-
PDF
- Split View
-
Views
-
Cite
Cite
Marjolein C de Jongh, Matthijs Bax, Khalil Ayan, Sakir Akin, Proning the extracorporeal membrane oxygenation plus Impella: a case report, European Heart Journal - Case Reports, Volume 8, Issue 4, April 2024, ytae165, https://doi.org/10.1093/ehjcr/ytae165
Close - Share Icon Share
Abstract
The prone position is recommended as supportive therapy in patients with moderate-to-severe acute respiratory distress syndrome (ARDS). However, little is known about prone position ventilation in patients with cardiogenic shock supported by extracorporeal membrane oxygenation (ECMO) plus Impella (ECPELLA) developing ARDS.
A 66-year-old man with severe left ventricular dysfunction was admitted to a non-academic ECMO centre for a high-risk coronary artery bypass grafting. He developed post-cardiotomy shock needing ECMO support. To improve left ventricular unloading, an Impella was inserted 2 days later. One day later, he developed ARDS and needed prone position ventilation with ECPELLA in situ. After 4 weeks, he was discharged from the intensive care unit.
Previous studies demonstrated that prone positioning could help avoid an additional venous cannula in veno-arterial ECMO patients, which is associated with mechanical complications. In this case, there was a promising role for unloading the left ventricle with Impella during veno-arterial ECMO and, for proning, the patient with cardiogenic shock developing ARDS during ECMO support without the need for an extra venous cannula.
It is technically possible to prone a patient with an ECMO plus Impella (ECPELLA) developing acute respiratory distress syndrome (ARDS).
Proning position with ECPELLA may avoid mechanical complications compared with veno-arterio-venous ECMO configuration in patients with cardiogenic shock developing ARDS
Introduction
The prone position is recommended as a supportive therapy in patients with moderate-to-severe acute respiratory distress syndrome (ARDS). Although small studies have found that proning in extracorporeal membrane oxygenation (ECMO) patients is safe and improves oxygenation,1 larger studies that address the effectiveness of proning in this population group are missing. Recently, ECMO and concomitant Impella support (‘ECPELLA’) have been increasingly used to treat cardiogenic shock by maintaining systemic circulation and unloading the left ventricle (LV). However, little is known about the prone position ventilation in patients under ECPELLA support. We hereby present the first case in the literature describing the prone position in a patient with ARDS following cardiogenic shock supported by ECPELLA.
Summary figure
| Date . | Event . |
|---|---|
| 10 May 2022 | The patient presents for a high-risk CABG due to severely impaired LV function resulting in post-cardiotomy shock and needing VA ECMO support |
| 12 May 2022 | Severe LV dysfunction despite VA ECMO support and inotropic infusions
|
| 13 May 2022 | Despite ECPELLA, the development of severe ARDS
|
| 14 May 2022 | ECPELLA in a prone position improved end-organ oxygenation |
| 16 May 2022 | Weaning from ECMO followed by explantation |
| 19 May 2022 | Persisting moderate ARDS managed by prone position ventilation |
| 20 May 2022 | Successful weaning from Impella under low dose inotropes (2 µg/kg/min dobutamine) |
| 21 May 2022 | Placement of tracheostomy tube due to ICU-acquired weakness
|
| 10 June 2022 | Discharge to the Cardiology Department |
| 1 July 2022 | Discharge from hospital to rehabilitation centre |
| 1 August 2022 | Discharge to home |
| Date . | Event . |
|---|---|
| 10 May 2022 | The patient presents for a high-risk CABG due to severely impaired LV function resulting in post-cardiotomy shock and needing VA ECMO support |
| 12 May 2022 | Severe LV dysfunction despite VA ECMO support and inotropic infusions
|
| 13 May 2022 | Despite ECPELLA, the development of severe ARDS
|
| 14 May 2022 | ECPELLA in a prone position improved end-organ oxygenation |
| 16 May 2022 | Weaning from ECMO followed by explantation |
| 19 May 2022 | Persisting moderate ARDS managed by prone position ventilation |
