 70/30 | 66.6/33.4 | 65/35 | 73/27 | 70/30 | |||||||
| Diabetes mellitus, % | 22 | 22.4 | 33.7 | 30.6 | 40.5 | 34.8 | 26 | |||||||
| Chronic (congestive) heart failure, % | 2.6 | 0 | 7.9 | 7 | ||||||||||
| Atrial fibrillation, % | 8 | 4.4 | 19.6 | 14.5 | 8 | |||||||||
| Coronary artery disease (CAD, CHD), % | 38.8 | 100 | 35.1 | 36 | ||||||||||
| Previous stroke, % | 7 | 6.4 | 7.8 | 9.2 | 7.9 (stroke, TIA) | 4 | ||||||||
| Previous myocardial infarction (STEMI/NSTE-ACS), % | 17 | 20.7 | 28.2 | 17.3 | 29.1 | 27.3 | 22 | |||||||
| Previous PCI, % | 22 | 22.8 | 35.6 | 18.7 | 24.4 | 22.9 | 22 | |||||||
| Previous CABG, % | 8.5 | 14.2 | 9.1 | 12.1 | 6.5 | 7 | ||||||||
| Arterial hypertension, % | 67.6 | 85.9 | 67.2 | 76.5 | 71.2 | 62 | ||||||||
| Peripheral arterial disease (PAD), % | 6.2 | 11.5 | 10.6 | 12 | ||||||||||
| Current smoking, % | 33 | 35.3 | 28.9 | 24.7 | 25.9 | 22.8 | 26 | |||||||
| Chronic kidney disease/renal impairment, % | 7.2 | 23.3 | 11.9 | 6.4 (severe) | 6.5 | |||||||||
| Antithrombotic pre-treatment: | ||||||||||||||
| Patients on chronic aspirin (ASA), % | 47.1 | 52.8 | 46.5 | 48.9 | 30 | |||||||||
| Patients on chronic clopidogrel/prasugrel/ticagrelor, % | 13/0.9/1.2 | 21.5/3.4/ 0 | 9.6/0.25/0 | 18.3/1/0 | 20/0.5/0 | |||||||||
| Patients on oral anticoagulation (VKA or NOAC), % | 5 | 8.1 | 12.3 (any AC), 8.5 (VKA) | 6 | ||||||||||
| ACS characteristics—Killip classes: I/II/III/IV, % | 70/19/5/7 | 87.1/8.7/2.2/1.9 | 90.3/8.7 (II/III)/1.0 | 15.1(II) 7.5 (III-IV) | 70.9/16.3/9.1/3.6 | 86.5/7.7/5.1/0.7 | 80.5/11/7/1 | |||||||
| Time from first medical contact to PCI, mean±SQ or median (IQR) hours | 4.6 (2.2 to 11.5) | 6.8 (3.0 to 19.0) | 27.4 (14.7 to 55) | |||||||||||
| Intervention during initial hospitalization | ||||||||||||||
| Coronary angiography, % | 100 | 86 | 100 | 85.1 | 66 | 79.6 | 91.5 | |||||||
| PCI, % | 75 | 81.8 | 79.3 | 66.8 (of pts with angio) | 47 | 50.6 | 66 | |||||||
| CABG, % | 6 | 2.7 | 3.3 | 13.4 (of pts with angio) | 3.6 | 5 | ||||||||
| PCI access radial/ femoral, % | 46/54 | 34/66 | 26.5 | 77/23 | 66/22 | |||||||||
| Repeat revascularization during same hospital stay, % | 8 | 4.3 | 0.2 | 9 | ||||||||||
| Treatment | ||||||||||||||
| (I) Pre-hospital pre-treatment for ACS | ||||||||||||||
| Patients with available data at this time point, n | 2181 | - | ||||||||||||
| Clopidogrel, % overall | 29 | 14.7 | 20 | |||||||||||
| Loading dose was given in … % | 100 | 86 | ||||||||||||
| Prasugrel, % overall | 5 | 1 | ||||||||||||
| Loading dose was given in … % | 100 | 89.5 | ||||||||||||
| Ticagrelor, % overall | 24 | 0 | ||||||||||||
| Loading dose was given in … % | 100 | 0 | ||||||||||||
| Aspirin (ASA), % | 97 | 46 | 28 | |||||||||||
| GPIIb/IIIa inhibitors, % | 0 | 0.2 | ||||||||||||
| Unfractionated heparin (UFH), % | 52 | 15 | 9 | |||||||||||
| Low molecular weight heparin (LMWH), % | 31 | 8 | 13 | |||||||||||
| Fondaparinux, % | 2 | 1 | ||||||||||||
| (II) Treatment in hospital | ||||||||||||||
| Patients with available data at this time point, n | 2181 | 5880 | 6777 | 1760 | - | |||||||||
| Clopidogrel , % overall | 14 | 61.5 | 81.2 | 88.8 | 93 | |||||||||
| Loading dose was given in … % | 98 | 65.3 | 68.9 | 71.5 | ||||||||||
| Prasugrel, % overall | 4 | 15.7 | 20.9 | 3.3 | 18 | |||||||||
| Loading dose was given in … % | 95 | 16.6 | 46.3 | 28 | ||||||||||
| Ticagrelor, % overall | 13 | 43.4 | 0 | 0 | ||||||||||
| Loading dose was given in … % | 97 | 0 | 0 | |||||||||||
| Switching from clopidogrel to prasugrel, % | 0 | 7.5 | 1.9 | 11 | ||||||||||
| Switching from clopidogrel to ticagrelor, % | 1 | 8.1 | 0 | 0 | ||||||||||
| Switching from ticagrelor/prasugrel to clopidogrel, % | 0 | 7.3 | 0.7 | - | 13 | |||||||||
| Aspirin (ASA), % | – | 97.2 | 100 | 97.4 | 99 | |||||||||
| GPIIb/IIIa inhibitors, % | 0 | 9.2 | 11.4 | 4.5 | 25 | |||||||||
| Unfractionated heparin, % | 68.6 | 94.9 | 8.1 | 50.5 | ||||||||||
| Low molecular weight heparin, % | 25.6 | 6.4 | 82.2 | 63.5 | ||||||||||
| Fondaparinux, % | 5.3 | 7.2 | 8.4 | 19 | ||||||||||
| (III) Information on treatment at hospital discharge (D)/after hospital discharge (after) | D | After | D | After | D | After | D | After | D | After | D | After | ||
| Patients with available data at these 2 time points, n | 5291 | 6777 | 549 | 484 | 1716 | 1749 | ||||||||
| Clopidogrel treatment at discharge/after discharge, % | 55.1 | 73.6 | 70 | 27 | 67.2 | 68 | ||||||||
| Prasugrel treatment at discharge/after discharge, % | 17.7 | 17.8 | 0.7 | 0 | 4.3 | 16 | ||||||||
| Ticagrelor treatment at discharge/after discharge, % | 27.1 | 1.1 | 1.2 | 0 | 0 | |||||||||
| Newcastle 2010 | Newcastle 2011 | Newcastle 2012 | Newcastle 2013 | SCAAR | SPUM | |||||||||
| Patient number (n) | 1356 | 1578 | 1445 | 1575 | 52319 | 931 | ||||||||
| Definition of (major) bleeding | Study specific | |||||||||||||
| Characteristics of patients | ||||||||||||||
| Age, mean (SD) | 65.8 (12.7) | 65.6 (12.8) | 65.7 (13) | 65.9 (12.7) | 68 (11) | 65 (12.3) | ||||||||
| >75 years, % | 34.4 | 33.4 | 37.4 | 32.9 | 28.6 | 24.9 | ||||||||
| Gender, males/females, % | 68/32 | 68/32 | 66/34 | 67/33 | 68/32 | 77/23 | ||||||||
| Diabetes mellitus, % | 18.9 | 19.5 | 22.8 | 25.1 | 25.2 | 21.6 | ||||||||
| Chronic (congestive) heart failure, % | 10.8 | 2.6 | ||||||||||||
| Atrial fibrillation, % | 8.2 | |||||||||||||
| Coronary artery disease (CAD, CHD), % | 28.6 | |||||||||||||
| Previous stroke, % | 8 | 7.3 | 8.37 | 7.4 | 9.4 | 2.7 | ||||||||
| Previous myocardial infarction (STEMI/NSTE-ACS), % | 28.8 | 26.5 | 30.2 | 27.6 | 24.2 | 20.5 | ||||||||
| Previous PCI, % | 15.9 | 16 | 17.7 | 20.8 | 15.5 | 21.8 | ||||||||
| Previous CABG, % | 6.9 | 7 | 8.7 | 7.1 | 9.5 | 8.3 | ||||||||
| Arterial hypertension, % | 56.8 | 65.7 | ||||||||||||
| Peripheral arterial disease (PAD), % | 5.8 | 6.1 | 7.7 | 8.4 | 5.2 | 8.6 | ||||||||
| Current smoking, % | 24.3 | 21.5 | 21.5 | 20.3 | 21 | 36.6 | ||||||||
| Chronic kidney disease/renal impairment, % | 3.1 | 3.7 | 4 | 6.2 | 21.4 | 0.9 | ||||||||
| Antithrombotic pre-treatment: | ||||||||||||||
| Patients on chronic aspirin (ASA), % | 37.8 | 42 | ||||||||||||
