-
PDF
- Split View
-
Views
-
Cite
Cite
Shyam S Kothari, Vishal Sharma, Kartik Patel, Gowtham Thakut, Rujuta Parikh, Late post-operative cardiogenic shock from left main coronary compression in tetralogy of Fallot with absent pulmonary valve: a case report, European Heart Journal - Case Reports, Volume 9, Issue 5, May 2025, ytaf190, https://doi.org/10.1093/ehjcr/ytaf190
Close - Share Icon Share
Abstract
Extrinsic left main coronary artery (LMCA) compression is well described in patients with severe pulmonary dilatation secondary to atrial septal defect, idiopathic pulmonary artery hypertension, and eisenmengerized patent ductus arteriosus. An inferiorly displaced origin of LMCA closer to the left coronary sinus and a dilated pulmonary artery (PA) trunk twice as much as aortic trunk increases risk of extrinsic compression. Such patients are prone to left ventricular ischaemia, malignant ventricular arrhythmia, and sudden cardiac death.
A 20-year-old girl presented with gradually worsening exertional dyspnoea for the last 5 years was diagnosed to have tetralogy of Fallot with absent pulmonary valve (TOF-APV). After undergoing intra-cardiac repair with bioprosthetic pulmonary valve implantation, she developed a broad complex right bundle branch block with right precordial Q waves (qRBBB), new-onset left ventricular dysfunction, acute decompensated heart failure, and elevated serum cardiac enzymes. Coronary angiogram and intravascular ultrasound examination showed a critically narrowed slit-like LMCA ostium. Percutaneous stenting of LMCA saw resolution of qRBBB and improvement in left ventricle function, after which patient stabilized and could be weaned off ventilatory support.
Left main coronary artery compression after surgical pulmonary valve replacement in TOF-APV occurring few days after the operation may pose diagnostic and therapeutic challenges. Exact mechanism for post-operative compression is not known. It can be hypothesized that the ionotropic agents used in post-operative period, or change in the geometric relationships of pulmonary artery and LMCA due to pulmonary valve implantation, brought out the compression. A case for prophylactic PA size reduction can be made to avoid the possibility of LMCA compression.
Tetralogy of Fallot with absent pulmonary valve can have dilated pulmonary arteries and can cause compression of surrounding structures. Pulmonary valve implantation can change the geometric relationship of pulmonary artery and can cause LMCA compression.
Left main coronary artery compression can often be missed and needs a high index of suspicion for the diagnosis. It may cause life-threatening acute decompensated heart failure and is a potentially reversible cause of left ventricle dysfunction.
Left main coronary artery compression may be expected in patients with aneurysmal dilatation of pulmonary arteries and, when undergoing a surgical correction for the underlying cause, may be considered for reduction of pulmonary artery size. It may be beneficial to accurately size the pulmonary arteries on a pre-operative computed tomography scan so that prophylactic surgical reduction may be considered if PA diameter is greater than 44.5 mm.
Introduction
Adult patients of tetralogy of Fallot with absent pulmonary valve (TOF-APV) are generally expected to have a smooth post-operative course after open heart surgery. However, left ventricular dysfunction in post-operative period in them might result from multiple causes. Extrinsic compression of left main coronary artery (LMCA) might be a treatable cause of such dysfunction in an appropriate context. It may not be realized that such a compression might occur days after extubation and might be intermittent. Therefore, such an occurrence is a diagnostic challenge. We report such a case in a 20-year-old girl operated for TOF-APV in whom LMCA compression manifested only in the late post-operative period.