| 20 May 2022 | Successful weaning from Impella under low dose inotropes (2 µg/kg/min dobutamine) |
| 21 May 2022 | Placement of tracheostomy tube due to ICU-acquired weakness
|
| 10 June 2022 | Discharge to the Cardiology Department |
| 1 July 2022 | Discharge from hospital to rehabilitation centre |
| 1 August 2022 | Discharge to home |
| Date . | Event . |
|---|---|
| 10 May 2022 | The patient presents for a high-risk CABG due to severely impaired LV function resulting in post-cardiotomy shock and needing VA ECMO support |
| 12 May 2022 | Severe LV dysfunction despite VA ECMO support and inotropic infusions
|
| 13 May 2022 | Despite ECPELLA, the development of severe ARDS
|
| 14 May 2022 | ECPELLA in a prone position improved end-organ oxygenation |
| 16 May 2022 | Weaning from ECMO followed by explantation |
| 19 May 2022 | Persisting moderate ARDS managed by prone position ventilation |
| 20 May 2022 | Successful weaning from Impella under low dose inotropes (2 µg/kg/min dobutamine) |
| 21 May 2022 | Placement of tracheostomy tube due to ICU-acquired weakness
|
| 10 June 2022 | Discharge to the Cardiology Department |
| 1 July 2022 | Discharge from hospital to rehabilitation centre |
| 1 August 2022 | Discharge to home |
| Date . | Event . |
|---|---|
| 10 May 2022 | The patient presents for a high-risk CABG due to severely impaired LV function resulting in post-cardiotomy shock and needing VA ECMO support |
| 12 May 2022 | Severe LV dysfunction despite VA ECMO support and inotropic infusions
|
| 13 May 2022 | Despite ECPELLA, the development of severe ARDS
|
| 14 May 2022 | ECPELLA in a prone position improved end-organ oxygenation |
| 16 May 2022 | Weaning from ECMO followed by explantation |
| 19 May 2022 | Persisting moderate ARDS managed by prone position ventilation |
| 20 May 2022 | Successful weaning from Impella under low dose inotropes (2 µg/kg/min dobutamine) |
| 21 May 2022 | Placement of tracheostomy tube due to ICU-acquired weakness
|
| 10 June 2022 | Discharge to the Cardiology Department |
| 1 July 2022 | Discharge from hospital to rehabilitation centre |
| 1 August 2022 | Discharge to home |
Case
A 66-year-old man was admitted to a non-academic ECMO centre for a high-risk coronary artery bypass grafting (CABG). His previous history included chronic obstructive pulmonary disease, primary hypogonadism, a metabolic syndrome for which he used testosterone gel, non-alcoholic fatty liver disease and a combined dyslipidaemia, depression for which he took venlafaxine. In addition, he was recently admitted to the intensive care unit (ICU) for 7 weeks due to coronavirus disease (COVID-19). After he was discharged, he continued to experience dyspnoea. A transthoracic echocardiogram showed severe worsening of his LV ejection function (LVEF 15%). Coronary angiography followed, and significant three-vessel disease was observed. Magnetic resonance imaging (MRI) showed diffuse viability and an LVEF of 21%. After he was discussed in the heart team for high-risk patients, he was accepted for a high-risk CABG within a few days.
During the CABG procedure, he experienced supraventricular tachycardia (SVT) with loss of output, for which he received a temporary pacemaker (and a Swan Ganz catheter was placed). After admission to the ICU for post-operative monitoring, the patient showed haemodynamic and respiratory stability, with the only remarkable finding being a pericardial murmur. After extubation, he became hypotensive and experienced SVT with progressive cardiogenic shock and high doses of inotropes, which resulted in the implantation of a veno-arterial (VA) ECMO. Hereafter, Harlequin syndrome, which manifests as differential oxygen saturation between upper and lower parts of the body due to peripheral VA ECMO, was not expected due to peri-operative echocardiography showing acceptable unloading under inotropes. However, 2 days later, there were signs of refractory end-organ hypoxia, and a transthoracic echo showed a dilated LV with insufficient opening of the aortic valve, leading to implantation of an Impella CP. On the same day, continuous veno-venous haemodialysis (CVVHD) was started due to acute kidney injury.
