-
PDF
- Split View
-
Views
-
Cite
Cite
Muhammad Umer, Matthew Peters, Hardeep Dholiya, Usman Sagheer, Sagar Bhandari, Shahab Ghafghazi, Mark S Slaughter, Dinesh K Kalra, Multiple recurrences of a left ventricular pseudoaneurysm: a case report, European Heart Journal - Case Reports, Volume 8, Issue 8, August 2024, ytae382, https://doi.org/10.1093/ehjcr/ytae382
Close - Share Icon Share
Abstract
Takotsubo syndrome (TTS) is being diagnosed more often with its increased recognition over the past 2 decades and with the availability of imaging such as point-of-care echocardiography and tissue characterization by cardiovascular magnetic resonance (CMR).
A young man developed pericarditis and was treated with steroids. A few weeks later, he suffered classic TTS and then presented a week later with the rare complication of apical myocardial rupture and a left ventricular (LV) pseudoaneurysm. He subsequently sustained two recurrences, likely secondary to the poor tensile strength of the repair in the region of necrotic myocardium.
Various features of both syndromes are discussed herein (myopericarditis and TTS) as well as their classic imaging findings with an emphasis on the echocardiographic diagnosis of an LV pseudoaneurysm and differentiating it from an aneurysm. Furthermore, we elucidate the classic imaging findings of CMR in myocarditis, myocardial infarction with non-obstructive coronary arteries, and TTS. Lastly, we discuss treatment options for LV pseudoaneurysms and strategies to prevent recurrence.
Acute viral pericarditis may mimic an acute coronary syndrome or MINOCA. However, myocardial involvement may not be fully evident in the early stages. Initial treatment should be guided by the clinical history and severity of cardiovascular involvement and typically consists of colchicine and non-steroidal anti-inflammatory agents.
This case emphasizes the importance of considering takotsubo syndrome (TTS) variants in chest pain presentation without obstructive coronary artery disease, following emotional or physical stressors, and rarely may be complicated by recurrences or even ventricular wall rupture.
Multimodality cardiac imaging, including echocardiography, cardiac CT, and CMR, plays a key role in diagnosing myopericarditis and TTS, as well as identifying rare complications. These modalities provide a comprehensive assessment, assist in accurate diagnosis, inform prognosis, and guide therapeutic decision-making.
The case highlights the importance of timely diagnosis of ventricular wall rupture and early surgical intervention using overlying supportive material like a patch to reduce the risk of recurrence. Close follow-up is vital due to the potential for recurrences despite surgical repair.
Primary specialties involved other than cardiology
Rheumatology, pathology, cardiothoracic surgery.
Introduction
Cardiac rupture is a rare but potentially fatal complication of takotsubo syndrome (TTS). Our understanding of the pathophysiology, triggers, risk stratification, management, and serious complications such as cardiogenic shock, malignant ventricular arrhythmias, and ventricular rupture associated with TTS continues to evolve. We report a case of a young man who developed left ventricular (LV) rupture and a pseudoaneurysm (PsA) as a complication of TTS who later had two more recurrences (at 6 and 17 months after the initial PsA repair).
Case presentation
A 32-year-old man presented to an outside facility with sharp left-sided chest pain for 3 days with associated mild dyspnoea and a low-grade fever. In the emergency department, he was febrile to 100.5°F and tachycardic (120 b.p.m.). On physical exam, his cardiopulmonary exam was benign with no murmur, rub, gallops, or rales auscultated. Serum troponin was normal, and electrocardiogram (EKG) showed minor diffuse ST elevations (Figure 1). Transthoracic echocardiogram (TTE) showed normal LV function and no pericardial effusion (see Supplementary material online, Video S1A and B). Given his lack of coronary risk factors and his symptoms, he was diagnosed with presumed acute viral pericarditis and was treated with oral prednisone 40 mg for 2 weeks. His symptoms abated over the ensuing week.
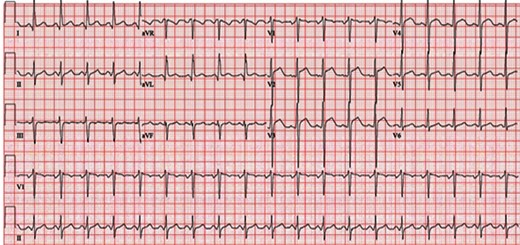
Electrocardiogram performed on initial presentation showing sinus tachycardia (120 b.p.m.) and J-point and ST elevation in precordial leads V2–V4.