Summary figure
| Day 0 | Evaluated for chronic dyspnoea and on presentation diagnosed to have TOF-APV. Electrocardiogram (ECG) showed right ventricular hypertrophy with QRS duration of 90 ms. Cardiomegaly was noted with aneurysmal dilatation of pulmonary arteries. Computed tomography pulmonary angiography measured main pulmonary artery, right and left pulmonary arteries to be 45.8, 27 mm and 35.4 mm, respectively, and no LMCA compression was noted |
| Day 3 | She underwent intra-cardiac repair with pulmonary valve implantation (transannular patch and EPIC 25 mm bioprosthetic valve). A routine post-operative echo showed adequate repair and extubated uneventfully |
| Day 6 | Developed acutely decompensated heart failure in step-down ICU and needed re-intubation. Review echo showed severe left ventricle (LV) dysfunction and started on appropriate medical treatment for heart failure with partial gradual betterment |
| Day 9 | Again, developed decompensated heart failure with progressive widening of QRS complex. ECG showed q right bundle branch block (qRBBB) with QRS duration 180 ms with deep T wave impression. Rise in cardiac biomarkers and poor biventricular function noted |
| Day 10 | Taken for coronary catheterization and found to have critical LMCA ostial compression. Treated with percutaneous DES implantation |
| Day 13 | Left ventricle function improved with gradual weaning off inotropes and ventilation |
| Day 20 | She was discharged with RBBB, QRS duration of 120 ms, and normal LV function |
| 3 months | Normal biventricular function and doing well |
| Day 0 | Evaluated for chronic dyspnoea and on presentation diagnosed to have TOF-APV. Electrocardiogram (ECG) showed right ventricular hypertrophy with QRS duration of 90 ms. Cardiomegaly was noted with aneurysmal dilatation of pulmonary arteries. Computed tomography pulmonary angiography measured main pulmonary artery, right and left pulmonary arteries to be 45.8, 27 mm and 35.4 mm, respectively, and no LMCA compression was noted |
| Day 3 | She underwent intra-cardiac repair with pulmonary valve implantation (transannular patch and EPIC 25 mm bioprosthetic valve). A routine post-operative echo showed adequate repair and extubated uneventfully |
| Day 6 | Developed acutely decompensated heart failure in step-down ICU and needed re-intubation. Review echo showed severe left ventricle (LV) dysfunction and started on appropriate medical treatment for heart failure with partial gradual betterment |
| Day 9 | Again, developed decompensated heart failure with progressive widening of QRS complex. ECG showed q right bundle branch block (qRBBB) with QRS duration 180 ms with deep T wave impression. Rise in cardiac biomarkers and poor biventricular function noted |
| Day 10 | Taken for coronary catheterization and found to have critical LMCA ostial compression. Treated with percutaneous DES implantation |
| Day 13 | Left ventricle function improved with gradual weaning off inotropes and ventilation |
| Day 20 | She was discharged with RBBB, QRS duration of 120 ms, and normal LV function |
| 3 months | Normal biventricular function and doing well |
| Day 0 | Evaluated for chronic dyspnoea and on presentation diagnosed to have TOF-APV. Electrocardiogram (ECG) showed right ventricular hypertrophy with QRS duration of 90 ms. Cardiomegaly was noted with aneurysmal dilatation of pulmonary arteries. Computed tomography pulmonary angiography measured main pulmonary artery, right and left pulmonary arteries to be 45.8, 27 mm and 35.4 mm, respectively, and no LMCA compression was noted |
| Day 3 | She underwent intra-cardiac repair with pulmonary valve implantation (transannular patch and EPIC 25 mm bioprosthetic valve). A routine post-operative echo showed adequate repair and extubated uneventfully |
| Day 6 | Developed acutely decompensated heart failure in step-down ICU and needed re-intubation. Review echo showed severe left ventricle (LV) dysfunction and started on appropriate medical treatment for heart failure with partial gradual betterment |
| Day 9 | Again, developed decompensated heart failure with progressive widening of QRS complex. ECG showed q right bundle branch block (qRBBB) with QRS duration 180 ms with deep T wave impression. Rise in cardiac biomarkers and poor biventricular function noted |