| Patients on chronic clopidogrel/prasugrel/ticagrelor, % | 5.7/0/0.3 | 14.5/0.5/0.4 | ||||||||||||
| Patients on oral anticoagulation (VKA or NOAC), % | 5.3 | 3.9 | ||||||||||||
| ACS characteristics—Killip classes: I/II/III/IV, % | 58.2/1.8/0.5/0.3 | 87.8/8.4/2/0.8 | ||||||||||||
| Time from first medical contact to PCI, mean±SQ or median (IQR), hours | 10.5 (8.0) | 6.2 | ||||||||||||
| Intervention during initial hospitalization | ||||||||||||||
| Coronary angiography, % | 86.5 | 90.6 | 84.2 | 86.8 | 100 | 100 | ||||||||
| PCI, % | 71.5 | 63.6 | 63 | 66.3 | 67.5 | 98.4 | ||||||||
| CABG, % | 6.9 | 1.6 | ||||||||||||
| PCI access radial/femoral, % | 82/18 | 83/17 | 82/18 | 81/19 | 66/34 | |||||||||
| Repeat revascularization during same hospital stay, % | 0.8 | 0.7 | 0.7 | 0.9 | 0.7 | 0 | ||||||||
| Treatment | ||||||||||||||
| (I) Pre-hospital treatment for ACS | ||||||||||||||
| Patients with available data at this time point, n | 52319 | 927 | ||||||||||||
| Clopidogrel, % overall | 48.9 | 14.5 | ||||||||||||
| Loading dose was given in … % | ||||||||||||||
| Prasugrel, % overall | 0.6 | 0.5 | ||||||||||||
| Loading dose was given in … % | ||||||||||||||
| Ticagrelor, % overall | 16.8 | 0.4 | ||||||||||||
| Loading dose was given in … % | ||||||||||||||
| Aspirin (ASA), % | 67 | 42 | ||||||||||||
| GPIIb/IIIa inhibitors, % | 0.5 | |||||||||||||
| Unfractionated heparin (UFH), % | 3 | |||||||||||||
| Low molecular weight heparin (LMWH), % | 4.7 | |||||||||||||
| Fondaparinux, % | 35.1 | |||||||||||||
| (II) Treatment in hospital | ||||||||||||||
| Patients with available data at this time point, n | 1827 | 1948 | 1945 | 1972 | 52319 | 926 | ||||||||
| Clopidogrel , % overall | 1.9 | 76.8 | ||||||||||||
| Loading dose was given in … % | 64.1 | 67.7 | 65.7 | 55.9 | 66 | |||||||||
| Prasugrel, % overall | 1.2 | 8.5 | ||||||||||||
| Loading dose was given in … % | 35.7 | 32.3 | 34.2 | 29.8 | 6 | |||||||||
| Ticagrelor, % overall | 1.5 | 9.5 | ||||||||||||
| Loading dose was given in … % | 0.05 | 0 | 0.05 | 14.3 | 9.2 | |||||||||
| Switching from clopidogrel to prasugrel, % | 1.7 | 0.5 | ||||||||||||
| Switching from clopidogrel to ticagrelor, % | 19.4 | 2.1 | ||||||||||||
| Switching from ticagrelor/prasugrel to clopidogrel, % | 3.3 | 0 | ||||||||||||
| Aspirin (ASA), % | 1.3 | 98.5 | ||||||||||||
| GPIIb/IIIa inhibitors, % | 5.4 | 19.5 | ||||||||||||
| Unfractionated heparin, % | 27 | 21.8 | 16.9 | 14.5 | 56.6 | 95.2 | ||||||||
| Low molecular weight heparin, % | 69.4 | 72.8 | 78.3 | 73.7 | 2 | 5.6 | ||||||||
| Fondaparinux, % | 7.1 | 7.8 | 12.4 | 11.4 | 0.3 | 5.2 | ||||||||
| (III) Information on treatment at hospital discharge (D)/after hospital discharge (after) | D | After | D | After | D | After | D | After | D | After | D | After | ||
| Patients with available data at these 2 time points, n | 1827 | 1948 | 1945 | 1972 | 52319 | 931 | ||||||||
| Clopidogrel treatment at discharge/after discharge, % | 63 | 64.1 | ||||||||||||
| Prasugrel treatment at discharge/after discharge, % | 64.1 | 67.7 | 65.7 | 55.9 | 0.9 | 14.5 | ||||||||
| Ticagrelor treatment at discharge/after discharge, % | 35.7 | 32.3 | 34.2 | 29.8 | 19.2 | 9.8 | ||||||||
F, female; M, male.
Characterization of the non-ST-elevation acute coronary syndrome cohorts
Total patient numbers in the different registries ranged between 586 (CZECH-2) and 52 319 (SCAAR). Mean patient age in the registries varied between 65 (AAPCI, SPUM, Newcastle) and 70 years (CZECH-2), with the other registries in between. Males were more frequent than females in all registries. Diabetes mellitus was frequently noted as a co-morbidity, in the range of 18.9 (Newcastle 2010) to 40.5% (CZECH-2). The prevalence of previously diagnosed coronary artery disease (CAD) varied substantially from 28.6 (SCAAR) to 100% [ATACS, with this rate due to the fact that CAD was an inclusion criterion] and prior MI rates ranged from 17 (AAPCI) to 30.2% (Newcastle 2012). Prior stroke ranged from 2.7 (SPUM) to 9.4% (SCAAR).
Chronic aspirin treatment rates at the time of the NSTE-ACS index event refer to a mixed population of individuals who had a first NSTE-ACS event and those who were previously known to have CAD. Only the latter group had an indication for chronic antiplatelet treatment. Unsurprisingly, given the substantial variations in patient characteristics, rates of chronic aspirin treatment as long-term treatment for CAD (unrelated to the index ACS event) varied between 30 (FAST-MI) and 52.8% (ATACS). Chronic treatment with P2Y12 inhibitors was reported in seven registries, with the highest rate seen in ATACS (24.9%).
Pre-hospital use of P2Y12 inhibitors (pre-treatment) was reported in CZECH-2 (14.7% of patients received clopidogrel), FAST-MI 2010 (20% clopidogrel, 1% prasugrel), SCAAR (48.9% clopidogrel, 0.6% prasugrel, 16.8% ticagrelor), and SPUM (14.5% clopidogrel, 0.5% prasugrel, 0.4% ticagrelor).
In hospital, almost all patients received loading doses of P2Y12 inhibitors for the treatment of the index NSTE-ACS event. Switching between drugs, most frequently from clopidogrel to prasugrel, was not widespread (0% in AAPCI to 11% in FAST-MI, for switching from clopidogrel to prasugrel). After the NSTE-ACS event, almost all patients in the registries received aspirin plus a P2Y12 receptor inhibitor (DAPT).
Time from first medical contact to PCI was reported in five registries, ranging from 4.6 (AAPCI/ADAPT) to 27.4 h (FAST-MI 2010). The great majority of patients received coronary angiography (66% in CZECH-2, 79.6% in DIOCLES, and 100% in AAPCI and ATACS, the latter being related to the inclusion criteria), and a substantial proportion received PCI (47% in CZECH-2 to 98.4% in SPUM, the latter being again rather high, mostly related to inclusion criteria). Where reported, radial access for PCI varied between 26.5 (ATACS) to 81–83% (Newcastle).
Outcomes
For various ischaemic and bleeding outcomes, event rates are presented descriptively for the NSTE-ACS cohort in total (Table 2) and by P2Y12 inhibitor (Table 3). Further, they are plotted against mean age of the patients in the various registries (Figure 2 and bubble plots in the Supplementary material online).
