-
PDF
- Split View
-
Views
-
Cite
Cite
Tomoko Machino-Ohtsuka, Miyako Igarashi, Naoto Kawamatsu, Tomoko Ishizu, Management of arrhythmia and heart failure in a 60-year-old with unrepaired D-transposition of the great arteries, atrial septal defect, and partial anomalous pulmonary venous return: a case report, European Heart Journal - Case Reports, Volume 9, Issue 4, April 2025, ytaf170, https://doi.org/10.1093/ehjcr/ytaf170
Close - Share Icon Share
Abstract
Dextro-transposition of the great arteries (d-TGA) typically results in death within the first year of life without surgery. Shunts can allow sufficient blood mixing and survival into adulthood; however, survival of patients with unrepaired d-TGA beyond 60 years has not been reported.
A 60-year-old woman with unrepaired d-TGA, an unrestricted atrial septal defect, and partial anomalous pulmonary venous return presented with recurrent syncope due to atrial tachycardia (AT) following two sessions of radiofrequency catheter ablation (RFCA) and sinus node dysfunction. She had a history of recurrent heart failure (HF) because of severe systemic right ventricle (sRV) dysfunction and tricuspid regurgitation. Multiple inducible ATs persisted even after repeated RFCA, making pacemaker implantation (PMI) and anti-arrhythmic therapy the preferred options. Although transvenous lead implantation was relatively contraindicated due to the presence of an intracardiac shunt, transvenous DDD PMI was deemed the most viable option considering the patient’s fragile haemodynamic state. Pacing studies indicated that sRV pacing was superior to left ventricular septal pacing, as it reduced the QRS duration and improved RV synchrony. Following successful sRV septal lead implantation and atrioventricular delay (AVD) optimization, HF symptoms improved, ATs were suppressed with amiodarone, and syncope resolved. The patient remained stable for 2 years with no thromboembolic events under apixaban therapy and no HF or arrhythmia recurrence.
Although continuous close monitoring is warranted, endocardial pacing, AVD optimization, and medication enabled effective stabilization over 2 years in this rare case of unrepaired d-TGA with bradytachyarrhythmia and sRV dysfunction.
Adult patients with unrepaired dextro-transposition of the great arteries (d-TGA) with concomitant shunt lesions are exceedingly rare. Individualized treatment approaches, beyond standard guidelines, may be necessary due to the complex pathophysiology.
Systemic right ventricular resynchronization through pacing site and atrioventricular delay optimization, along with medical therapy, can successfully stabilize heart failure and arrhythmia for over 2 years in a 60-year-old patient with unrepaired d-TGA, the oldest reported case in the literature.
Introduction
Dextro-transposition of the great arteries (d-TGA) is a congenital cyanotic heart disease in which the aorta arises from the morphologic right ventricle (RV) and the pulmonary artery from the morphologic left ventricle (LV).1 This anomaly creates parallel circulations, and without surgical intervention, approximately 90% of affected infants do not survive past their first year.1 Concomitant intracardiac shunting can allow for limited blood mixing of oxygenated and deoxygenated blood, potentially extending survival; however, such cases are exceedingly rare.1 Most affected adults without surgery exhibit complex adaptations, making each case unique in terms of clinical management and outcomes.2–5
Here, we report the case of a 60-year-old patient with unrepaired d-TGA, atrial septal defect (ASD), and partial anomalous pulmonary venous return (PAPVR) presenting with arrhythmia and heart failure (HF), and discuss our decision-making approach in this challenging case.
Summary figure
Case presentation
A 60-year-old woman with unrepaired d-TGA, secundum ASD, and PAPVR was admitted to our institution because of recurrent syncope. The patient had central cyanosis at birth, but early growth and development had been normal. She was misdiagnosed with tetralogy of Fallot and underwent left Blalock–Taussig shunting at 4 years of age. At 25 years of age, she was diagnosed with d-TGA, non-restrictive ASD, and PAPVR with the right pulmonary veins draining into the superior vena cava. Surgical correction was considered, but the procedure was deemed too risky owing to systemic right ventricle (sRV) dysfunction. At 52 years of age, she developed HF triggered by atrial tachycardia (AT) originating from the right atrium (RA). She underwent radiofrequency catheter ablation (RFCA), and diuretics and carvedilol (1.25 mg/day) were initiated. At 57 years of age, her HF worsened, leading to two hospital readmissions and referral to our adult congenital heart disease (ACHD) clinic. Iron-deficiency anaemia disproportionate to observed cyanosis was noted, and HF improved with iron supplementation and initiation of empagliflozin (10 mg) treatment. However, at the age of 58 years, she had recurrent presyncope due to bradycardia–tachycardia syndrome and underwent RFCA for RA-origin ATs. The long pauses resolved after carvedilol was discontinued, eliminating the need for pacemaker implantation (PMI).