| Day 10 | Taken for coronary catheterization and found to have critical LMCA ostial compression. Treated with percutaneous DES implantation |
| Day 13 | Left ventricle function improved with gradual weaning off inotropes and ventilation |
| Day 20 | She was discharged with RBBB, QRS duration of 120 ms, and normal LV function |
| 3 months | Normal biventricular function and doing well |
| Day 0 | Evaluated for chronic dyspnoea and on presentation diagnosed to have TOF-APV. Electrocardiogram (ECG) showed right ventricular hypertrophy with QRS duration of 90 ms. Cardiomegaly was noted with aneurysmal dilatation of pulmonary arteries. Computed tomography pulmonary angiography measured main pulmonary artery, right and left pulmonary arteries to be 45.8, 27 mm and 35.4 mm, respectively, and no LMCA compression was noted |
| Day 3 | She underwent intra-cardiac repair with pulmonary valve implantation (transannular patch and EPIC 25 mm bioprosthetic valve). A routine post-operative echo showed adequate repair and extubated uneventfully |
| Day 6 | Developed acutely decompensated heart failure in step-down ICU and needed re-intubation. Review echo showed severe left ventricle (LV) dysfunction and started on appropriate medical treatment for heart failure with partial gradual betterment |
| Day 9 | Again, developed decompensated heart failure with progressive widening of QRS complex. ECG showed q right bundle branch block (qRBBB) with QRS duration 180 ms with deep T wave impression. Rise in cardiac biomarkers and poor biventricular function noted |
| Day 10 | Taken for coronary catheterization and found to have critical LMCA ostial compression. Treated with percutaneous DES implantation |
| Day 13 | Left ventricle function improved with gradual weaning off inotropes and ventilation |
| Day 20 | She was discharged with RBBB, QRS duration of 120 ms, and normal LV function |
| 3 months | Normal biventricular function and doing well |
Case presentation
A 20-year-old girl presented with gradually worsening exertional dyspnoea for the last 5 years. On examination, pulse 88 b.p.m., blood pressure 112/74 mmHg, and oximetric saturation of 95% were noted. She was average built with height of 154 cm and weight 50 kg. Cardiac examination showed grade 4/6 ejection systolic murmur and low frequency diastolic murmur of pulmonary regurgitation.
Electrocardiogram showed sinus rhythm, prominent right ventricular hypertrophy with rsR′ in V1 and QRS duration of 90 ms. Chest X-ray showed enlarged cardiac silhouette with aneurysmally dilated pulmonary arteries (PA) (Figure 1). On echocardiography, there was a cono-ventricular ventricular septal defect with anteriorly displaced conal septum and aortic override. Pulmonary valve was rudimentary with severe annular and infundibular narrowing with a gradient of 70 mmHg. Biventricular systolic function was normal. Computed tomography (CT) scan showed aneurysmally dilated pulmonary main PA mildly compressing the left main bronchus. No LMCA compression was noticed. The size of the main PA and right and left PAs were 45.8, 27 mm and 35.4 mm, respectively.
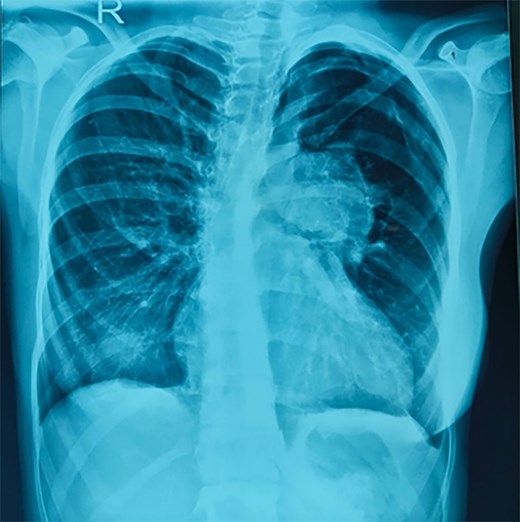
Chest X-ray on admission showing cardiomegaly and aneurysmal dilatation of pulmonary arteries.
She underwent open surgical intra-cardiac repair with pulmonary valve implantation (transannular patch and EPIC 25 mm bioprosthetic valve). A routine post-operative echo showed adequate repair. Post-operatively, she was extubated on the third day and shifted to a step-down intensive care unit. The patient had to be re-intubated as she had severe dyspnoea and hypotension. A review echo done showed global hypokinesia of the LV with ejection fraction of 30%. She was gradually weaned off the inotropes with improvement in her LV function and was extubated. However, she again went into severe hypotension and required re-intubation with echo again showing severe biventricular dysfunction without any residual RV outflow obstruction or pulmonary regurgitation. She developed a broad complex q right bundle branch block (qRBBB) with deep T wave inversions in anterior chest leads with left axis deviation (Figure 2). Her troponin I levels were 3965 ng/L (normal value < 1.9 ng/L).