Endpoints in the total non-ST-segment elevation acute coronary syndrome cohorts
| . | AAPCI/ADAPT . | AMIS Plus . | ATACS . | BLITZ-4 . | CZECH-2 . | DIOCLES . | FAST-MI 2010 . | Newcastle . | Newcastle 2010 . | Newcastle 2011 . | Newcastle 2012 . | Newcastle 2013 . | SCAAR . | SPUM . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All-cause death | ||||||||||||||
| In-hospital | 2.8 | 2.41 | 1.65 | 2.08 | 4.79 | 2.94 | 2.49 | 1.07 | 1.18 | 1.2 | 0.76 | 1.14 | 1.15 | 0.97 |
| 30 days | 3.23 | 6.65 | 3.66 | 2.96 | 1.76 | 1.61 | ||||||||
| 180 days | 7.16 | 3.66 | ||||||||||||
| 1 year | 3.14 | 9.73 | 5.26 | 4.55 | ||||||||||
| CV death | ||||||||||||||
| In-hospital | 1.28 | 0.97 | ||||||||||||
| 30 days | 1.5 | |||||||||||||
| 180 days | ||||||||||||||
| 1 year | 3.25 | |||||||||||||
| CV events | ||||||||||||||
| In-hospital | 0.6 | 2.04 | ||||||||||||
| 30 days | 2.26 | |||||||||||||
| 180 days | ||||||||||||||
| 1 year | 4.23 | 9.63 | ||||||||||||
| Stroke | ||||||||||||||
| In-hospital | 0.32 | 0.49 | 0.21 | 0.58 | 0 | 0.79 | 0.11 | 0.21 | ||||||
| 30 days | 1.13 | 0.18 | 0.29 | 0.43 | ||||||||||
| 180 days | 1.11 | 0.98 | ||||||||||||
| 1 year | 1.52 | 1.19 | ||||||||||||
| Recurrent MI | ||||||||||||||
| In-hospital | 0.28 | 0.56 | 0.18 | 0.97 | 0.68 | 2.77 | 1.27 | 0.86 | ||||||
| 30 days | 0.72 | 0.9 | 5.43 | 1.07 | ||||||||||
| 180 days | 3.96 | 8.28 | ||||||||||||
| 1 year | 3.55 | 9.78 | 3.57 | |||||||||||
| Repeat PCI | ||||||||||||||
| In-hospital | 8.3 | 4.31 | 0.17 | 0.86 | ||||||||||
| 30 days | 1.29 | |||||||||||||
| 180 days | ||||||||||||||
| 1 year | 5.74 | |||||||||||||
| Fatal/life-threating bleeding | ||||||||||||||
| In-hospital | 0.02 | 0 | 0.01 | 0 | ||||||||||
| 30 days | 0.11 | |||||||||||||
| 180 days | ||||||||||||||
| 1 year | 2.06 | |||||||||||||
| Major bleeding | ||||||||||||||
| In-hospital | 1.56 | 0.94 | 0.68 | 2.77 | 1.83 | 0.96 | 0 | |||||||
| 30 days | 1.08 | 0 | ||||||||||||
| 180 days | ||||||||||||||
| 1 year | 2.06 | |||||||||||||
| Minor bleeding | ||||||||||||||
| In-hospital | 2.27 | 0.21 | ||||||||||||
| 30 days | 0.21 | |||||||||||||
| 180 days | ||||||||||||||
| 1 year | 4.44 | |||||||||||||
| . | AAPCI/ADAPT . | AMIS Plus . | ATACS . | BLITZ-4 . | CZECH-2 . | DIOCLES . | FAST-MI 2010 . | Newcastle . | Newcastle 2010 . | Newcastle 2011 . | Newcastle 2012 . | Newcastle 2013 . | SCAAR . | SPUM . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All-cause death | ||||||||||||||
| In-hospital | 2.8 | 2.41 | 1.65 | 2.08 | 4.79 | 2.94 | 2.49 | 1.07 | 1.18 | 1.2 | 0.76 | 1.14 | 1.15 | 0.97 |
| 30 days | 3.23 | 6.65 | 3.66 | 2.96 | 1.76 | 1.61 | ||||||||
| 180 days | 7.16 | 3.66 | ||||||||||||
| 1 year | 3.14 | 9.73 | 5.26 | 4.55 | ||||||||||
| CV death | ||||||||||||||
| In-hospital | 1.28 | 0.97 | ||||||||||||
| 30 days | 1.5 | |||||||||||||
| 180 days | ||||||||||||||
| 1 year | 3.25 | |||||||||||||
| CV events | ||||||||||||||
| In-hospital | 0.6 | 2.04 | ||||||||||||
| 30 days | 2.26 | |||||||||||||
| 180 days | ||||||||||||||
| 1 year | 4.23 | 9.63 | ||||||||||||
| Stroke | ||||||||||||||
| In-hospital | 0.32 | 0.49 | 0.21 | 0.58 | 0 | 0.79 | 0.11 | 0.21 | ||||||
| 30 days | 1.13 | 0.18 | 0.29 | 0.43 | ||||||||||
| 180 days | 1.11 | 0.98 | ||||||||||||
| 1 year | 1.52 | 1.19 | ||||||||||||
| Recurrent MI | ||||||||||||||
| In-hospital | 0.28 | 0.56 | 0.18 | 0.97 | 0.68 | 2.77 | 1.27 | 0.86 | ||||||
| 30 days | 0.72 | 0.9 | 5.43 | 1.07 | ||||||||||
| 180 days | 3.96 | 8.28 | ||||||||||||
| 1 year | 3.55 | 9.78 | 3.57 | |||||||||||
| Repeat PCI | ||||||||||||||
| In-hospital | 8.3 | 4.31 | 0.17 | 0.86 | ||||||||||
| 30 days | 1.29 | |||||||||||||
| 180 days | ||||||||||||||
| 1 year | 5.74 | |||||||||||||
| Fatal/life-threating bleeding | ||||||||||||||
| In-hospital | 0.02 | 0 | 0.01 | 0 | ||||||||||
| 30 days | 0.11 | |||||||||||||
| 180 days | ||||||||||||||
| 1 year | 2.06 | |||||||||||||
| Major bleeding | ||||||||||||||
| In-hospital | 1.56 | 0.94 | 0.68 | 2.77 | 1.83 | 0.96 | 0 | |||||||
| 30 days | 1.08 | 0 | ||||||||||||
| 180 days | ||||||||||||||
| 1 year | 2.06 | |||||||||||||
| Minor bleeding | ||||||||||||||
| In-hospital | 2.27 | 0.21 | ||||||||||||
| 30 days | 0.21 | |||||||||||||
| 180 days | ||||||||||||||
| 1 year | 4.44 | |||||||||||||
Numbers show the incidence rates of various effectiveness and safety (bleeding) outcomes at various time points, in the total NSTE-ACS populations in each study (across treatments). Empty fields show that the respective parameter has not been collected at this time point in a given registry. No summary statistics across all studies were generated.
Endpoints in the total non-ST-segment elevation acute coronary syndrome cohorts
| . | AAPCI/ADAPT . | AMIS Plus . | ATACS . | BLITZ-4 . | CZECH-2 . | DIOCLES . | FAST-MI 2010 . | Newcastle . | Newcastle 2010 . | Newcastle 2011 . | Newcastle 2012 . | Newcastle 2013 . | SCAAR . | SPUM . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All-cause death | ||||||||||||||
| In-hospital | 2.8 | 2.41 | 1.65 | 2.08 | 4.79 | 2.94 | 2.49 | 1.07 | 1.18 | 1.2 | 0.76 | 1.14 | 1.15 | 0.97 |
| 30 days | 3.23 | 6.65 | 3.66 | 2.96 | 1.76 | 1.61 | ||||||||
| 180 days | 7.16 | 3.66 | ||||||||||||
| 1 year | 3.14 | 9.73 | 5.26 | 4.55 | ||||||||||
| CV death | ||||||||||||||
| In-hospital | 1.28 | 0.97 | ||||||||||||
| 30 days | 1.5 | |||||||||||||
| 180 days | ||||||||||||||
| 1 year | 3.25 | |||||||||||||
| CV events | ||||||||||||||
| In-hospital | 0.6 | 2.04 | ||||||||||||
| 30 days | 2.26 | |||||||||||||
| 180 days | ||||||||||||||
| 1 year | 4.23 | 9.63 | ||||||||||||
| Stroke | ||||||||||||||
| In-hospital | 0.32 | 0.49 | 0.21 | 0.58 | 0 | 0.79 | 0.11 | 0.21 | ||||||
| 30 days | 1.13 | 0.18 | 0.29 | 0.43 | ||||||||||
| 180 days | 1.11 | 0.98 | ||||||||||||
| 1 year | 1.52 | 1.19 | ||||||||||||
| Recurrent MI | ||||||||||||||
| In-hospital | 0.28 | 0.56 | 0.18 | 0.97 | 0.68 | 2.77 | 1.27 | 0.86 | ||||||
| 30 days | 0.72 | 0.9 | 5.43 | 1.07 | ||||||||||
| 180 days | 3.96 | 8.28 | ||||||||||||
| 1 year | 3.55 | 9.78 | 3.57 | |||||||||||
| Repeat PCI | ||||||||||||||
| In-hospital | 8.3 | 4.31 | 0.17 | 0.86 | ||||||||||
| 30 days | 1.29 | |||||||||||||
| 180 days | ||||||||||||||
| 1 year | 5.74 | |||||||||||||