At 60 years of age, she was readmitted for recurrent syncope with HF [New York Heart Association (NYHA) class III], finger clubbing, and a 3/6 pansystolic murmur at the third left sternal border (blood pressure, 95/56 mmHg; heart rate, 66 bpm; oxygen saturation, 83% on room air). Electrocardiography on admission showed sinus rhythm, right axis deviation, and right bundle branch block; 14-day continuous patch monitor electrocardiography indicated frequent ATs (with heart rate, 200 bpm) causing recurrent syncope owing to reduced cardiac output (Figure 1). Non-sustained or sustained ventricular tachycardia was not observed; however, a 3.3 s sinus arrest was recorded. Transthoracic echocardiography revealed decreased contraction and asynchrony of the sRV along with significant tricuspid regurgitation (TR) (Supplementary material online, Video). Cardiac magnetic resonance imaging showed a sRV ejection fraction of 38%, severe TR (regurgitant fraction, 49%), and a low cardiac index [(CI), 1.23 L/min/m2] (Figure 2A). Due to the patient’s chronic kidney disease (estimated glomerular filtration rate, 28 mL/min/1.73 m²), gadolinium-based contrast agents could not be administered. Cardiac catheterization revealed mildly elevated sRV end-diastolic pressure (11 mmHg), but no evidence of pulmonary hypertension or significant pulmonary valve stenosis (Figure 2B). Transoesophageal echocardiography confirmed a large 35 mm secundum ASD with a bidirectional shunt.
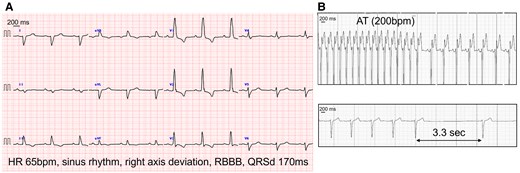
(A) 12-lead electrocardiogram showing sinus rhythm with a heart rate of 65 bpm, right axis deviation, and right bundle branch block with a QRS duration of 170 ms. (B) Atrial tachycardia at 200 bpm (top panel) and a sinus arrest of 3.3 s (bottom panel) recorded using a 14-day electrocardiography patch monitor. AT, atrial tachycardia; QRSd, QRS duration; RBBB, right bundle branch block.
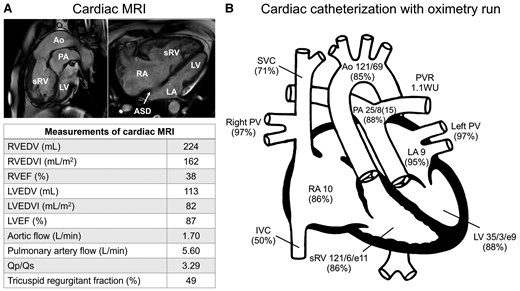
(A) Cardiac magnetic resonance imaging showing the aorta arising from the enlarged systemic right ventricle, the pulmonary artery originating from the left ventricle, and a large secundum atrial septal defect. The lower table summarizes the measurements. (B) Intracardiac pressure (upper panel, mmHg) and oxygen saturation (lower panel, %) data obtained using cardiac catheterization. ASD, atrial septal defect; Ao, aorta; IVC, inferior vena cava; LA, left atrium; LV, left ventricle; LVEDV, left ventricular end-diastolic volume; LVEDVI, left ventricular end-diastolic volume index; LVEF, left ventricular ejection fraction; MRI, magnetic resonance imaging; PA, pulmonary artery; PV, pulmonary vein; PVR, pulmonary vascular resistance; Qp/Qs, pulmonary-to-systemic flow ratio; RA, right atrium; RVEDV, right ventricular end-diastolic volume; RVEDVI, right ventricular end-diastolic volume index; RVEF, right ventricular ejection fraction; sRV, systemic right ventricle; SVC, superior vena cava; WU, Wood units.
Although clinical AT originating from the RA was successfully ablated after the previous RFCA, multiple inducible ATs persisted; therefore, RFCA alone was considered insufficient for arrhythmia control. Sinus node dysfunction was also observed, necessitating the consideration of PMI and anti-arrhythmic therapy as better options. Although transvenous lead placement is generally contraindicated in patients with intracardiac shunts because of the risk of embolism, severe sRV dysfunction and low CI rendered surgical lead implantation too risky. Syncope during AT required atrial anti-tachycardia pacing, and endocardial leads were preferred for their superior thresholds and reliability. Furthermore, with both right bundle branch and left posterior fascicular blocks, the patient was at risk of developing an advanced atrioventricular block from anti-arrhythmic therapy. Accordingly, transvenous DDD PMI was planned under conscious sedation and local anaesthesia.