Six weeks later, he again presented to the same outlying emergency department with chest pressure and dyspnoea. He recounted an episode of chest pain, described as sudden-onset pressure in the middle of his chest, about 7 days prior after a heated argument with a family member. This pain, as well as dyspnoea, waxed and waned over the subsequent few days but worsened on the day of presentation. In the emergency department, his heart rate was 104 b.p.m., and he was hypotensive with a blood pressure of 99/72 mmHg, was tachypnoeic with a respiratory rate of 26 breaths/minute, and had an oxygen saturation of 91% on room air. His cardiovascular physical exam, with the exception of his low-grade tachycardia, was again normal, but he appeared anxious. EKG showed sinus tachycardia and ST elevations in the anterior leads (Figure 2). Serum troponin I levels were mildly elevated (1.6 ng/mL; normal ≤ 0.04 ng/mL). Urine toxicology screen was negative. Urgent TTE identified normal to increased basal wall contractility, mildly reduced overall LV ejection fraction (LVEF) of 45%, apical hypokinesis, and an apical PsA (Figure 3A, Supplementary material online, Videos S2 and S3). He was taken emergently to the cardiac catheterization laboratory where coronary angiography showed normal coronaries with no thrombus, dissection, plaque rupture, or slow flow identified. He was transferred to our tertiary care university hospital. After transfer and initial evaluation, the differential diagnosis included TTS, myocardial infarction in the absence of obstructive coronary artery disease (from embolism or spasm), and myocarditis. Myocarditis was considered less likely during this presentation due to the absence of a viral prodrome, fever, systemic features, or classic cardiovascular magnetic resonance (CMR) findings. The diagnosis of TTS was favoured due to the patient’s history of a recent emotional stressor as well as the findings of normal coronaries in addition to classic wall motion abnormalities including focal apical hypokinesis. Computed tomography angiography (CTA) and CMR (Figure 3B and C, Supplementary material online, Video S4) confirmed the presence of a large PsA at the LV apex with to-and-fro blood flow between the LV and PsA cavity. Myocardial oedema in the distal walls and hypokinesis were visualized; however, no late gadolinium enhancement (LGE) was noted which again supported the diagnosis of TTS. The patient underwent emergent surgical repair of the PsA with primary oversewing of the myocardial defect (1.5 cm) with 4–0 Prolene sutures in a cerclage fashion. Next, full-thickness, pledgeted 4–0 Prolene sutures were placed to completely close the defect. The PsA sac was resected, leaving a small rim, which was then closed over the defect repair using a 4–0 Prolene horizontal mattress sutures followed by a running stitch. Histopathology showed necrosis of the adjacent myocardium without any classic features of myocarditis (no lymphocytic infiltrate or giant cells) or evidence of Chagas disease, amyloid or iron deposition, vasculitis, myocyte hypertrophy, or myocyte disarray (Figure 3D). He did well postoperatively and was discharged home.
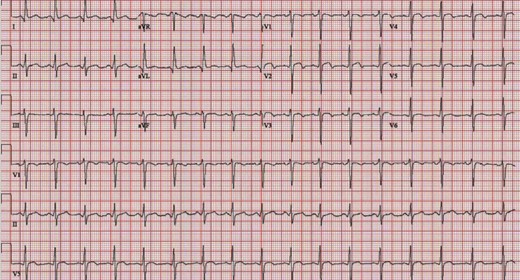
Electrocardiogram on first rupture showing sinus tachycardia (104 b.p.m.) and J-point and ST elevation in precordial leads V2–4 with T-wave inversion in V1–V4.
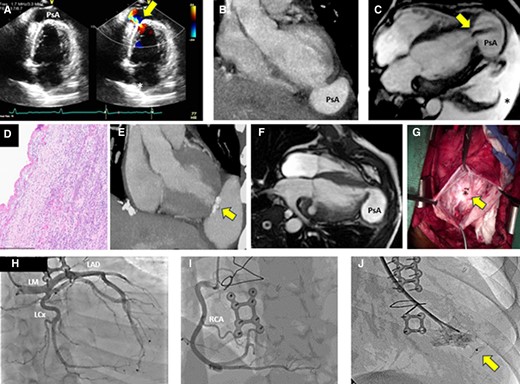
(A) TTE apical two-chamber view (first rupture) shows apical PsA and colour Doppler demonstrates to-and-fro flow (arrow). (B) CTA (first rupture), in the coronal view, shows contrast collection (PsA) at the LV apex. (C) CMR (1.5 T) SSFP sequence four-chamber image (first rupture) showing a large (55 × 39 mm) apical PsA with dephasing signal from flow into the PsA cavity (arrow) and a large left pleural effusion (asterisk). (D) Histopathological examination (first rupture) with H&E stain shows no evidence of vasculitis or myocarditis. (E) CTA shows recurrent PsA (second rupture). There are also calcifications noted (arrow) at the prior surgical repair site. (F) Six months later (second rupture), the patient had the development of a new PsA cavity (67 × 52 mm) adjacent to the site of surgical repair of the initial rupture. (G) Apical defect (15 mm) seen during the second rupture after evacuation of the haematoma. (H and I) Coronary angiography (third rupture) shows normal left and right coronary arteries. (J) An Amplatzer occluder (12 mm) was successfully implanted to seal the defect in the LV apex during the third rupture (arrow). TTE, transthoracic echocardiogram; PsA, pseudoaneurysm; LV, left ventricular; CTA, computed tomography angiography; CMR, cardiovascular magnetic resonance; SSFP, steady-state free precession; H&E, haematoxylin and eosin; LM, left main; LAD, left anterior descending artery; LCx, left circumflex artery; RCA, right coronary artery.