| Fatal/life-threating bleeding | ||||||||||||||
| In-hospital | 0.02 | 0 | 0.01 | 0 | ||||||||||
| 30 days | 0.11 | |||||||||||||
| 180 days | ||||||||||||||
| 1 year | 2.06 | |||||||||||||
| Major bleeding | ||||||||||||||
| In-hospital | 1.56 | 0.94 | 0.68 | 2.77 | 1.83 | 0.96 | 0 | |||||||
| 30 days | 1.08 | 0 | ||||||||||||
| 180 days | ||||||||||||||
| 1 year | 2.06 | |||||||||||||
| Minor bleeding | ||||||||||||||
| In-hospital | 2.27 | 0.21 | ||||||||||||
| 30 days | 0.21 | |||||||||||||
| 180 days | ||||||||||||||
| 1 year | 4.44 | |||||||||||||
| . | AAPCI/ADAPT . | AMIS Plus . | ATACS . | BLITZ-4 . | CZECH-2 . | DIOCLES . | FAST-MI 2010 . | Newcastle . | Newcastle 2010 . | Newcastle 2011 . | Newcastle 2012 . | Newcastle 2013 . | SCAAR . | SPUM . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All-cause death | ||||||||||||||
| In-hospital | 2.8 | 2.41 | 1.65 | 2.08 | 4.79 | 2.94 | 2.49 | 1.07 | 1.18 | 1.2 | 0.76 | 1.14 | 1.15 | 0.97 |
| 30 days | 3.23 | 6.65 | 3.66 | 2.96 | 1.76 | 1.61 | ||||||||
| 180 days | 7.16 | 3.66 | ||||||||||||
| 1 year | 3.14 | 9.73 | 5.26 | 4.55 | ||||||||||
| CV death | ||||||||||||||
| In-hospital | 1.28 | 0.97 | ||||||||||||
| 30 days | 1.5 | |||||||||||||
| 180 days | ||||||||||||||
| 1 year | 3.25 | |||||||||||||
| CV events | ||||||||||||||
| In-hospital | 0.6 | 2.04 | ||||||||||||
| 30 days | 2.26 | |||||||||||||
| 180 days | ||||||||||||||
| 1 year | 4.23 | 9.63 | ||||||||||||
| Stroke | ||||||||||||||
| In-hospital | 0.32 | 0.49 | 0.21 | 0.58 | 0 | 0.79 | 0.11 | 0.21 | ||||||
| 30 days | 1.13 | 0.18 | 0.29 | 0.43 | ||||||||||
| 180 days | 1.11 | 0.98 | ||||||||||||
| 1 year | 1.52 | 1.19 | ||||||||||||
| Recurrent MI | ||||||||||||||
| In-hospital | 0.28 | 0.56 | 0.18 | 0.97 | 0.68 | 2.77 | 1.27 | 0.86 | ||||||
| 30 days | 0.72 | 0.9 | 5.43 | 1.07 | ||||||||||
| 180 days | 3.96 | 8.28 | ||||||||||||
| 1 year | 3.55 | 9.78 | 3.57 | |||||||||||
| Repeat PCI | ||||||||||||||
| In-hospital | 8.3 | 4.31 | 0.17 | 0.86 | ||||||||||
| 30 days | 1.29 | |||||||||||||
| 180 days | ||||||||||||||
| 1 year | 5.74 | |||||||||||||
| Fatal/life-threating bleeding | ||||||||||||||
| In-hospital | 0.02 | 0 | 0.01 | 0 | ||||||||||
| 30 days | 0.11 | |||||||||||||
| 180 days | ||||||||||||||
| 1 year | 2.06 | |||||||||||||
| Major bleeding | ||||||||||||||
| In-hospital | 1.56 | 0.94 | 0.68 | 2.77 | 1.83 | 0.96 | 0 | |||||||
| 30 days | 1.08 | 0 | ||||||||||||
| 180 days | ||||||||||||||
| 1 year | 2.06 | |||||||||||||
| Minor bleeding | ||||||||||||||
| In-hospital | 2.27 | 0.21 | ||||||||||||
| 30 days | 0.21 | |||||||||||||
| 180 days | ||||||||||||||
| 1 year | 4.44 | |||||||||||||
Numbers show the incidence rates of various effectiveness and safety (bleeding) outcomes at various time points, in the total NSTE-ACS populations in each study (across treatments). Empty fields show that the respective parameter has not been collected at this time point in a given registry. No summary statistics across all studies were generated.
Endpoints in the non-ST-segment elevation acute coronary syndrome cohorts by P2Y12 receptor inhibitor-based dual antiplatelet therapy
| Treatment . | AAPCI/ADAPT . | AMIS plus . | ATACS . | DIOCLES . | SCAAR . | SPUM . | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | P . | T . | C . | P . | T . | C . | P . | T . | C . | P . | T . | C . | P . | T . | C . | P . | T . | C . |
| All-cause death | ||||||||||||||||||
| In-hospital | 1.08 | 2.11 | 2.22 | 1.64 | 1.31 | 3.05 | 1.18 | 1.68 | 2.22 | 1.24 | 0.94 | 0.93 | ||||||
| 30 days | 3.02 | 1.46 | 1.26 | 1.45 | 0 | 1.01 | ||||||||||||
| 180 days | 6.3 | 2.58 | 2.57 | 3.02 | ||||||||||||||
| 1 year | 0.61 | 2.38 | 3.72 | 3.37 | 3.39 | 4.5 | 0 | 4.22 | ||||||||||
| CV death | ||||||||||||||||||
| In-hospital | 1.06 | 0.56 | 1.57 | |||||||||||||||
| 30 days | 0 | 0.34 | ||||||||||||||||
| 180 days | ||||||||||||||||||
| 1 year | 0.61 | 0 | 2.05 | 0 | 1.86 | |||||||||||||
| CV events | ||||||||||||||||||
| In-hospital | 1.08 | 0.5 | 0.55 | 2.22 | 0.67 | |||||||||||||
| 30 days | 2.96 | 1.68 | ||||||||||||||||
| 180 days | ||||||||||||||||||
| 1 year | 5.93 | 8.78 | ||||||||||||||||
| Stroke | ||||||||||||||||||
| In-hospital | 0.54 | 0.37 | 0.22 | 0.23 | 0.44 | 0.58 | 0.08 | 0.24 | 0.72 | 0 | 0.17 | |||||||
| 30 days | 0.22 | 0.04 | 0.29 | 0 | 0.34 | |||||||||||||
| 180 days | 0.94 | 0.45 | 0.27 | 1.01 | ||||||||||||||
| 1 year | 5.14 | 1.01 | 0.4 | 1.53 | 0 | 1.18 | ||||||||||||
| Recurrent MI | ||||||||||||||||||
| In-hospital | 0.54 | 0.12 | 0.33 | 0.7 | 0.37 | 0.62 | 0.24 | 0.17 | 2.8 | 2.22 | 0.34 | |||||||
| 30 days | 6.97 | 1.64 | 5.91 | 2.22 | 0.5 | |||||||||||||
| 180 days | 4.02 | 8.76 | 2.49 | 9.54 | ||||||||||||||
| 1 year | 6.13 | 1.85 | 3.24 | 11.24 | 2.98 | 11.55 | 1.48 | 3.04 | ||||||||||
| Repeat PCI | ||||||||||||||||||
| In-hospital | 9.19 | 8.67 | 7.98 | 4.87 | 4.12 | 1.48 | 0.5 | |||||||||||
| 30 days | 2.22 | 1.01 | ||||||||||||||||
| 180 days | ||||||||||||||||||
| 1 year | 2.96 | 5.74 | ||||||||||||||||
| Fatal/life-threating bleeding | ||||||||||||||||||
| In-hospital | 0 | 0.06 | 0 | 0 | 0.02 | 0 | 0 | 0 | ||||||||||
| 30 days | 0 | 0 | ||||||||||||||||
| 180 days | ||||||||||||||||||
| 1 year | 0 | 1.52 | ||||||||||||||||
| Major bleeding | ||||||||||||||||||
| In-hospital | 2.16 | 1.36 | 1.22 | 0.63 | 1.03 | 2.93 | 0.45 | 0.84 | 0.94 | 0 | 0 | |||||||
| 30 days | 0 | 0 | ||||||||||||||||
| 180 days | ||||||||||||||||||
| 1 year | 0.74 | 1.35 | ||||||||||||||||
| Minor bleeding | ||||||||||||||||||
| In-hospital | 0 | 0.17 | ||||||||||||||||
| 30 days | 0 | 0.17 | ||||||||||||||||
| 180 days | ||||||||||||||||||
| 1 year | 2.22 | 5.07 | ||||||||||||||||
| Treatment . | AAPCI/ADAPT . | AMIS plus . | ATACS . | DIOCLES . | SCAAR . | SPUM . | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | P . | T . | C . | P . | T . | C . | P . | T . | C . | P . | T . | C . | P . | T . | C . | P . | T . | C . |
| All-cause death | ||||||||||||||||||
| In-hospital | 1.08 | 2.11 | 2.22 | 1.64 | 1.31 | 3.05 | 1.18 | 1.68 | 2.22 | 1.24 | 0.94 | 0.93 | ||||||
| 30 days | 3.02 | 1.46 | 1.26 | 1.45 | 0 | 1.01 | ||||||||||||
| 180 days | 6.3 | 2.58 | 2.57 | 3.02 | ||||||||||||||
| 1 year | 0.61 | 2.38 | 3.72 | 3.37 | 3.39 | 4.5 | 0 | 4.22 | ||||||||||
| CV death | ||||||||||||||||||
