-
PDF
- Split View
-
Views
-
Cite
Cite
Béatrice Susanne Kahl, Manfred Marx, Matthias Gass, Dominik Wiedemann, Ina Michel-Behnke, Mechanical circulatory support as bridge to recovery in an 8-year-old girl with tachycardia-induced cardiomyopathy due to atypical atrioventricular nodal re-entrant tachycardia: a case report, European Heart Journal - Case Reports, Volume 8, Issue 10, October 2024, ytae509, https://doi.org/10.1093/ehjcr/ytae509
Close - Share Icon Share
Abstract
Incessant tachycardias can severely impair cardiac function, which is known as tachycardia-induced cardiomyopathy (TIC). The cornerstone of successful therapy is heart rate control. Otherwise, heart failure requiring mechanical circulatory support (MCS) and ultimately heart transplantation may evolve. We report a case of successful weaning from MCS after severe TIC due to the very rarely incessant atypical atrioventricular nodal re-entrant tachycardia (aAVNRT) with subsequent successful radiofrequency ablation (RFA).
An 8-year-old girl was transferred to our unit with severely impaired cardiac function and went into circulatory collapse, including cardiopulmonary resuscitation. Stabilization was possible only by MCS, first by venoarterial extracorporeal membrane oxygenation, switched to long-term MCS (Berlin Heart EXCOR® Pediatric). However, pharmacological control of heart rate allowed myocardial recovery and finally the device was successfully explanted. As TIC was causative for the cardiomyopathy, the patient underwent invasive electrophysiological mapping and subsequent curative ablation of an aAVNRT.
This case report describes technical considerations of both the special electrophysiological aspects of this rare tachycardia and the weaning protocol from a pulsatile ventricular assist device in a young child who finally recovered from TIC. Pharmacological heart rate control delayed curative RFA until explantation of the MCS.
Tachycardia-induced cardiomyopathy requiring mechanical circulatory support has the potential to recover after heart rate control.
Berlin Heart Excor ventricular assist device offers the application of detailed weaning protocols in children and allows bridge to recovery.
Introduction
In children, the most common incessant tachycardias that may lead to dilated cardiomyopathy [tachycardia-induced cardiomyopathy (TIC)] are ectopic atrial tachycardia and permanent junctional re-entrant tachycardia (PJRT).1,2 Only rarely incessant atypical atrioventricular nodal re-entrant tachycardias (aAVNRTs) have been described in this context.3,4
Due to substantial left ventricular (LV) function impairment, 16% of patients require mechanical circulatory support (MCS) at initial presentation and or during the subsequent clinical course.2
Tachycardia-induced cardiomyopathy is potentially reversible if pharmacological heart rate (HR) control or conversion to sinus rhythm (SR) can be achieved consistently.5 If not, it is reasonable to consider early catheter ablation (CA). After long-term MCS, weaning can rarely be achieved; however, in patients with TIC, successful weaning has been described.6
We report a young child with TIC related to incessant aAVNRT, requiring MCS with subsequent CA.
Case presentation
An 8-year-old girl (22 kg, 126 cm), suffering from malaise for 3 weeks, was admitted with supraventricular tachycardia unresponsive to adenosine. There was no history of chronic disease, episodes of palpitations, or syncope. She presented pale, awake, orientated, and eupnoeic. Heart rate was 235/min and blood pressure was 69/56 mmHg. Electrocardiogram showed narrow complex tachycardia with a cycle length (CL) of 260 ms with negative p-waves in leads II, III, and aVF and inferior QRS axis (interval between R-wave and P-wave, RP 180 ms and interval between P-wave and R-wave, PR 80 ms) (Figure 1). Echocardiogram revealed normal intra-cardiac anatomy without valve abnormalities, severely dilated LV [LV end-diastolic diameter (LVEDD) 60.6 mm, z-score +4.41], moderate mitral valve regurgitation (MVR), severely reduced LV, and moderately reduced right ventricular function (LV fraction shortening 11.2%) (Supplementary material online, Video 1). N-terminal pro-brain natriuretic peptide level exceeded 35 000 pg/mL. Another attempt with adenosine (6 mg) failed; however, a few minutes later, she spontaneously converted to SR with 130/min followed by immediate circulatory collapse. Advanced life support failed to achieve stable cardiopulmonary function and venoarterial extracorporeal membrane oxygenation (ECMO) was installed. Three days later, ECMO was switched to the pulsatile Berlin Heart EXCOR® Pediatric biventricular assist device (BiVAD) using 30 mL ventricles. Anticoagulation with bivalirudin, clopidogrel, and aspirin was administered according to ACTION harmonized protocols.7 To facilitate patient’s mobility, the primary device was switched to the mobile driving unit EXCOR® Active 2 weeks later. Cardiomyopathy causes other than TIC were ruled out with LV endomyocardial biopsy (site of pump implantation). Histological and immunohistochemical examinations and search for the viral genome as well as genetic analysis (whole exome sequencing, including all candidate genes for dilatative cardiomyopathy and ion channel diseases) revealed negative results. Cardiac magnetic resonance (CMR) imaging was technically not feasible on ECMO or VAD, after explantation radiologists refused CMR because of the metallic parts within the chest.