Six months after his operation, he returned to our hospital with sudden-onset dyspnoea and chest pain. Computed tomography angiography of the chest did not identify a pulmonary embolism but did visualize a recurrent perforation of the LV apex with PsA formation lateral to the previous repair (Figure 3E). Cardiovascular magnetic resonance confirmed the presence of a recurrent apical LV PsA (Figure 3F, Supplementary material online, Video S5). He underwent redo sternotomy and surgical repair of the rupture. Intraoperatively, an additional defect was identified lateral to the previous repair (Figure 3G). This defect was closed by oversewing with 3–0 Prolene in multiple layers and then sealing with BioGlue adhesive with the PsA sac again resected. He was discharged after a week in stable condition. Histopathology was again unrevealing and showed thinned-out myocardium at the margin of the PsA. As an outpatient, a comprehensive rheumatologic, immunologic, and haematological workup for malignancy, collagen vascular diseases, and autoimmune diseases with extensive serologies and a bone marrow biopsy was obtained. This workup returned negative.
The patient again presented to our emergency department 11 months after his second operation with chest pain after doing well since discharge. A third rupture (or second recurrence) was identified. Repeat coronary angiography showed normal coronaries (Figure 3H and I). After discussion with the multidisciplinary Heart Team, the patient opted for percutaneous closure of this third defect with an Amplatzer device (Figure 3J). Since this procedure 10 months ago, he has done well and is asymptomatic, walking one mile daily.
Discussion
The patient’s initial presentation was likely due to acute viral pericarditis with mild subclinical myocarditis. His EKG and symptoms were consistent with pericarditis, and the lack of troponin elevation suggested the absence of significant myocyte injury (albeit the troponin level obtained in this case was not a high-sensitivity assay). Furthermore, evaluation was performed early in the disease course when full-blown myocardial involvement may not have manifested yet. The administration of steroids early on may have paradoxically increased the chance of apical myocardial rupture when he presented 6 weeks later. European Society of Cardiology guidelines recommend colchicine with or without non-steroidal anti-inflammatory drugs (NSAIDs) as the first-line agents for the management of pericarditis.1 Corticosteroids are not recommended in viral pericarditis, as they may reactivate viral infections and lead to ongoing inflammation. Cardiovascular magnetic resonance can be useful in diagnosing myocarditis in the early to mid-disease course by identifying oedema, inflammation, and subepicardial or mid-wall LGE patterns, typical for cases of myocarditis. Endomyocardial biopsy remains the definitive diagnostic test but is not often performed or needed in typical cases due to the risks inherent to the procedure as well as the patchy nature of the disease2,3 (Table 1).
Clinical, laboratory, and imaging features that help differentiate between TTS (takotsubo syndrome), myocarditis, and acute myocardial infarction (AMI)
| . | Favours TTS . | Favours myocarditis . | Favours AMI . |
|---|---|---|---|
| Typical presentation | Post-menopausal woman with chest pain after an emotional or physical stressor | Young or middle-aged adult with viral prodrome, chest pain, and ECG abnormalities. Drug-induced cases usually associated with known offending cardiotoxic agents (e.g. immune checkpoint inhibitors) | Middle-aged or elderly adults with traditional risk factors and symptoms suggesting acute ischaemia |
| Systemic features | Usually none | Based on underlying aetiology—in viral myocarditis—fever, and systemic symptoms involving respiratory and gastrointestinal systems | Rales or poor peripheral perfusion may be seen in large MI |
| Aetiology | Catecholamine surge related to emotional or physical stress | Viral or bacterial infection, autoimmune diseases, and drug reactions | Atherothrombotic CAD precipitated by atherosclerotic plaque disruption, thrombus, or coronary dissection |
| Electrocardiogram | Reversible STE, STD, LBBB, T-wave inversion, and/or QTc prolongation | Non-specific ST and T-wave changes, diffuse PR depression with or without STE | Hyperacute T-waves or T-wave inversion, STD or STE, pathologic Q-waves |
| Biomarkers | Mildly elevated troponin and significantly elevated serum natriuretic peptides. ECG changes more dramatic than troponin elevation would suggest | Mildly elevated troponin and significantly elevated inflammatory markers | Significantly elevated troponin and mildly elevated serum natriuretic peptides |
| Coronary artery involvement | Normal or non-obstructive CAD | Normal or non-obstructive CAD | Obstructive CAD, usually plaque rupture or erosion leading to thrombotic occlusion |