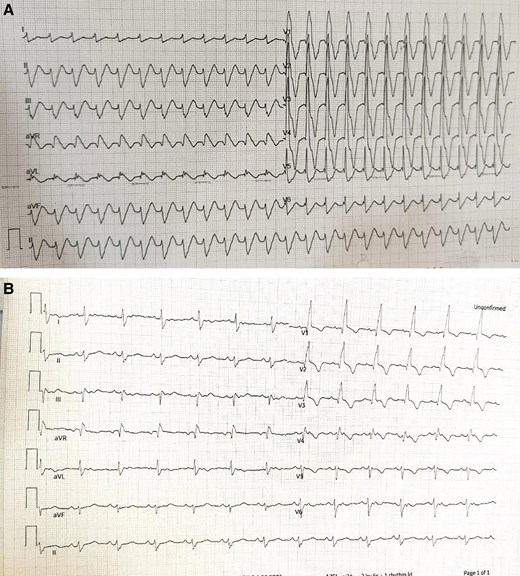
(A) Event electrocardiogram (ECG) showing q right bundle branch block (qRBBB) with QRS duration 180 ms and deep T wave inversion in anterior chest leads. (B) Immediate post-operative ECG, showing sinus rhythm with RBBB with QRS duration 120 ms.
This sequence of events was suspicious of myocardial ischaemia, and she was taken for coronary angiography. Left main coronary artery showed critically narrowed ostium (Figure 3) and normal distal coronary arteries. Intravascular ultrasound (IVUS) examination showed slit-like opening of LMCA ostium (Figure 4) with extrinsic compression. A 4*12 mm quantum apex non-compliant balloon was inflated to check LMCA distensibility. A 4.5*18 mm Resolute Onyx drug eluting stent was placed from left main ostium to proximal left anterior descending (LAD) artery, keeping left circumflex (LCx) artery as side branch by provisional technique of bifurcation stenting. Proximal optimization was done with 5*8 non-compliant balloon (see Supplementary material online, Video, Figure S1). Post-stenting IVUS showed a well-expanded stent (Figure 5).
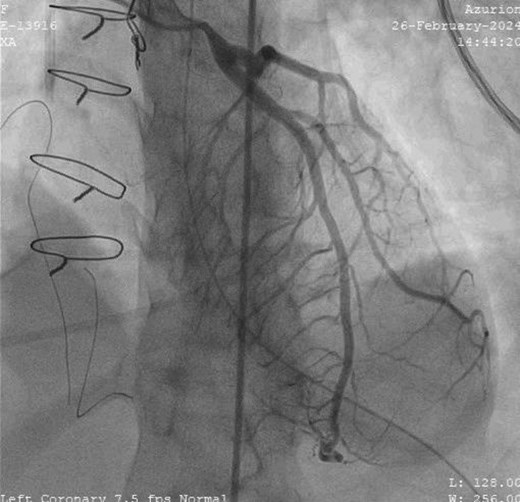
Invasive coronary angiogram in left anterior oblique cranial view showing narrowed left main coronary artery ostium with no coronary artery disease.

Intravascular ultrasound examination showed slit-like narrowing of left main coronary artery ostium without any atherosclerotic or thrombotic disease.
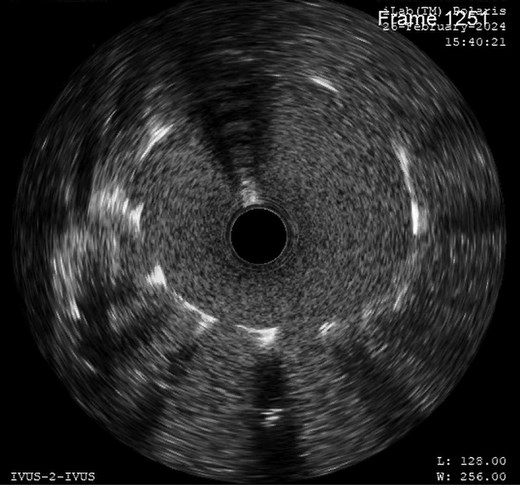
Post-stenting intravascular ultrasound showed covered ostium and well-expanded stent without any recoil.