To improve sRV dysfunction, a pacing study was conducted before PMI to determine the optimal pacing site for improving sRV synchrony (Figure 3). Baseline ECG showed a QRS duration (QRSd) of 170 ms with a right bundle branch block. Although a Purkinje potential was recorded at the LV septal pacing site, the pacing prolonged the QRSd (to 180 ms), worsening sRV dyssynchrony. Conversely, sRV pacing narrowed the QRS to 120 ms and preserved sRV synchrony. Although the sRV-free wall was initially targeted based on these results, accessing it was difficult because of sRV enlargement. Intraoperative difficulties—including reduction in blood pressure and oxygen saturation and significant bleeding within the pacemaker pocket—necessitated continuous catecholamine infusion to maintain haemodynamic stability. Ultimately, a thin lumenless lead (SelectSecure™; Medtronic Inc., Minneapolis, USA) was successfully implanted in the sRV septum. Post-PMI, amiodarone 100 mg and bisoprolol 1.25 mg were initiated, and atrioventricular delay was optimized based on Doppler echocardiographic findings, as well as CI measurements obtained using non-invasive electrical cardiometry (AESCULON® mini; Osypka Medical, Berlin, Germany) (Figure 4). With improved sRV synchrony and right ventricle inflow pattern, the CI increased from 1.47 L/min/m2 during atrial pacing and ventricular sensing to 1.87 L/min/m2 with a 160 ms AV delay during atrial and ventricular pacing as the final setting.
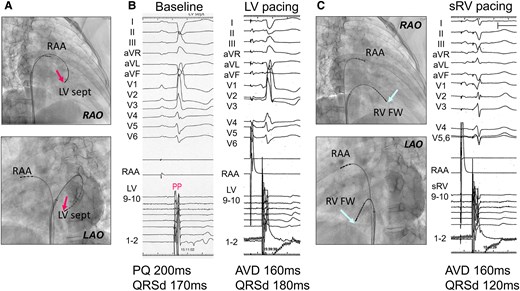
Data obtained from the pacing study prior to pacemaker implantation. (A) Electrode catheter placed at the left ventricular septum via the atrial septal defect (red arrows). (B) Although a Purkinje potential was recorded at the left ventricular septal pacing site, indicating that the conduction system was being captured, left ventricular pacing unexpectedly resulted in a prolonged QRS duration. (C) Electrode catheter placed at the systemic right ventricle-free wall (light blue arrows), showing a shortened QRS duration during systemic right ventricle pacing. AVD, atrioventricular delay; FW, free wall; LAO, left anterior oblique; LV, left ventricular; PP, Purkinje potential; QRSd, QRS duration; RAA, right atrial appendage; RAO, right anterior oblique; sRV, systemic right ventricle.
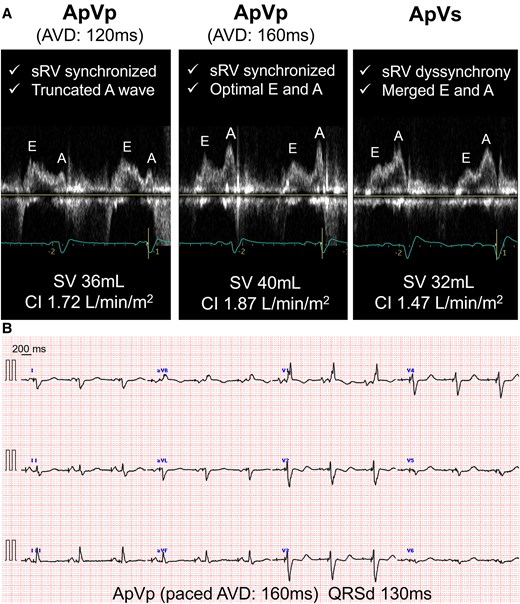
(A) Optimization of atrioventricular delay using non-invasive electrical cardiometry and echocardiography. Systemic right ventricular synchrony, transmitral flow patterns, stroke volume, and cardiac index were assessed during atrioventricular delay adjustments in DDD mode at 70 bpm and delays of 120 ms (ApVp), 160 ms (ApVp), and 300 ms (ApVs). The optimal atrioventricular delay was 160 ms. (B) The final setting at an atrioventricular delay of 160 ms resulted in a narrowed QRS duration of 130 ms on 12-lead electrocardiography. AVD, atrioventricular delay; CI, cardiac index; QRSd, QRS duration; sRV, systemic right ventricle; SV, stroke volume.
Following these interventions, the ATs were suppressed, syncope resolved, and HF improved (Figure 5). At 62 years of age, the patient had remained stable for 2 years without arrhythmia, HF exacerbation, or thromboembolic events while on apixaban (5 mg/day), empagliflozin (25 mg/day), spironolactone (25 mg/day), bisoprolol (0.625 mg/day), amiodarone (50 mg/day), furosemide (30 mg/day), tolvaptan (7.5 mg/day), and pimobendan (5 mg/day). Pimobendan, approved in Japan for chronic HF, was used to manage low-output symptoms.