| Ventricular function | Transient LV dysfunction extending beyond a single epicardial coronary distribution, typically circumferential apical hypokinesia/akinesia/dyskinesia (apical ballooning) with basal hyperkinesis (less likely involving mid and basal LV; rarely focal) | Normal or global LV systolic dysfunction, occasionally regional wall motion abnormalities | Regional wall motion abnormalities in a coronary artery distribution |
| Ventricular structure/tissue characterization | Diffuse and transmural LV myocardial oedema matching wall motion abnormalities but notable absence of LGE | Mid-wall or subepicardial oedema, with LGE (88%) in a patchy pattern not in a coronary distribution, pericardial enhancement may be present | Transmural or subendocardial oedema with LGE in a coronary distribution |
| LV outflow tract obstruction | Due to hypercontractility of the base (14–25%) | Absent | Absent |
| Pericardial involvement | Unusual | Pericarditis with pericardial effusion is common | Unusual |
| Complications: aneurysm, pseudoaneurysm, intramyocardial haemorrhage, and ventricular wall rupture | Rare and generally diagnosed post-mortem; risk factors include advanced age, females, HTN, persistent STE, absence of T-wave inversion in lateral leads, higher LVEF, and low frequency of beta-blocker use | Rare | Seen in 5–10% of cases, especially with delayed revascularization, advanced age, females, HTN, persistent STE |
| . | Favours TTS . | Favours myocarditis . | Favours AMI . |
|---|---|---|---|
| Typical presentation | Post-menopausal woman with chest pain after an emotional or physical stressor | Young or middle-aged adult with viral prodrome, chest pain, and ECG abnormalities. Drug-induced cases usually associated with known offending cardiotoxic agents (e.g. immune checkpoint inhibitors) | Middle-aged or elderly adults with traditional risk factors and symptoms suggesting acute ischaemia |
| Systemic features | Usually none | Based on underlying aetiology—in viral myocarditis—fever, and systemic symptoms involving respiratory and gastrointestinal systems | Rales or poor peripheral perfusion may be seen in large MI |
| Aetiology | Catecholamine surge related to emotional or physical stress | Viral or bacterial infection, autoimmune diseases, and drug reactions | Atherothrombotic CAD precipitated by atherosclerotic plaque disruption, thrombus, or coronary dissection |
| Electrocardiogram | Reversible STE, STD, LBBB, T-wave inversion, and/or QTc prolongation | Non-specific ST and T-wave changes, diffuse PR depression with or without STE | Hyperacute T-waves or T-wave inversion, STD or STE, pathologic Q-waves |
| Biomarkers | Mildly elevated troponin and significantly elevated serum natriuretic peptides. ECG changes more dramatic than troponin elevation would suggest | Mildly elevated troponin and significantly elevated inflammatory markers | Significantly elevated troponin and mildly elevated serum natriuretic peptides |
| Coronary artery involvement | Normal or non-obstructive CAD | Normal or non-obstructive CAD | Obstructive CAD, usually plaque rupture or erosion leading to thrombotic occlusion |
| Ventricular function | Transient LV dysfunction extending beyond a single epicardial coronary distribution, typically circumferential apical hypokinesia/akinesia/dyskinesia (apical ballooning) with basal hyperkinesis (less likely involving mid and basal LV; rarely focal) | Normal or global LV systolic dysfunction, occasionally regional wall motion abnormalities | Regional wall motion abnormalities in a coronary artery distribution |
| Ventricular structure/tissue characterization | Diffuse and transmural LV myocardial oedema matching wall motion abnormalities but notable absence of LGE | Mid-wall or subepicardial oedema, with LGE (88%) in a patchy pattern not in a coronary distribution, pericardial enhancement may be present | Transmural or subendocardial oedema with LGE in a coronary distribution |
| LV outflow tract obstruction | Due to hypercontractility of the base (14–25%) | Absent | Absent |
| Pericardial involvement | Unusual | Pericarditis with pericardial effusion is common | Unusual |
| Complications: aneurysm, pseudoaneurysm, intramyocardial haemorrhage, and ventricular wall rupture | Rare and generally diagnosed post-mortem; risk factors include advanced age, females, HTN, persistent STE, absence of T-wave inversion in lateral leads, higher LVEF, and low frequency of beta-blocker use | Rare | Seen in 5–10% of cases, especially with delayed revascularization, advanced age, females, HTN, persistent STE |
LV, left ventricular; LVEF, left ventricular ejection fraction; CAD, coronary artery disease; LGE, late gadolinium enhancement; LBBB, left bundle branch block; STE, ST segment elevation; STD, ST segment depression; HTN, hypertension.