| In-hospital | 1.06 | 0.56 | 1.57 | |||||||||||||||
| 30 days | 0 | 0.34 | ||||||||||||||||
| 180 days | ||||||||||||||||||
| 1 year | 0.61 | 0 | 2.05 | 0 | 1.86 | |||||||||||||
| CV events | ||||||||||||||||||
| In-hospital | 1.08 | 0.5 | 0.55 | 2.22 | 0.67 | |||||||||||||
| 30 days | 2.96 | 1.68 | ||||||||||||||||
| 180 days | ||||||||||||||||||
| 1 year | 5.93 | 8.78 | ||||||||||||||||
| Stroke | ||||||||||||||||||
| In-hospital | 0.54 | 0.37 | 0.22 | 0.23 | 0.44 | 0.58 | 0.08 | 0.24 | 0.72 | 0 | 0.17 | |||||||
| 30 days | 0.22 | 0.04 | 0.29 | 0 | 0.34 | |||||||||||||
| 180 days | 0.94 | 0.45 | 0.27 | 1.01 | ||||||||||||||
| 1 year | 5.14 | 1.01 | 0.4 | 1.53 | 0 | 1.18 | ||||||||||||
| Recurrent MI | ||||||||||||||||||
| In-hospital | 0.54 | 0.12 | 0.33 | 0.7 | 0.37 | 0.62 | 0.24 | 0.17 | 2.8 | 2.22 | 0.34 | |||||||
| 30 days | 6.97 | 1.64 | 5.91 | 2.22 | 0.5 | |||||||||||||
| 180 days | 4.02 | 8.76 | 2.49 | 9.54 | ||||||||||||||
| 1 year | 6.13 | 1.85 | 3.24 | 11.24 | 2.98 | 11.55 | 1.48 | 3.04 | ||||||||||
| Repeat PCI | ||||||||||||||||||
| In-hospital | 9.19 | 8.67 | 7.98 | 4.87 | 4.12 | 1.48 | 0.5 | |||||||||||
| 30 days | 2.22 | 1.01 | ||||||||||||||||
| 180 days | ||||||||||||||||||
| 1 year | 2.96 | 5.74 | ||||||||||||||||
| Fatal/life-threating bleeding | ||||||||||||||||||
| In-hospital | 0 | 0.06 | 0 | 0 | 0.02 | 0 | 0 | 0 | ||||||||||
| 30 days | 0 | 0 | ||||||||||||||||
| 180 days | ||||||||||||||||||
| 1 year | 0 | 1.52 | ||||||||||||||||
| Major bleeding | ||||||||||||||||||
| In-hospital | 2.16 | 1.36 | 1.22 | 0.63 | 1.03 | 2.93 | 0.45 | 0.84 | 0.94 | 0 | 0 | |||||||
| 30 days | 0 | 0 | ||||||||||||||||
| 180 days | ||||||||||||||||||
| 1 year | 0.74 | 1.35 | ||||||||||||||||
| Minor bleeding | ||||||||||||||||||
| In-hospital | 0 | 0.17 | ||||||||||||||||
| 30 days | 0 | 0.17 | ||||||||||||||||
| 180 days | ||||||||||||||||||
| 1 year | 2.22 | 5.07 | ||||||||||||||||
Numbers show the incidence rates of various effectiveness and safety (bleeding) outcomes at various time points, for prasugrel (P), ticagrelor (T), and clopidogrel (C). Empty fields show that the respective parameter has not been collected at this time point. No summary statistics across all studies were generated.
Endpoints in the non-ST-segment elevation acute coronary syndrome cohorts by P2Y12 receptor inhibitor-based dual antiplatelet therapy
| Treatment . | AAPCI/ADAPT . | AMIS plus . | ATACS . | DIOCLES . | SCAAR . | SPUM . | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | P . | T . | C . | P . | T . | C . | P . | T . | C . | P . | T . | C . | P . | T . | C . | P . | T . | C . |
| All-cause death | ||||||||||||||||||
| In-hospital | 1.08 | 2.11 | 2.22 | 1.64 | 1.31 | 3.05 | 1.18 | 1.68 | 2.22 | 1.24 | 0.94 | 0.93 | ||||||
| 30 days | 3.02 | 1.46 | 1.26 | 1.45 | 0 | 1.01 | ||||||||||||
| 180 days | 6.3 | 2.58 | 2.57 | 3.02 | ||||||||||||||
| 1 year | 0.61 | 2.38 | 3.72 | 3.37 | 3.39 | 4.5 | 0 | 4.22 | ||||||||||
| CV death | ||||||||||||||||||
| In-hospital | 1.06 | 0.56 | 1.57 | |||||||||||||||
| 30 days | 0 | 0.34 | ||||||||||||||||
| 180 days | ||||||||||||||||||
| 1 year | 0.61 | 0 | 2.05 | 0 | 1.86 | |||||||||||||
| CV events | ||||||||||||||||||
| In-hospital | 1.08 | 0.5 | 0.55 | 2.22 | 0.67 | |||||||||||||
| 30 days | 2.96 | 1.68 | ||||||||||||||||
| 180 days | ||||||||||||||||||
| 1 year | 5.93 | 8.78 | ||||||||||||||||
| Stroke | ||||||||||||||||||
| In-hospital | 0.54 | 0.37 | 0.22 | 0.23 | 0.44 | 0.58 | 0.08 | 0.24 | 0.72 | 0 | 0.17 | |||||||
| 30 days | 0.22 | 0.04 | 0.29 | 0 | 0.34 | |||||||||||||
| 180 days | 0.94 | 0.45 | 0.27 | 1.01 | ||||||||||||||
| 1 year | 5.14 | 1.01 | 0.4 | 1.53 | 0 | 1.18 | ||||||||||||
| Recurrent MI | ||||||||||||||||||
| In-hospital | 0.54 | 0.12 | 0.33 | 0.7 | 0.37 | 0.62 | 0.24 | 0.17 | 2.8 | 2.22 | 0.34 | |||||||
| 30 days | 6.97 | 1.64 | 5.91 | 2.22 | 0.5 | |||||||||||||
| 180 days | 4.02 | 8.76 | 2.49 | 9.54 | ||||||||||||||
| 1 year | 6.13 | 1.85 | 3.24 | 11.24 | 2.98 | 11.55 | 1.48 | 3.04 | ||||||||||
| Repeat PCI | ||||||||||||||||||
| In-hospital | 9.19 | 8.67 | 7.98 | 4.87 | 4.12 | 1.48 | 0.5 | |||||||||||
| 30 days | 2.22 | 1.01 | ||||||||||||||||
| 180 days | ||||||||||||||||||
| 1 year | 2.96 | 5.74 | ||||||||||||||||
| Fatal/life-threating bleeding | ||||||||||||||||||
| In-hospital | 0 | 0.06 | 0 | 0 | 0.02 | 0 | 0 | 0 | ||||||||||
| 30 days | 0 | 0 | ||||||||||||||||
| 180 days | ||||||||||||||||||
| 1 year | 0 | 1.52 | ||||||||||||||||
| Major bleeding | ||||||||||||||||||
| In-hospital | 2.16 | 1.36 | 1.22 | 0.63 | 1.03 | 2.93 | 0.45 | 0.84 | 0.94 | 0 | 0 | |||||||
| 30 days | 0 | 0 | ||||||||||||||||
| 180 days | ||||||||||||||||||
| 1 year | 0.74 | 1.35 | ||||||||||||||||
| Minor bleeding | ||||||||||||||||||
| In-hospital | 0 | 0.17 | ||||||||||||||||
| 30 days | 0 | 0.17 | ||||||||||||||||
| 180 days | ||||||||||||||||||
| 1 year | 2.22 | 5.07 | ||||||||||||||||
| Treatment . | AAPCI/ADAPT . | AMIS plus . | ATACS . | DIOCLES . | SCAAR . | SPUM . | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | P . | T . | C . | P . | T . | C . | P . | T . | C . | P . | T . | C . | P . | T . | C . | P . | T . | C . |
| All-cause death | ||||||||||||||||||
| In-hospital | 1.08 | 2.11 | 2.22 | 1.64 | 1.31 | 3.05 | 1.18 | 1.68 | 2.22 | 1.24 | 0.94 | 0.93 | ||||||
| 30 days | 3.02 | 1.46 | 1.26 | 1.45 | 0 | 1.01 | ||||||||||||
| 180 days | 6.3 | 2.58 | 2.57 | 3.02 | ||||||||||||||
| 1 year | 0.61 | 2.38 | 3.72 | 3.37 | 3.39 | 4.5 | 0 | 4.22 | ||||||||||
| CV death | ||||||||||||||||||
| In-hospital | 1.06 | 0.56 | 1.57 | |||||||||||||||
| 30 days | 0 | 0.34 | ||||||||||||||||
| 180 days | ||||||||||||||||||
| 1 year | 0.61 | 0 | 2.05 | 0 | 1.86 | |||||||||||||
| CV events | ||||||||||||||||||
| In-hospital | 1.08 | 0.5 | 0.55 | 2.22 | 0.67 | |||||||||||||
| 30 days | 2.96 | 1.68 | ||||||||||||||||
| 180 days | ||||||||||||||||||
| 1 year | 5.93 | 8.78 | ||||||||||||||||
| Stroke | ||||||||||||||||||
| In-hospital | 0.54 | 0.37 | 0.22 | 0.23 | 0.44 | 0.58 | 0.08 | 0.24 | 0.72 | 0 | 0.17 | |||||||
| 30 days | 0.22 | 0.04 | 0.29 | 0 | 0.34 | |||||||||||||
| 180 days | 0.94 | 0.45 | 0.27 | 1.01 | ||||||||||||||
| 1 year | 5.14 | 1.01 | 0.4 | 1.53 | 0 | 1.18 | ||||||||||||
| Recurrent MI | ||||||||||||||||||