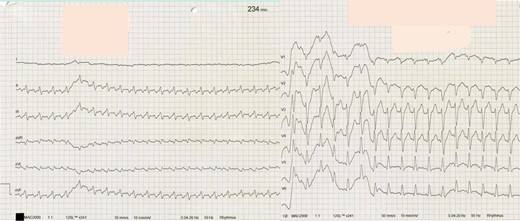
Twelve-lead electrocardiogram with narrow complex tachycardia with a cycle length of 260 ms with negative p-waves in leads II, III, and aVF and inferior QRS axis (interval between R-wave and P-wave, RP 180 ms, interval between P-wave and R-wave, PR 80 ms).
With continuous administration of amiodarone and esmolol, stable SR was achieved on Day 5 after VAD implantation; however, the patient never succeeded in full switch to oral medication without returning to tachycardia. Six weeks after VAD implantation, improved heart function with LV ejection fraction (LVEF) of 57% supported prospective weaning from MCS7 (Figure 2). Echocardiography revealed normalized LVEDD (39 mm, z + 0.01), LVEF (54%), and only mild MVR. After one night with intentionally reduced pump rates of 55/min (left) and 50/min (right), heart function was still normal; therefore, pump rates were reduced by 5/min every 5 min and finally paused for 15 min under vigorous monitoring. On Day 3 of the weaning protocol, haemodynamic parameters were invasively measured after gradually decreasing pump rates until the complete pump stop. Cardiac output index was 3.85 L/kg ∗ min after 25 min of pump stop. Pulmonary vascular resistance index increased to 3 wood units and the ratio pulmonary/systemic vascular resistance rose from 0.11 to 0.19. End-diastolic LV pressure stayed at 13 mmHg. Lactate levels remained normal and echocardiographic parameters unchanged. Consequently, BIVAD was explanted and electrophysiology study (EPS) was performed 1 week later. The clinically documented tachycardia (CL 300 ms, RP interval 230 ms, and PR interval 70 ms) was reproduced by programmed atrial stimulation, and programmed singular ventricular extra beats revealed no proceeding and no reset of tachycardia supporting the diagnosis of aAVNRT. During tachycardia, local activation time mapping was performed with a TactiCath® steerable contact force ablation catheter (Abbott®). The earliest atrial activation was found at the roof of the coronary sinus ostium, where irrigated radiofrequency ablation (RFA) (40°C/43 W) led to the termination of tachycardia within 2 s at the first attempt (total RFA time 120 s, energy 4600 J) (Figure 3). Antiarrhythmic drugs were stopped immediately after EPS/RFA.
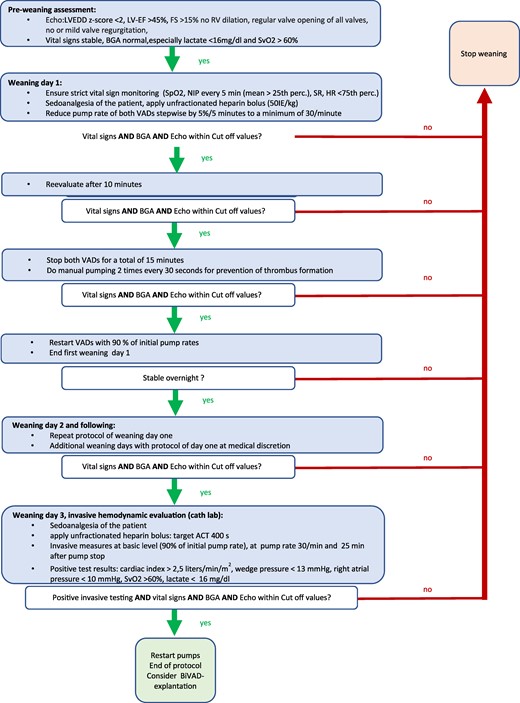
Weaning protocol. ACT, activated clotting time; BIVAD, biventricular assist device; cvBGA, central venous blood gas; EF, ejection fraction; FS, fractional shortening; HR, heart rate; LVEDD, left ventricular end-diastolic diameter; NIP, non-invasive blood pressure; perc., percentile; RV, right ventricle; SpO2, pulsoxymetric saturation; SR, sinus rhythm; SvO2, central venous saturation; VAD, ventricular assist device.