Clinical, laboratory, and imaging features that help differentiate between TTS (takotsubo syndrome), myocarditis, and acute myocardial infarction (AMI)
| . | Favours TTS . | Favours myocarditis . | Favours AMI . |
|---|---|---|---|
| Typical presentation | Post-menopausal woman with chest pain after an emotional or physical stressor | Young or middle-aged adult with viral prodrome, chest pain, and ECG abnormalities. Drug-induced cases usually associated with known offending cardiotoxic agents (e.g. immune checkpoint inhibitors) | Middle-aged or elderly adults with traditional risk factors and symptoms suggesting acute ischaemia |
| Systemic features | Usually none | Based on underlying aetiology—in viral myocarditis—fever, and systemic symptoms involving respiratory and gastrointestinal systems | Rales or poor peripheral perfusion may be seen in large MI |
| Aetiology | Catecholamine surge related to emotional or physical stress | Viral or bacterial infection, autoimmune diseases, and drug reactions | Atherothrombotic CAD precipitated by atherosclerotic plaque disruption, thrombus, or coronary dissection |
| Electrocardiogram | Reversible STE, STD, LBBB, T-wave inversion, and/or QTc prolongation | Non-specific ST and T-wave changes, diffuse PR depression with or without STE | Hyperacute T-waves or T-wave inversion, STD or STE, pathologic Q-waves |
| Biomarkers | Mildly elevated troponin and significantly elevated serum natriuretic peptides. ECG changes more dramatic than troponin elevation would suggest | Mildly elevated troponin and significantly elevated inflammatory markers | Significantly elevated troponin and mildly elevated serum natriuretic peptides |
| Coronary artery involvement | Normal or non-obstructive CAD | Normal or non-obstructive CAD | Obstructive CAD, usually plaque rupture or erosion leading to thrombotic occlusion |
| Ventricular function | Transient LV dysfunction extending beyond a single epicardial coronary distribution, typically circumferential apical hypokinesia/akinesia/dyskinesia (apical ballooning) with basal hyperkinesis (less likely involving mid and basal LV; rarely focal) | Normal or global LV systolic dysfunction, occasionally regional wall motion abnormalities | Regional wall motion abnormalities in a coronary artery distribution |
| Ventricular structure/tissue characterization | Diffuse and transmural LV myocardial oedema matching wall motion abnormalities but notable absence of LGE | Mid-wall or subepicardial oedema, with LGE (88%) in a patchy pattern not in a coronary distribution, pericardial enhancement may be present | Transmural or subendocardial oedema with LGE in a coronary distribution |
| LV outflow tract obstruction | Due to hypercontractility of the base (14–25%) | Absent | Absent |
| Pericardial involvement | Unusual | Pericarditis with pericardial effusion is common | Unusual |
| Complications: aneurysm, pseudoaneurysm, intramyocardial haemorrhage, and ventricular wall rupture | Rare and generally diagnosed post-mortem; risk factors include advanced age, females, HTN, persistent STE, absence of T-wave inversion in lateral leads, higher LVEF, and low frequency of beta-blocker use | Rare | Seen in 5–10% of cases, especially with delayed revascularization, advanced age, females, HTN, persistent STE |
| . | Favours TTS . | Favours myocarditis . | Favours AMI . |
|---|---|---|---|
| Typical presentation | Post-menopausal woman with chest pain after an emotional or physical stressor | Young or middle-aged adult with viral prodrome, chest pain, and ECG abnormalities. Drug-induced cases usually associated with known offending cardiotoxic agents (e.g. immune checkpoint inhibitors) | Middle-aged or elderly adults with traditional risk factors and symptoms suggesting acute ischaemia |
| Systemic features | Usually none | Based on underlying aetiology—in viral myocarditis—fever, and systemic symptoms involving respiratory and gastrointestinal systems | Rales or poor peripheral perfusion may be seen in large MI |
| Aetiology | Catecholamine surge related to emotional or physical stress | Viral or bacterial infection, autoimmune diseases, and drug reactions | Atherothrombotic CAD precipitated by atherosclerotic plaque disruption, thrombus, or coronary dissection |
| Electrocardiogram | Reversible STE, STD, LBBB, T-wave inversion, and/or QTc prolongation | Non-specific ST and T-wave changes, diffuse PR depression with or without STE | Hyperacute T-waves or T-wave inversion, STD or STE, pathologic Q-waves |
| Biomarkers | Mildly elevated troponin and significantly elevated serum natriuretic peptides. ECG changes more dramatic than troponin elevation would suggest | Mildly elevated troponin and significantly elevated inflammatory markers | Significantly elevated troponin and mildly elevated serum natriuretic peptides |
| Coronary artery involvement | Normal or non-obstructive CAD | Normal or non-obstructive CAD | Obstructive CAD, usually plaque rupture or erosion leading to thrombotic occlusion |
| Ventricular function | Transient LV dysfunction extending beyond a single epicardial coronary distribution, typically circumferential apical hypokinesia/akinesia/dyskinesia (apical ballooning) with basal hyperkinesis (less likely involving mid and basal LV; rarely focal) | Normal or global LV systolic dysfunction, occasionally regional wall motion abnormalities | Regional wall motion abnormalities in a coronary artery distribution |
| Ventricular structure/tissue characterization | Diffuse and transmural LV myocardial oedema matching wall motion abnormalities but notable absence of LGE | Mid-wall or subepicardial oedema, with LGE (88%) in a patchy pattern not in a coronary distribution, pericardial enhancement may be present | Transmural or subendocardial oedema with LGE in a coronary distribution |
| LV outflow tract obstruction | Due to hypercontractility of the base (14–25%) | Absent | Absent |
| Pericardial involvement | Unusual | Pericarditis with pericardial effusion is common | Unusual |
| Complications: aneurysm, pseudoaneurysm, intramyocardial haemorrhage, and ventricular wall rupture | Rare and generally diagnosed post-mortem; risk factors include advanced age, females, HTN, persistent STE, absence of T-wave inversion in lateral leads, higher LVEF, and low frequency of beta-blocker use | Rare | Seen in 5–10% of cases, especially with delayed revascularization, advanced age, females, HTN, persistent STE |
LV, left ventricular; LVEF, left ventricular ejection fraction; CAD, coronary artery disease; LGE, late gadolinium enhancement; LBBB, left bundle branch block; STE, ST segment elevation; STD, ST segment depression; HTN, hypertension.