| In-hospital | 0.54 | 0.12 | 0.33 | 0.7 | 0.37 | 0.62 | 0.24 | 0.17 | 2.8 | 2.22 | 0.34 | |||||||
| 30 days | 6.97 | 1.64 | 5.91 | 2.22 | 0.5 | |||||||||||||
| 180 days | 4.02 | 8.76 | 2.49 | 9.54 | ||||||||||||||
| 1 year | 6.13 | 1.85 | 3.24 | 11.24 | 2.98 | 11.55 | 1.48 | 3.04 | ||||||||||
| Repeat PCI | ||||||||||||||||||
| In-hospital | 9.19 | 8.67 | 7.98 | 4.87 | 4.12 | 1.48 | 0.5 | |||||||||||
| 30 days | 2.22 | 1.01 | ||||||||||||||||
| 180 days | ||||||||||||||||||
| 1 year | 2.96 | 5.74 | ||||||||||||||||
| Fatal/life-threating bleeding | ||||||||||||||||||
| In-hospital | 0 | 0.06 | 0 | 0 | 0.02 | 0 | 0 | 0 | ||||||||||
| 30 days | 0 | 0 | ||||||||||||||||
| 180 days | ||||||||||||||||||
| 1 year | 0 | 1.52 | ||||||||||||||||
| Major bleeding | ||||||||||||||||||
| In-hospital | 2.16 | 1.36 | 1.22 | 0.63 | 1.03 | 2.93 | 0.45 | 0.84 | 0.94 | 0 | 0 | |||||||
| 30 days | 0 | 0 | ||||||||||||||||
| 180 days | ||||||||||||||||||
| 1 year | 0.74 | 1.35 | ||||||||||||||||
| Minor bleeding | ||||||||||||||||||
| In-hospital | 0 | 0.17 | ||||||||||||||||
| 30 days | 0 | 0.17 | ||||||||||||||||
| 180 days | ||||||||||||||||||
| 1 year | 2.22 | 5.07 | ||||||||||||||||
Numbers show the incidence rates of various effectiveness and safety (bleeding) outcomes at various time points, for prasugrel (P), ticagrelor (T), and clopidogrel (C). Empty fields show that the respective parameter has not been collected at this time point. No summary statistics across all studies were generated.
Ischaemic outcomes
All-cause death rates were from 0.76 (Newcastle 2012) to 4.79% (CZECH-2) in-hospital, based on data from 84 053 patients for this time point; from 1.61 (SPUM) to 6.65% (CZECH-2) at 30 days; from 3.66 (SCAAR) to 7.16% (DIOCLES) at 180 days; and from 3.14 (AMIS Plus) to 9.73% (FAST-MI 2010) at 1 year (Figure 1).
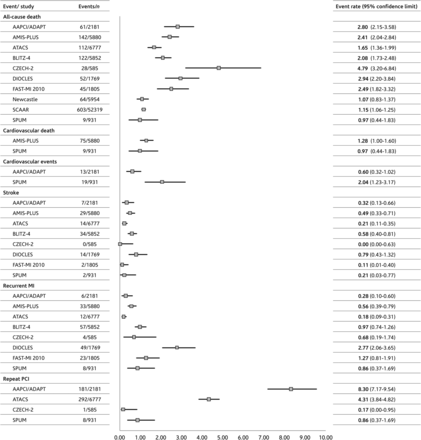
The column on the left displays the endpoints and the registries with available data in the non-ST-segment elevation acute coronary syndrome cohort for the respective endpoint at the end of hospitalization period. The column ‘Events/n’ shows the number of events and the number of patients in the non-ST-segment elevation acute coronary syndrome cohort (denominator). The column ‘Event rate (95% confidence interval)’ provides the underlying data for the graph. Boxes in the graph visualize the event rate, and the horizontal lines the 95% confidence intervals.
Cardiovascular death rates were reported only in two registries: they were 0.97 (SPUM) and 1.28% (AMIS-Plus) in-hospital; 1.5% at 30 days (data from SPUM only); and 3.25% (data from SPUM only) at 1 year.
For cardiovascular non-fatal ischaemic events, rates were 0.6% (AAPCI/ADAPT) and 2.04% (SPUM) in-hospital; 2.26% (SPUM data only) at 30 days; and 4.23 (AMIS-Plus) and 9.63% (SPUM) at 1 year.
Stroke events were reported in all registries with the exception of the Newcastle registry. They ranged from 0 (CZECH-2) to 0.79% (DIOCLES) in-hospital. Post-discharge stroke events ranged from 0.18 (CZECH-2) to 1.13% (BLITZ-4) at 30 days; were 0.98 (SCAAR) and 1.11% (DIOCLES) at 180 days; and were 1.19 (SPUM) and 1.52% (SCAAR) at 1 year.
Recurrent in-hospital MI reported by eight registries ranged between 0.18 (ATACS) and 2.77% (DIOCLES). After discharge, the recurrent MI rate was between 0.72 (BLITZ-4) and 5.43% (SCAAR) at 30 days; 3.96 (DIOCLES) and 8.28% (SCAAR) at 180 days; and 3.55 (AMIS Plus) and 9.78% (SCAAR) at 1 year (no 1-year data from other registries were available).
Repeat PCI rates varied widely between 0.17 (CZECH-2) and 8.3% (AAPCI/ADAPT) in-hospital; 1.29% at 30 days (SPUM, no data from other registries available); and 5.74% at 1 year (SPUM, no data from other registries available). No data for repeated PCI were available at 180 days from any registry.
Outcomes by dual antiplatelet therapy
Ischaemic endpoints according to the use of the three P2Y12 inhibitors are displayed in Figure 2. Data from 3199 patients on prasugrel, 36 336 on clopidogrel, and 11 906 on ticagrelor were available for the analysis of all-cause death in hospital.

The graphs show the unadjusted event rate (%) on the y-axis and the mean patient age on the x-axis. Each bubble represents a P2Y12 group (green, prasugrel; blue, clopidogrel; pink, ticagrelor) of the named registry, and the size of the bubbles visualize the patient number of the P2Y12 group. The patient number of each treatment group and further demographic and treatment information is shown in Supplementary material online, Table S1. In the analysis by dual antiplatelet therapy group, patients in the ticagrelor group were substantially older than those in the prasugrel group, and those in the clopidogrel group were even older.
The univariate analyses showed that patients on prasugrel, besides being younger, also had lower event rates compared with those on ticagrelor and, to an even greater extent, those on clopidogrel.
The named figures in this manuscript and additional 28 bubble plot graphs in the Supplementary material online display the various ischaemic outcomes at the different time points.
Bleeding
The studies used various bleeding definitions: AAPCI, CZECH-2, and FAST-MI used the definition of TIMI,5 and, after 2012, AMIS-Plus used BARC.6 ATACS used the definition of GUSTO,7 and the other registries used unspecified or proprietary definitions as displayed in Table 1. Overall, the data on the various bleeding types and documentation time points were less complete than the data on ischaemic outcomes. FAST-MI 2010, SPUM, and SCAAR were the only registries to report various degrees of bleeding (Tables 2 and 3, bottom), and SPUM was the only registry that reported bleeding event rates beyond the hospitalization phase.
Bleeding events in hospital by endpoint type and registry are summarized in Figure 3. Data on fatal/life-threatening bleeding during hospitalization were available from four studies (AMIS-Plus, SCAAR, SPUM, and FAST-MI 2010). Rates during this in-hospital time frame fell within a narrow range, between 0 (FAST-MI 2010 and SPUM) and 0.02% (AMIS Plus). At 30 days post-discharge, the rate in SPUM (the only study with data for this time frame) was 0.11%, and at 1 year, 2.06% (data from SPUM only; no data at 180 days).