Further respiratory failure was seen the following day and suspected for the development of an ARDS caused by pneumonia. X-ray showed pleural effusion and atelectasis in addition (Figure 1). We decided to prone the patient despite prolonged protective ventilation because of the decreased PF ratio (<100 mmHg). This finding was never described before in the literature, our country, or our centre. At that moment, ECMO blood flow was 2.7 L/min, and fractional oxygen needed 80% (Table 1). The Impella unloaded the ventricle with 2.9 L/min (performance level 6). We mechanically ventilated the patient with a maximum FIO2 of 100%, giving 10 cmH20 positive end-expiratory pressure with peak pressures of 23 cmH20.
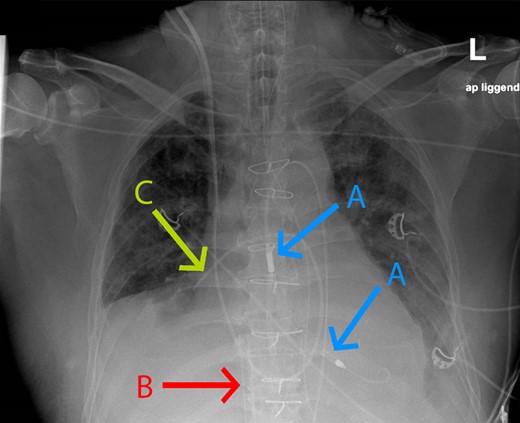
Chest X-ray of the patient with acute respiratory distress syndrome right before the prone position. Arrow A = Impella; Arrow B = extracorporeal membrane oxygenation cannula in the inferior vena cava; Arrow C = Schwan Ganz catheter in the right pulmonary artery.
| . | PEEP . | Oxygenation index . | PF ratio . | Impella: P -stand . | Impella: cardiac output (L/min) . | ECMO: Output (L/min) . | Cardiac output (L/min) (Schwan Ganz) . | PCWP (Schwan Ganz) . | Maximum inotropic dose . |
|---|---|---|---|---|---|---|---|---|---|
| Before proning | 12 | 14.4 | 11 | P6 | 3 | 2.0 | 7.5 | 10 | Dobutamine 4γ + noradrenalin 0.1γ |
| During proning | 10 | 5.8 | 27 | P6 | 2.9 | 2.7 | —a | —a | Dobutamine 4γ + noradrenalin 0.14γ |
| After proning | 10 | 8.5 | 18 | P6 | 2.8 | 2.7 | —a | —a | Dobutamine 4γ + noradrenalin 0.14γ |
| . | PEEP . | Oxygenation index . | PF ratio . | Impella: P -stand . | Impella: cardiac output (L/min) . | ECMO: Output (L/min) . | Cardiac output (L/min) (Schwan Ganz) . | PCWP (Schwan Ganz) . | Maximum inotropic dose . |
|---|---|---|---|---|---|---|---|---|---|
| Before proning | 12 | 14.4 | 11 | P6 | 3 | 2.0 | 7.5 | 10 | Dobutamine 4γ + noradrenalin 0.1γ |
| During proning | 10 | 5.8 | 27 | P6 | 2.9 | 2.7 | —a | —a | Dobutamine 4γ + noradrenalin 0.14γ |
| After proning | 10 | 8.5 | 18 | P6 | 2.8 | 2.7 | —a | —a | Dobutamine 4γ + noradrenalin 0.14γ |
ECMO, extracorporeal membrane oxygenation; FIO2%, fraction of inspired oxygen in percentage; PCWP, pulmonary capillary wedge pressure; PEEP, positive end-expiratory pressure; PF, PaO2/FiO2.
aNo data are available due to the removal of dysfunctional Schwan Ganz in the prone position.