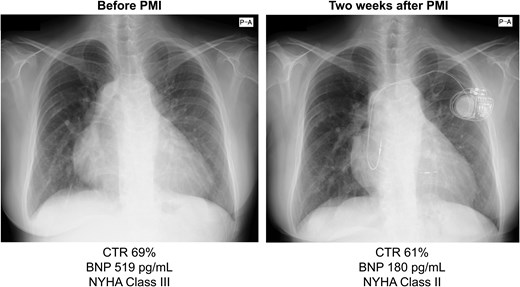
Chest radiographs obtained before and after pacemaker implantation indicate a reduction in the cardiothoracic ratio, along with a decrease in brain natriuretic peptide levels and improvement in NYHA classification. BNP, brain natriuretic peptide; CTR, cardiothoracic ratio; NYHA, New York Heart Association.
Discussion
The standard treatment for d-TGA is arterial switch surgery in early infancy, which greatly improves survival and long-term outcomes.6 Without surgery, long-term survival is rare and usually depends on additional shunts allowing blood mixing.1 This is the first report of a patient with unrepaired d-TGA surviving beyond 60 years.2–5 In cases of unrepaired d-TGA with only ASD, mean survival period is 1.34 years1; thus, the combination of PAPVR and non-restrictive ASD likely contributed to this patient’s unusually prolonged survival.
The ventricular pacing site plays a crucial role in systemic ventricular synchrony and function.6,7 In this case, a prior pacing study helped identify the optimal ventricular pacing site, narrowing the QRS duration and improving sRV synchrony to some extent. Additionally, DDD settings were adjusted, and AV delay optimization improved diastolic ventricular filling and stroke volumes as reported previously.8
Transvenous lead placement is relatively contraindicated in patients with intracardiac shunts because of the risk of arterial embolism,6,9 which cannot be fully mitigated by warfarin or aspirin, and data on direct oral anticoagulants remain limited.10,11 Furthermore, surgical options are often high-risk for patients with complex ACHD and cardiac dysfunction, leaving few viable alternatives. However, recent reports have shown favourable outcomes in patients with intracardiac shunts who underwent transvenous or leadless PMI combined with appropriate anticoagulant therapy.12–15 In the present case, apixaban successfully prevented embolic events for 2 years without complications. Although continued monitoring is necessary, this approach may represent a viable alternative for high-risk patients.
In addition, there is no evidence-based pharmacological therapy specifically for the treatment of sRV dysfunction.6 In this case, among HF medications, those with vasodilatory effects were avoided due to the potential risk of exacerbating high-output HF. Instead, we opted for mineralocorticoid receptor antagonists, sodium-glucose co-transporter 2 inhibitors, and very low-dose beta-blockers, which were effective in stabilizing HF. The unique haemodynamic characteristics of each ACHD case should also be carefully evaluated when selecting pharmacologic therapies.
Conclusions
Endocardial DDD PMI with careful planning successfully stabilized arrhythmia and HF for over 2 years in this rare case of unrepaired d-TGA. Our findings also indicate that the management of patients with ACHD, each with their own unique and complex pathophysiology, might occasionally require individualized decision-making beyond the standard guidelines.
Lead author biography

Tomoko Machino-Ohtsuka graduated from the University of Tsukuba School and obtained her PhD. She has specialized in echocardiography as a cardiologist and has been involved in the care of adult congenital heart disease patients at the University of Tsukuba Hospital since 2016. Her current main areas of interest are infective endocarditis and valvular disease in adult congenital heart disease.
Supplementary material
Supplementary material is available at European Heart Journal – Case Reports online.
Acknowledgements
The authors thank the patient for consenting to her case details being shared for scientific publication, and acknowledge the efforts of the treatment teams involved in the care of the patient at the hospital.
Consent: Written informed consent was obtained from the patient for the submission and publication of this case report, including all images and related content, in accordance with the COPE guidelines.
Funding: None declared.
Data availability
The data underlying this article are available in the article and its online Supplementary material.
References
Author notes
Conflict of interest. None declared.
- antithymoglobulin
- cardiac arrhythmia
- syncope
- atrial tachycardia
- partial anomalous pulmonary venous connection
- radiofrequency catheter ablation
- atrial septal defect
- heart failure
- right ventricle
- adult
- surgical procedures, operative
- survivin
- periodic paralysis, potassium-sensitive cardiodysrhythmic type
- qrs complex duration
- arterial tortuosity syndrome
- d-transposition of the great vessels





Comments