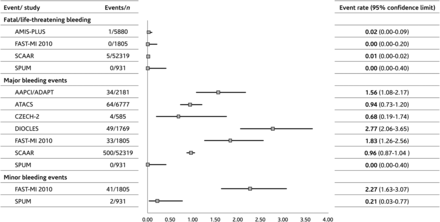
The column on the left displays the endpoints and the registries with available data in the non-ST-segment elevation acute coronary syndrome cohort for the respective endpoint at the end of hospitalization period. The column ‘Events/n’ shows the number of events and the number of patients in the non-ST-segment elevation acute coronary syndrome cohort (denominator). The column ‘Event rate (95% confidence interval)’ provides the underlying data for the graph. Boxes in the graph visualize the event rate, and the horizontal lines the 95% confidence intervals.
For major bleeding events, the database was richer. Seven studies reported major bleeding events in-hospital, which occurred in up to 2.77% of patients (DIOCLES). Rates at 30 days post-discharge were available from only two studies (0% in SPUM and 1.08% in CZECH-2). One-year data were available only for SPUM; the rate was 2.06%.
Minor bleeding events were reported in two studies for the in-hospital period. The minor bleeding rates during this period were 0.21 (SPUM) and 2.27% (FAST-MI 2010). At 30 days, the rate was 0.21% (SPUM), and at 1 year, it was 4.44% (SPUM, no data from other studies were available).
Bleedings outcomes by dual antiplatelet therapy
Bleeding event patterns were inconsistent across registries for the three P2Y12 inhibitors in the incidence of bleeding rates for fatal/life-threatening, major, or minor bleeding in hospital in the univariate analyses. Whereas in AAPCI/ADAPT, the major bleeding rates were highest for prasugrel (2.16%) and lowest for clopidogrel (1.22%); the opposite was found in SCAAR (prasugrel 0.45% vs. clopidogrel 0.94%). In ATACS, major bleeding rates were higher for clopidogrel compared with prasugrel (1.03 vs. 0.63%).
The bubble plot graphs in the Supplementary material online display the various bleeding outcomes at different time points accounting for patient age.
Discussion
The main results of this contemporary review on the characteristics and outcomes of patients with NSTE-ACS treated with DAPT were in line with those for the STEMI cohort.4 Overall, the rates for death and various other ischaemic outcomes, as well as bleeding events, were similar or somewhat higher than those recorded in the Phase III studies of the various P2Y12 inhibitors. There were important differences in use and patient selection between clopidogrel, prasugrel, and ticagrelor, which were associated with differences in ischaemic outcomes. No clear pattern across studies emerged for the P2Y12 receptor inhibitors with regard to bleeding rates for fatal/life-threatening, major, or minor bleeding in hospital.
All registries documented patients on clopidogrel, as expected, since the drug has been in use for 15 years for PCI. Prasugrel was also reported from all registries, although in DIOCLES, the numbers were too low for robust analyses and thus are not reported here. Ticagrelor, as it was introduced into clinical practice most recently, was documented only in a limited number of registries (in AAPCI, AMIS-Plus, and SCAAR).
Use of P2Y12 receptor inhibitors in certain patient groups
The baseline characteristics of patients in the various registries suggest that the product labelling for individual P2Y12 inhibitors is closely followed. Prasugrel was predominantly used in younger patients when compared with ticagrelor, and patients on clopidogrel formed the oldest population.
Age is a central factor in major cardiovascular risk equations, such as TIMI or GRACE scores, and is closely related to ischaemic and bleeding events in patients with NSTE-ACS.8,9
As younger patients have fewer co-morbidities and are generally less ill or at lower cardiovascular risk, outcomes in the P2Y12 inhibitor subgroups have to be interpreted with great caution if not adjusted for age. Against this background, the PIRAEUS data can be used to obtain a general overview on the current treatment approaches for NSTE in Europe and comparative data within the three DAPT regimens, but not between regimens.
According to the product labelling, ticagrelor should be used with caution in patients with a history of asthma and/or chronic obstructive pulmonary disease (due to a relatively high incidence of dyspnoea) and also in patients with renal impairment (due to creatinine level increases).10 These side effects have not been systematically assessed in the registries contributing to PIRAEUS.
Prasugrel also has a restricted labelling as it is contraindicated in patients with prior transient ischaemic attack (TIA) or stroke. The drug is generally not recommended in elderly patients (≥75 years); however, following individual benefit/risk evaluation, if treatment is considered necessary, a maintenance dose of 5 mg/day should be used after a 60 mg loading dose. Further, in patients with low body weight (<60 kg), a reduced 5 mg maintenance dose should be used.11
Probably owing to these restrictions of the two newer P2Y12 receptor antagonists, clopidogrel was given to the older and sicker population, despite the fact that the ESC NSTE-ACS guidelines overall give preference to prasugrel and ticagrelor.2 The current ESC guidelines provide no recommendation for or against pre-treatment with ticagrelor or clopidogrel, as the optimal timing of administration in NSTE-ACS patients scheduled for an invasive strategy has not been adequately investigated to date with ticagrelor or clopidogrel. Based on the ACCOAST results, pre-treatment with prasugrel is not recommended.1 In ACCOAST, prasugrel given at the time of PCI vs. given as pre-treatment resulted in reduced bleeding complications while anti-ischaemic efficacy was preserved.12
Event rates overall
An obvious finding across the NSTE-ACS cohorts in the described registries was that the event rates shortly after the ACS event, irrespective of event type, were lower compared with those reported in the STEMI cohorts in the same registries (STEMI data were reported elsewhere3,4). For example, while all-cause mortality in hospital in STEMI patients was 5.68% in AAPCI, 4.15% in AMIS-Plus, 5.16% in SCAAR, and 2.01% in SPUM, the corresponding rates in NSTE-ACS patients were 2.8% in AAPCI, 2.41% in AMIS-Plus, 1.15% in SCAAR, and 0.97% in SPUM. This pattern was consistent across studies for the other ischaemic event types and the bleeding events. At 1-year follow-up, the all-cause death rates in FAST-MI in STEMI patients were lower than in NSTE-ACS patients (7.13 vs. 9.73%), but not in SCAAR (9.58 vs. 5.26%) or SPUM (4.89 vs. 4.55%). In SPUM, the only registry that provided data for all ischaemic and bleeding endpoints, the overall event rates in STEMI patients at 1 year did not deviate substantially from those of the NSTE-ACS patients.
Between registries, differences in reported outcomes were profound. The range of all-cause mortality in the in-hospital period varied widely, between 0.76% in Newcastle 2012 and 4.79% in CZECH-2, suggesting a selection bias in some registries. Stroke rates in hospital were in a closer range, between 0% in CZECH-2 and 0.79% in DIOCLES, but for repeat PCI, the differences were enormous, between 0.17% in CZECH-2 and 8.3% in AAPCI. The latter endpoint depends on the setting and the clinical decision rules of the respective centre and is therefore investigator-driven. The CZECH-2 registry differs from most other registries in that there was no centre or patient exclusion (all hospitals participated and documented all eligible patients); thus, patients admitted to resuscitation units or to small community hospitals without the availability of a cardiologist were also included. This might have contributed to the higher event rates.
Overall, across all analysed registries, in-hospital and follow-up mortality rates associated with treatment of NSTE-ACS with PCI were similar or somewhat higher compared with the rates observed in Phase III studies such as TRITON-TIMI 38 and PLATO for the individual drugs. (Those trials reported on 15- and 12-month outcomes (TRITON) or 12-month outcomes (PLATO), which are not available in most registries, so comparisons are difficult.) This finding could be explained by the inclusion of consecutive (less selected) and thus more ill patients in registries when compared with clinical trials.