| . | PEEP . | Oxygenation index . | PF ratio . | Impella: P -stand . | Impella: cardiac output (L/min) . | ECMO: Output (L/min) . | Cardiac output (L/min) (Schwan Ganz) . | PCWP (Schwan Ganz) . | Maximum inotropic dose . |
|---|---|---|---|---|---|---|---|---|---|
| Before proning | 12 | 14.4 | 11 | P6 | 3 | 2.0 | 7.5 | 10 | Dobutamine 4γ + noradrenalin 0.1γ |
| During proning | 10 | 5.8 | 27 | P6 | 2.9 | 2.7 | —a | —a | Dobutamine 4γ + noradrenalin 0.14γ |
| After proning | 10 | 8.5 | 18 | P6 | 2.8 | 2.7 | —a | —a | Dobutamine 4γ + noradrenalin 0.14γ |
| . | PEEP . | Oxygenation index . | PF ratio . | Impella: P -stand . | Impella: cardiac output (L/min) . | ECMO: Output (L/min) . | Cardiac output (L/min) (Schwan Ganz) . | PCWP (Schwan Ganz) . | Maximum inotropic dose . |
|---|---|---|---|---|---|---|---|---|---|
| Before proning | 12 | 14.4 | 11 | P6 | 3 | 2.0 | 7.5 | 10 | Dobutamine 4γ + noradrenalin 0.1γ |
| During proning | 10 | 5.8 | 27 | P6 | 2.9 | 2.7 | —a | —a | Dobutamine 4γ + noradrenalin 0.14γ |
| After proning | 10 | 8.5 | 18 | P6 | 2.8 | 2.7 | —a | —a | Dobutamine 4γ + noradrenalin 0.14γ |
ECMO, extracorporeal membrane oxygenation; FIO2%, fraction of inspired oxygen in percentage; PCWP, pulmonary capillary wedge pressure; PEEP, positive end-expiratory pressure; PF, PaO2/FiO2.
aNo data are available due to the removal of dysfunctional Schwan Ganz in the prone position.
Two days later, we could explant the ECMO. Three days later, a computed tomography (CT) scan showed ground-glass opacification in all the lobes of the right lung and pleural effusion and atelectasis of the left lower lung (Figure 2). We proned the patient again due to respiratory failure, partially caused by immobilization of sputum while the Impella was unloading the ventricle with 2.5 L/min (performance level 4). Finally, after the second session of prone positioning, the patient’s respiratory metrics were stabilized, and we could wean off the Impella (1 day later) and stop the sedation. He could be woken up, but due to his ICU-acquired weakness, we performed a percutaneous tracheostomy to wean him from ventilatory support. He stayed for another 19 days in the ICU to wean from mechanical ventilation and to recover from ICU-acquired weakness. In addition, we could stop CVVHD, and there was no need for chronic renal replacement therapy. He was discharged to the cardiac care unit (CCU) for further optimization of his condition. After discharge, his cardiac function increased from severe LV dysfunction to moderate–severe LV dysfunction with an LV ejection fraction of 15% and 35%, respectively. At the CCU, he developed a fever, and a CT scan showed increased pleural effusion, which was deemed post-cardiac injury related. He received a thoracic drain and treatment with diuretics, leading to a stabilization of his clinical condition. He could be discharged to a revalidation facility without further complications. Five months after his discharge from the hospital, ambulatory rehabilitation could be started with swimming, walking, and cycling progression.
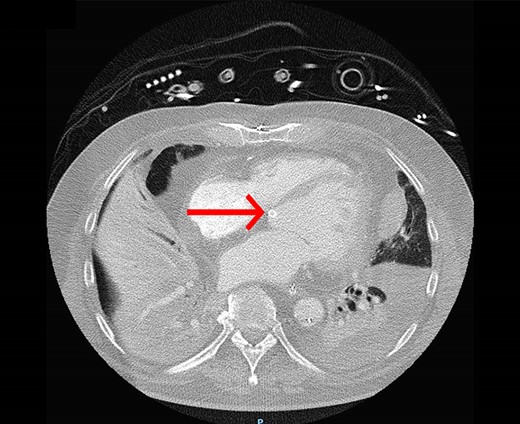
The computed tomography (CT) scan shows ground-glass opacification in the right lung, pleural effusion, and atelectasis of the left lower lung. The red arrow indicates the position of the Impella in the left ventricle.