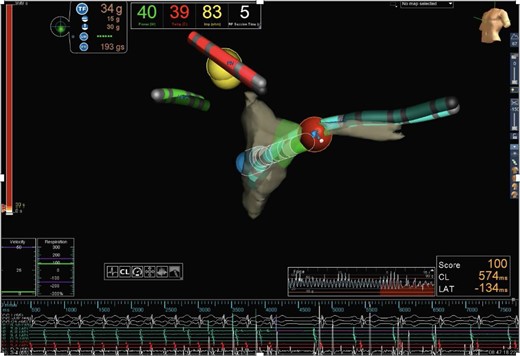
Activation mapping using radiofrequency ablation catheter (TactiCath® Contact Force Ablation Catheter) and EnSite Precision Cardiac Mapping System®, Abbott. At the roof of the coronary sinus ostium, the earliest atrial activation was documented. Irrigated radiofrequency ablation (40°C/43 W) was performed in this region, leading to tachycardia termination within 2 s at the first attempt. Anatomic orientation: the green catheter with the labeling ‘RA’ is located in the right atrium; the red catheter with the labeling ‘RV’ is located in the right ventricle; the turquoise catheter with the labeling “CS” is located in the CS; and the red dot marks the ablation region at the roof of the CS ostium.
Two months after admission, 18 days after BIVAD explantation, and 5 days after aAVNRT ablation the girl was discharged fully mobilized with normalized LVEF and without clinical or neurological sequelae (Supplementary material online, Video 2). Twenty-four months after the initial presentation, heart function was completely normal without recurrence of aAVNRT.
Discussion
This case report presents the successful weaning of a young child with TIC from MCS with subsequent successful RFA of aAVNRT. Patients with this arrhythmia are often asymptomatic for various periods despite constantly increased HR. Thus, the onset of the girl’s symptoms with discomfort did not necessarily indicate the onset of tachycardia but rather severe impairment of myocardial function.
Short-term ECMO therapy as a bridge to recovery seems a reasonable approach; however, the duration of MCS support is usually not predictable at the time of implantation. Long-term VAD therapy lowers the risk of haemostaseological complications and difficulties with the mobilization of the patient as well.
Although EPS under MCS has been described,8,9 in our patient, recovery of cardiac function came along with increasing episodes of lowered HR and SR. Given these findings, RFA, which would have been unreasonably complex on BiVAD, was postponed.
In paediatric TIC patients, the overall median time to recovery has been reported with 51 days for LVEF and 71 days for LVEDD, whereas the time to recovery in patients on MCS is significantly shorter. In general, 13% of children show myocardial recovery on Berlin Heart EXCOR®.7,10 Potential candidates for weaning from VAD can be identified by various echocardiographic parameters.11 A 5-min pump-off time is considered possible, if regular opening of the aortic and pulmonary valve, competent AV valves, and a normal LVEDD z-scores (<+2.0) are given.6 In our patient, LVEDD was 39 mm (z + 0.01) and LVEF 54%. Nevertheless, echocardiographic assessment of myocardial function on VADs remains challenging. From published weaning protocols, we adopted the haemodynamic cut-off values published by Miera et al.10–13 Ventricular assist device support was reduced to pump stop on 3 consecutive days as described in Figure 2. Since echocardiography, vital signs and invasive testing were promising, BIVAD was successfully explanted, and EPS/RFA was performed 1 week later.
Patients with AVNRT do have dual AV node with fast and slow conducting fibres capable of ante- and retrograde conduction. Only 6.4% are considered aAVNRT with specifically delayed retrograde atrial activation (His to atrial interval > 70 ms),4 favourable for maintaining incessant tachycardia very similar to PJRT. Reliable differentiation is only possible by EPS.2 Atypical atrioventricular nodal re-entrant tachycardias are associated with lower ablation success rates, and the optimal technique is not established.8,14 In our patient, cooled RFA was effective within 2 s of the first attempted lesion (40°/42 W) (Figure 3).
Conclusion
In conclusion, this case report presents the successful weaning of a young child with TIC from MCS with subsequent successful RFA of an aAVNRT. Tachycardia-induced cardiomyopathy, secondary to incessant aAVNRT, is very likely reversible, if HR or rhythm control is achievable. The use of MCS might significantly reduce the time to recovery and herewith mitigates the necessity of HTX. Combined echocardiographic and invasive assessment during MCS helps to define candidates for weaning. To the best of our knowledge, this is the first paediatric report of successful weaning from the new Berlin Heart EXCOR® Active driving unit after TIC due to aAVNRT.
Lead author biography

Dr Béatrice Susanne Kahl graduated from the Medical University of Vienna in July 2018. She then started her residency in paediatrics and adolescent medicine at the Medical University of Vienna with specialty training in paediatric cardiology at the Pediatric Heart Center Vienna, Austria.
Supplementary material
Supplementary material is available at European Heart Journal – Case Reports online.
Consent: The authors confirm that written consent for submission and publication of this case report including images and associated text has been obtained from the minor patient’s parents in line with the COPE guidance.
Funding
None declared.
Data availability
The data underlying this article are available in the article and its online Supplementary material.
References
Author notes
Conflict of interest. The authors declare no conflicts of interest.




Comments