Her LV dysfunction improved, and she was weaned off ventilatory support on third day. A CT coronary angiogram showed patent stent in both systole and diastole. She was discharged in stable condition on tenth day. Her electrocardiogram (ECG) at discharge shows qRBBB with QRS duration of 140 ms and axis of 110 degree, and echocardiogram at discharge showed normal biventricular function. On follow-up at 3 months, patient was in excellent health with normal biventricular function on echo.
Discussion
Left main coronary artery compression can often be missed and needs a high index of suspicion for the diagnosis. It is reported in up to 6% of patients with dilated PA due to any aetiology even without signs and symptoms of myocardial ischaemia.1 The dilatation of main PA to >40 mm diameter might result in downward displacement and reduction in take-off angle of LMCA and might lead to extrinsic compression.1–3
Left main coronary artery compression was not suspected in the pre-operative CT in our patient. The patient never had any angina, or ventricular dysfunction. Why did the LMCA compression manifest in the post-operative period can only be speculated. It can be hypothesized that the ionotropic agents used in post-operative period, or change in the geometric relationships of pulmonary artery and LMCA due to pulmonary valve implantation, brought out the compression. Retrospectively, it seems likely that PA size reduction could have possibly averted the LMCA compression. It is suggested that prophylactic surgical PA reduction may be considered when the diameter of PA is greater than 44.5 mm.4 However, reduction of PA was not done as there was only mild bronchial compression and no respiratory symptoms.
Similar LMCA compression has been described in patients with valved conduits. Such obstruction might present early in the post-operative period or years later due to calcification and dilatation of the conduits.5 A direct injury and straddling of the coronary artery, abutting of a metal strut of the bioprosthetic valve on a coronary, or impingement by the sewing ring of valve can compress the coronary artery, but would be obvious in the early post-operative period.6 In patients undergoing percutaneous pulmonary valve replacement, a balloon interrogation to avoid coronary compression is mandatory, but instances of late compression are known.7
Left main coronary artery stenting in the setting of extrinsic compression seems effective. Stenting is relatively low risk due to absence of athero-thrombotic disease and hence lower rates of in-stent restenosis. A study demonstrated that 5-year clinical outcomes were favourable with no case of probable or possible stent thrombosis and stent recoil in 2% patients. In-stent restenosis and target vessel revascularization was reported in 9.8%, but the risk was lower with DES as compared with bare metal stent.8 Another study reported long-term follow-up of 4.5 years that have shown adequate results in this subgroup of patients with pulmonary hypertension.9 Ten-year follow-up data show comparable mortality, target lesion, and target vessel revascularization between CABG and LMCA stenting in isolated left main disease or when SYNTAX score is less than 32.10 Therefore, standard dual antiplatelet therapy for 1 year followed by single antiplatelet therapy is planned for our patient with regular clinical follow-up. Of course, IVUS guidance, as done in our patient, is important in assessing the stent expansion and exclude acute recoil of the vessel, as angiography alone may be misleading.11
Conclusion
Left main coronary artery compression should be remembered as a treatable cause of post-operative LV dysfunction in an appropriate context. Left main coronary artery stenting seems to offer an acceptable alternative to reoperation.
Lead author biography

Dr Rujuta Parikh, MD, DM (Interventional Cardiology), BJ Medical College, and assistant professor, U.N. Mehta Institute of Cardiology and Research Centre, Ahmedabad.
Supplementary material
Supplementary material is available at European Heart Journal – Case Reports online.
Consent: The authors confirm that written consent for submission and publication of this case report including images and associated text has been obtained from the patient in line with the COPE guidelines.
Funding: This work has not received grant from any funding agency, commercial, or not for profit sectors.
Data availability
The data underlying this article cannot be shared publicly due to the privacy of individuals that participated in the study. The data will be shared on reasonable request to the corresponding author.
References
Author notes
All authors contributed equally to the study.
Conflict of interest. None declared.
- stents
- coronary angiography
- ventricular function, left
- tetralogy of fallot
- congenital absence of pulmonic valve
- pulmonary artery
- ventricular dysfunction, left
- right bundle-branch block
- left coronary artery
- cardiogenic shock
- intravascular ultrasonography
- bioprosthesis
- dilatation, pathologic
- postoperative period
- surgical procedures, operative
- diagnosis
- heart
- acute decompensated heart failure
- compression
- pulmonary valve implantation
- left main coronary artery compression




Comments