Discussion
As far as we know, this case report demonstrated the first post-cardiotomy patient mechanically ventilated in a prone position while supported by an ECPELLA. Previous studies, predominantly in veno-venous ECMO patients, showed the efficiency and safety of proning position.1–4 However, only a couple of case and observational studies demonstrate the effectiveness of the prone position in patients with a VA ECMO.5,6 These studies showed that prone positioning could help avoid an additional venous cannula, which can potentially decrease arterial blood flow and cause mechanical complications. In experienced centres, there is increasing use of more advanced strategies by cannulation of three large vessels (‘triple’ cannulation), resulting in veno-veno-arterial or veno-arterio-venous (VAV) cannulation. Veno-veno-arterial ECMO intends to improve drainage and unloading, and it represents a very potent method to provide circulatory and respiratory support simultaneously. Such triple cannulation expands the field of application at the expense of the increased complexity of ECMO systems.7 Although VAV ECMO represents a rescue strategy in critically ill patients with combined respiratory failure and cardio-circulatory shock, the use is complex and is highly associated with complications.8,9 Our clinical case is unique, as we did not convert our VA ECMO supported by Impella for LV unloading, ECPELLA, to VAV ECMO, and may show that the conventional treatment of ARDS targeting homogeneous and protective ventilation of the lungs in a prone position may prevent mortality as reported before.10 In addition, we operated knowing this patient had a pre-operative severe LV systolic function. At the same time, other studies have not reported about the LV function (just) before implanting VA ECMO.5,6 Finally, we were able to wean the patient from ECPELLA in a total of 4 days, compared with an average of 8 days, as reported by Genty et al.5 in VA ECMO patients. It is worth mentioning that we started unloading the LV with relatively high P-levels after Impella was inserted. This was done as a response to increased dilation of the LV in combination with severe mitral regurgitation (MR). However, this is not according to our standard care, where a gradual increase of P-levels is recommended and also to not start unloading the LV at levels above P4 (estimated flow output of 1.5–2 L/min) on top of VA ECMO since unloading too quickly could increase cardiopulmonary flow eventually leading to Harlequin syndrome causing ARDS.
Harlequin syndrome11 is a rare complication of peripheral VA ECMO that can occur when LVF is recovering, but the lungs are still poorly functioning. It is known that unloading the LV with Impella on top of VA ECMO could lead to improved cardiopulmonary circulation.12,13 However, Impella, especially in the case of higher P-levels, could mimic potentially recovering LVF, which can cause the mixing of deoxygenated blood from severely damaged lungs with oxygenated blood from the VA ECMO in the aortic arch. This is a potential way of competition between VA ECMO and Impella flow and could worsen multi-organ failure, which we have successfully managed by proning the patient. Therefore, Harlequin syndrome should be considered in the early phases of unloading the LV by Impella on top of VA ECMO. This potentially unexpected side effect of starting Impella with higher P-levels could have contributed to the worsening of the lung function and other factors, such as atelectasis, pneumonia, severe LV dysfunction, and severe MR. There is an unmet need for more studies in unloading after VA ECMO.
Conclusion
The clinical benefit of unloading the LV with mechanical circulatory support during ECMO support has not yet been verified in a randomized study. However, in this case, there was a promising role for unloading the LV with Impella during VA ECMO, even initially leading to Harlequin syndrome, worsening the ARDS, resulting in successfully proning on ECPELLA.
Lead author biography
 Marjolein C. de Jongh is a cardiologist in training at Haga Teaching Hospital, the Hague, the Netherlands. Her main areas of interest include cardiovascular imaging with a focus on Cardiac MRI and computing in cardiology. She is a PhD student in Interventional Cardiac MRI.
Marjolein C. de Jongh is a cardiologist in training at Haga Teaching Hospital, the Hague, the Netherlands. Her main areas of interest include cardiovascular imaging with a focus on Cardiac MRI and computing in cardiology. She is a PhD student in Interventional Cardiac MRI.
Acknowledgements
The authors thank Dr Ralph Nowitzky for his contribution to this case report and treatment of the patient at the intensive care. They also thank Prof Dr Osama Soliman for proofreading the manuscript.
Consent: The authors confirm that the written consent for submission and publication of this case report, including images and associated text, has been obtained from the patient in line with COPE guidance. Informed consent was obtained from the patient to publish this case report (and the accompanying images).
Funding: None declared.
Data availability
The data that support the findings of this case report are available upon request from the corresponding author.
References
Author notes
Conflict of interest: None declared.




Comments