Takotsubo syndrome, also known as stress myocardial injury or cardiomyopathy, is an acute and usually reversible neurogenic myocardial stunning and heart failure syndrome in the absence of atherosclerotic coronary artery disease (CAD).4,5 The estimated prevalence in the USA is around ∼14 000 cases per year, accounting for 1–2% of all admissions suspected to be due to acute coronary syndrome.6,7 The exact pathophysiology of TTS remains unclear, but evidence points towards catecholamine-induced cardiotoxicity and microvascular dysfunction.8 Takotsubo syndrome predominantly affects post-menopausal women (82%), and the clinical presentation may be indistinguishable from acute myocardial infarction (AMI). Ventricular wall rupture is rare (<1%) but is a well-described complication of TTS and may be fatal.9 Cases of recurrent TTS have also been described. While it is possible that our patient had recurrent episodes of the initial pathophysiological process that ultimately resulted in recurrent wall injury and progressive thinning, it is more likely that the tissue at the site of the initial rupture was necrotic and friable which led to failure of the sutures to hold due to poor tensile strength. This likely predisposed the patient to recurrent rupture in this region of thinned-out myocardium, especially when considering the lack of use of overlying supportive material like a patch.10
Transthoracic echocardiogram during the first recurrence identified an apical PsA with to-and-fro flow into the apical extra-cardiac cavity that was contained only by the pericardium. As opposed to a true LV aneurysm which is comprised of the LV wall (albeit necrosed or scarred), a PsA is a through-and-through rupture or breach through all three layers of the LV wall (endocardium, myocardium, and epicardium) and is thus bound only by clotted blood, fibrin, and the surrounding tissues such as the pericardium and/or mediastinal tissues.11 Consequently, PsAs have a higher risk of rupture than aneurysms (estimated to be between 30 and 45%)12 and prompt surgical closure with primary repair or a patch is recommended.13 Aneurysms have a wide neck and are usually seen in the anterior wall and apex. With the widespread use of prompt revascularization after MI over the past 3–4 decades, the incidence of LV aneurysms has declined. Pseudoaneurysms, conversely, are more common in the lateral and inferior walls and usually have a narrow neck (<40% of the maximal diameter of the PsA) and may occur after failed reperfusion, delay in seeking care after an MI, or after trauma or cardiac surgery.
Multimodality imaging plays a vital role in the diagnosis of these clinically overlapping syndromes.14 Transthoracic echocardiogram is the first-line imaging modality15,16 and is widely available and portable, while CMR can help differentiate TTS from myocarditis and AMI17–19 (Table 2). In myocarditis, the LV global systolic function is usually mildly reduced or low normal, and regional wall motion abnormalities may be seen. Myocarditis typically has mid-wall or subepicardial LGE distribution patterns that do not follow a coronary distribution as well as oedema. Pericardial involvement is not infrequent, especially in the form of a small pericardial effusion and/or myopericarditis. Cardiovascular magnetic resonance of cases of AMI typically shows regional wall motion abnormalities, oedema, and LGE corresponding to the infarct-related coronary distribution. The LGE starts subendocardially and spreads outwards, becoming more transmural with increasing severity of ischaemic injury. In contrast, TTS presents as distal LV and apical ballooning (75–80%), and CMR shows transmural LV myocardial oedema in the areas of wall motion abnormalities and a characteristic lack of LGE. An increasing number of variants are being recognized, including mid-ventricular TTS (10–20%), basal LV (5%), or rarely biventricular and focal segmental (both at <0.5%, respectively). Our patient had the classical type of TTS with basal hypercontractility. Apical hypokinesis or injury could not be fully ascertained because of the rupture of the thinned apical myocardium at the time of presentation.