Bleeding events
Bleeding events were not standardized across registries, and in some registries, the definitions were not given. Indeed, there is a lack of uniformity in bleeding definitions and the timing of reporting among recent ACS and PCI clinical trials and registries,6 and uncritical comparisons of the absolute bleeding rates may be misleading in the interpretation of the safety of the various P2Y12 antagonists. Quinlan et al. listed the following as factors that explain most of the variability in reported bleeding rates: different definitions of major bleeding, timing of reporting the primary outcome of major bleeding, and rates of coronary artery bypass graft (CABG) surgery.13 They illustrated this by comparing the bleeding rates in randomized studies on high-dose clopidogrel (CURRENT 2010), prasugrel (TRITON TIMI-38), and ticagrelor (PLATO) using the same bleeding definition (i.e. TIMI major bleeding) at the same points in time. When restricting the time period to the first 30 days after the ACS event, a time point at which this information was available for all three trials, the TIMI major bleeding rates were 1.0% for prasugrel (vs. 0.9% for clopidogrel) in TRITON-TIMI 38, 1.4% for ticagrelor (vs. 1.0% for clopidogrel) in PLATO, and 0.9% for high-dose clopidogrel (vs. 0.6% for standard-dose clopidogrel) in CURRENT 2010.13
In the registries analysed here, the unadjusted major bleeding rate (in hospital) was lower on prasugrel compared with ticagrelor in SCAAR but, conversely, was higher in AAPCI/ADAPT. In contrast to the findings in AAPCI/ADAPT, in the ATACS, SCAAR, and SPUM registries, bleeding rates in the clopidogrel group were higher compared with the newer P2Y12 receptor inhibitors. The latter finding is in contrast to all major randomized studies that included comparisons between clopidogrel and the newer P2Y12 receptor inhibitors, and is most likely explained by patient selection (older, more co-morbid patients on clopidogrel).13
Limitations
We aimed to provide a very current picture (‘snapshot’) of contemporary treatment patterns and excluded registry data older than 5 years. Thus, we therefore cannot report on secular trends. There were substantial differences between registries in terms of study setting, eligibility of patients, site selection, and definition of endpoints, including bleeding events, which limits the comparability of results. We did not formally assess nor adjust or weigh the risk of bias in the various observational studies (transfer of raw data was not possible due to data protection). Also, we did not perform a formal meta-analysis of the registries on the various endpoints due to large heterogeneity in the settings, patient characteristics, and outcome definitions. Not all of the previously identified as suitable registries3 provided data in the agreed structured format, and therefore such data could not be analysed for the purpose of this paper. Data were not differentiated between NSTEMI and unstable angina (UA), and some registries were limited to NSTEMI. Lost-to-follow-up rates in most registries were high after 30 days follow-up. The statistical handling of such data sets is challenging, as a conservative approach (all lost-to-follow-up cases counted as affected by an event) will dramatically overestimate the incidence of rare events (such as fatal bleeding or death), while another approach that restricts the analysis to those patients who can be followed (alive and able to report events reliably) will underestimate the true event rates.
Conclusions
PIRAEUS provides a comprehensive picture about the actual outcomes of NSTE-ACS patients as they are currently treated under real-life conditions, and thus complements the data from randomized controlled Phase III trials (RCTs) of the various P2Y12 receptor inhibitors. Overall, in the registries, death rates and various other ischaemic outcomes as well as bleeding events were similar or somewhat higher than in the RCTs. This may reflect the fact that consecutive and more ill patients were included in the registries.
Notably, the registries that provided information about NSTE-ACS and UA patients showed considerable differences in setting as well as patient and treatment selection. The ischaemic outcomes for the three P2Y12 inhibitors differed enormously between registries, most likely driven by the differences in patients' baseline characteristics. Interpretation of bleeding rates is difficult given the differences between registries. These differences include, among others, different definitions, different CABG-related interventions, and different femoral/radial access rates.
One important lesson from PIREAUS is that, in future registries, data collection should be performed in a more standardized way with respect to endpoints, definitions, and time points, to enable further robust common analyses.
Supplementary material
Funding
Meetings of the PIRAEUS group, and medical writing of the first draft of the present article by 3P Consulting, Germany, were funded by Daiichi Sankyo GmbH Europe and Eli Lilly.
Conflict of interest: A.B.: consulting fees from AstraZeneca. J.A.B.: consulting fees from AstraZeneca, Bayer, Daiichi-Sankyo, and Menarini. The DIOCLES Registry was funded by an unrestricted research grant from Daiichi-Sankyo to the Spanish Society of Cardiology. A.C.: research grants from Abbott Vascular, Medtronic, Biomenco, and Spanish Society of Cardiology; and consulting/lecturer fees from Abbott Vascular, Medtronic, Boston Scientific, Daiichi-Sankyo, Eli-Lilly, AstraZeneca, Ferrer International, and Menarini. M.J.C.: received honoraria for advisory boards or as speaker/chairman at scientific congresses from the following companies: AstraZeneca, Bayer, Boehringer Ingelheim, Daiichi Sankyo, Eli Lilly, Sanofi, and The Medicines Company. N.D.: received research grants from Amgen, Astra-Zeneca, Bayer, Daiichi-Sankyo, Eli-Lilly, GSK, Merck, Novartis, and Sanofi; and lecture or consulting fees from Amgen, AstraZeneca, Bayer, Bristol-Myers Squibb, Boehringer Ingelheim, Daiichi-Sankyo, Eli-Lilly, GlaxoSmithKline, MSD, Novartis, Novo-Nordisk, Pfizer, Roche, Sanofi, Servier, and The Medicines Company. L.D.L.: received honoraria for advisory boards or as speaker/chairman at scientific congresses from the following companies: AstraZeneca, Bayer, Boehringer Ingelheim, Daiichi Sankyo, Eli Lilly, Menarini, and The Medicines Company. J.D.: consulting and lectures fees from Astra Zeneca, Daiichi-Sankyo, Eli-Lilly, MSD, and Servier. D.E.: lectures fees from Astra Zeneca, Eli-Lilly, and The Medicines Company. P.G.: received fees and honorarium from Daiichi Sankyo, Eli Lilly, Astra Zeneca, Bayer, BMS PFIZER, Boehringer Ingelheim, and The Medicines Company. J.W.J.: received research grants from and/or was speaker (with or without lecture fees) on (CME accredited) meetings sponsored by Amgen, Astellas, Anthera, Astra Zeneca, Bayer, Biotronik, Boston Scientific, Correvio, Daiichi Sankyo, Lilly, Genzyme, Medtronic, Merck-Schering-Plough, Pfizer, Orbus Neich, Novartis, Roche, Servier, Sanofi Aventis, The Medicines Company, the Netherlands Heart Foundation, the Interuniversity Cardiology Institute of the Netherlands, and the European Community Framework KP7 Programme. S.M.K.: received honoraria from Eli Lilly for PIRAEUS meeting. G.L.: received fees from Astra Zeneca, Bristol Myers Squibb, Daiichi Sankyo, Lilly, MSD, Servier, and Pfizer as speaker and advisory boards. M.L.: received fees as speaker or Advisory Board member from Aspen, Astra Zeneca, BMS, Boehringer, Bayer, Daiichi Sankyo, Eli Lilly, Sanofi, and Pfizer. J.L.S.: advisor, honoraria from Astra Zeneca, Lilly-Daichi Sankio, Amgen, Menarini, Berlin Chemie AG, Boehringer Ingelheim, and Bristol-Myers Squibb; and research grants from Astra Zeneca, BMS, Servier, Bayer, and Pfizer. T.F.L.: research grants to the institution from AstraZeneca, Bayer, Biosensors, Biotronik, Boston Scientific, Medtronic, MSD, Roche, and Servier, including lecture fees. C.M.M.: research grants to the institution from Eli Lilly, AstraZeneca, Roche, MSD, Medtronic, St. Jude Medical, Sanofi, and Pfizer; and lecture fees from Eli Lilly, Daiichi-Sankyo, AstraZeneca, Roche, and MSD. G.M.: research grants to the institution or consulting/lecture fees from Acuitude, ADIR, Amgen, AstraZeneca, Bayer, Berlin Chemie AG, Boehringer Ingelheim, Bristol-Myers Squibb, Brigham Women's Hospital, Cardiovascular Research Foundation, Celladon, CME resources, Daiichi-Sankyo, Eli-Lilly, Europa, Fédération Française de Cardiologie, Gilead, Hôpitaux Universitaires Genève, ICAN, Janssen-Cilag, Lead-Up, Medcon International, Menarini, Medtronic, MSD, Pfizer, Recor, Sanofi-Aventis, Stentys, The Medicines Company, TIMI Study Group, Universitat Basel, WebMD, and Zoll Medical. F.W.: speakers honoraria and consultancy fees from Astra Zeneca, Lilly, Daiichi Sankyo, BMS, and Pfizer. C.F.M.W.: participated in Advisory Boards for Eli Lilly and Daiichi Sankyo. P.W.: received occasional speakers' honoraria and consultancy fees from AstraZeneca, Daiichi Sankyo, and Eli Lilly. A.Z.: received research support and lecture fees from Sanofi; and lecture fees and member of advisory boards for Astra Zeneca, Lilly, and Daiichi-Sankyo. U.Z.: reports personal fees from Astra Zeneca, during the conduct of the study; and personal fees from Astra Zeneca, grants and personal fees from Daiichi Sankyo, grants and personal fees from Eli Lilly, personal fees from Bayer Healthcare, personal fees from The Medicines Company, grants and personal fees from Sanofi, grants and personal fees from Novartis, personal fees from Boehringer Ingelheim, and personal fees from MSD, outside the submitted work.
Acknowledgements
Yasuyuki Matsushita, PhD, from Daiichi Sankyo GmbH Europe performed the statistical analyses. We thank Claudia Copeland, PhD, New Orleans, LA, USA for proofreading major parts of the current manuscript.
References