The role of cardiovascular magnetic resonance (CMR) in the differential diagnosis and prognostication of takotsubo syndrome (TTS), myopericarditis, and acute myocardial infarction (AMI) or myocardial infarction with non-obstructive coronary arteries (MINOCA)
| . | TTS . | Myopericarditis . | AMI or MINOCA . |
|---|---|---|---|
| T1 mapping and ECV | T1 values are elevated in both the affected hypokinetic LV myocardium and hyperdynamic regions. The number of segments with elevated T1 values correlates with delayed LV function recovery. Elevated T1 values in the hyperdynamic myocardium inversely correlate with lower LVEF. Significantly elevated T1 values indicate the risk of fatal complications such as cardiac rupture. T1 mapping offers superior diagnostic performance for detecting myocardial oedema and predicting LV wall motion recovery compared to T2-weighted imaging in TTS ECV is also elevated in the affected myocardium | Elevated global or regional T1 values ECV > 35% is independently associated with MACE | Elevated T1 values indicate infarcted and/or oedematous myocardium in a coronary distribution Native T1 values within the infarct core are inversely correlated with MVO extent, LV remodelling, and all-cause mortality |
| T2 mapping | Native T2 values are elevated in both the affected myocardium and the hyperdynamic regions. Elevated native T2 values in the hyperdynamic myocardium inversely correlate with lower LVEF | Elevated global or regional native T2 values | T2 mapping quantifies the extent of oedema or area at risk in the ischaemic zone, while T2* mapping detects and quantifies IMH |
| LGE | The absence of LGE is an important finding that differentiates TTS from myocarditis and AMI | Patchy or diffuse subepicardial or mid-wall LGE in a non-coronary distribution, as well as pericardial LGE The presence of LGE is associated with higher all-cause mortality and cardiac death | Subendocardial or transmural LGE in a coronary distribution CMR can detect MVO in 60% of patients within the first week after STEMI. MVO is a strong prognostic indicator in the medium and long term, independent of infarct size and LVEF, predicting increased risk of MACE and cardiovascular death |
| . | TTS . | Myopericarditis . | AMI or MINOCA . |
|---|---|---|---|
| T1 mapping and ECV | T1 values are elevated in both the affected hypokinetic LV myocardium and hyperdynamic regions. The number of segments with elevated T1 values correlates with delayed LV function recovery. Elevated T1 values in the hyperdynamic myocardium inversely correlate with lower LVEF. Significantly elevated T1 values indicate the risk of fatal complications such as cardiac rupture. T1 mapping offers superior diagnostic performance for detecting myocardial oedema and predicting LV wall motion recovery compared to T2-weighted imaging in TTS ECV is also elevated in the affected myocardium | Elevated global or regional T1 values ECV > 35% is independently associated with MACE | Elevated T1 values indicate infarcted and/or oedematous myocardium in a coronary distribution Native T1 values within the infarct core are inversely correlated with MVO extent, LV remodelling, and all-cause mortality |
| T2 mapping | Native T2 values are elevated in both the affected myocardium and the hyperdynamic regions. Elevated native T2 values in the hyperdynamic myocardium inversely correlate with lower LVEF | Elevated global or regional native T2 values | T2 mapping quantifies the extent of oedema or area at risk in the ischaemic zone, while T2* mapping detects and quantifies IMH |
| LGE | The absence of LGE is an important finding that differentiates TTS from myocarditis and AMI | Patchy or diffuse subepicardial or mid-wall LGE in a non-coronary distribution, as well as pericardial LGE The presence of LGE is associated with higher all-cause mortality and cardiac death | Subendocardial or transmural LGE in a coronary distribution CMR can detect MVO in 60% of patients within the first week after STEMI. MVO is a strong prognostic indicator in the medium and long term, independent of infarct size and LVEF, predicting increased risk of MACE and cardiovascular death |
ECV, extracellular volume; LGE, late gadolinium enhancement; LV, left ventricular; LVEF, left ventricular ejection fraction; MACE, major adverse cardiovascular events; MVO, microvascular obstruction; STEMI, ST elevation myocardial infarction; IMH, intramyocardial haemorrhage.
The role of cardiovascular magnetic resonance (CMR) in the differential diagnosis and prognostication of takotsubo syndrome (TTS), myopericarditis, and acute myocardial infarction (AMI) or myocardial infarction with non-obstructive coronary arteries (MINOCA)
| . | TTS . | Myopericarditis . | AMI or MINOCA . |
|---|---|---|---|
| T1 mapping and ECV | T1 values are elevated in both the affected hypokinetic LV myocardium and hyperdynamic regions. The number of segments with elevated T1 values correlates with delayed LV function recovery. Elevated T1 values in the hyperdynamic myocardium inversely correlate with lower LVEF. Significantly elevated T1 values indicate the risk of fatal complications such as cardiac rupture. T1 mapping offers superior diagnostic performance for detecting myocardial oedema and predicting LV wall motion recovery compared to T2-weighted imaging in TTS ECV is also elevated in the affected myocardium | Elevated global or regional T1 values ECV > 35% is independently associated with MACE | Elevated T1 values indicate infarcted and/or oedematous myocardium in a coronary distribution Native T1 values within the infarct core are inversely correlated with MVO extent, LV remodelling, and all-cause mortality |
| T2 mapping | Native T2 values are elevated in both the affected myocardium and the hyperdynamic regions. Elevated native T2 values in the hyperdynamic myocardium inversely correlate with lower LVEF | Elevated global or regional native T2 values | T2 mapping quantifies the extent of oedema or area at risk in the ischaemic zone, while T2* mapping detects and quantifies IMH |
| LGE | The absence of LGE is an important finding that differentiates TTS from myocarditis and AMI | Patchy or diffuse subepicardial or mid-wall LGE in a non-coronary distribution, as well as pericardial LGE The presence of LGE is associated with higher all-cause mortality and cardiac death | Subendocardial or transmural LGE in a coronary distribution CMR can detect MVO in 60% of patients within the first week after STEMI. MVO is a strong prognostic indicator in the medium and long term, independent of infarct size and LVEF, predicting increased risk of MACE and cardiovascular death |
| . | TTS . | Myopericarditis . | AMI or MINOCA . |
|---|---|---|---|
| T1 mapping and ECV | T1 values are elevated in both the affected hypokinetic LV myocardium and hyperdynamic regions. The number of segments with elevated T1 values correlates with delayed LV function recovery. Elevated T1 values in the hyperdynamic myocardium inversely correlate with lower LVEF. Significantly elevated T1 values indicate the risk of fatal complications such as cardiac rupture. T1 mapping offers superior diagnostic performance for detecting myocardial oedema and predicting LV wall motion recovery compared to T2-weighted imaging in TTS ECV is also elevated in the affected myocardium | Elevated global or regional T1 values ECV > 35% is independently associated with MACE | Elevated T1 values indicate infarcted and/or oedematous myocardium in a coronary distribution Native T1 values within the infarct core are inversely correlated with MVO extent, LV remodelling, and all-cause mortality |
| T2 mapping | Native T2 values are elevated in both the affected myocardium and the hyperdynamic regions. Elevated native T2 values in the hyperdynamic myocardium inversely correlate with lower LVEF | Elevated global or regional native T2 values | T2 mapping quantifies the extent of oedema or area at risk in the ischaemic zone, while T2* mapping detects and quantifies IMH |
| LGE | The absence of LGE is an important finding that differentiates TTS from myocarditis and AMI | Patchy or diffuse subepicardial or mid-wall LGE in a non-coronary distribution, as well as pericardial LGE The presence of LGE is associated with higher all-cause mortality and cardiac death | Subendocardial or transmural LGE in a coronary distribution CMR can detect MVO in 60% of patients within the first week after STEMI. MVO is a strong prognostic indicator in the medium and long term, independent of infarct size and LVEF, predicting increased risk of MACE and cardiovascular death |
ECV, extracellular volume; LGE, late gadolinium enhancement; LV, left ventricular; LVEF, left ventricular ejection fraction; MACE, major adverse cardiovascular events; MVO, microvascular obstruction; STEMI, ST elevation myocardial infarction; IMH, intramyocardial haemorrhage.
When coronary angiography fails to show an infarct-related culprit lesion and CMR visualizes transmural myocardial oedema without LGE, the diagnosis of TTS is favoured over AMI or myocarditis. Unfortunately, the diagnosis was delayed for our patient as he was admitted initially to an outlying facility and treated with steroids for suspected pericarditis. European Society of Cardiology guidelines on pericardial disease do not recommend corticosteroids (class III) as the first-line treatment of viral pericarditis.1 They may be used in low doses when contraindications or a failure of response exist to colchicine, aspirin, or NSAIDs, or in the case of autoimmune diseases after infectious causes have been excluded. This patient likely had viral myopericarditis, which along with steroid administration weakened the myocardial wall and increased the risk of LV rupture, as seen when he developed TTS a little over a month later.20
Serious complications are uncommon in TTS but include cardiogenic shock, thromboembolism, ventricular arrhythmias, and cardiac rupture (CR).7,21 Risk factors for CR include older age, persistent ST elevations, hypertension, higher LVEF, elevated LV outflow tract gradient, and less frequent use of beta-blockers. To our knowledge, there have been no reports of recurrent LV PsA following a case of TTS. Multiple factors contributed to PsA recurrence in our patient. It is likely that the necrotic and friable myocardium after the initial episode of TTS led to recurrent ruptures due to poor tissue integrity at the site of suture repair in the thin apical wall. In addition, incomplete excision of the necrotic material during the original procedure and the lack of use of a supportive patch or synthetic material overlying the repair may have led to further weakening of the initial repair.
Currently, there is no evidence to guide long-term drug therapy to prevent recurrence and fatal complications.22 Beta-blockers are recommended for patients with increased sympathetic tone, anxiety, persistent symptoms, and recurrent episodes. Patients with CR have higher fluctuation of intracardiac pressure, and beta-blockers may protect against LV rupture. Guideline-directed medical therapy for LV systolic dysfunction such as renin–angiotensin inhibitors can reduce the risk of recurrence, although studies have shown modest benefit in terms of 1-year survival. Addressing anxiety, depression, and substance use, which are more prevalent in younger males, can reduce recurrences and improve long-term outcomes.
The management of recurrent PsA requires a multidisciplinary approach to determine the optimal timing of the intervention, assess surgical risk, and decide between surgical repair and percutaneous closure. Cardiac imaging modalities, including echocardiography, cardiac computed tomography, and CMR, play a vital role in precisely localizing the recurrent rupture in relation to the prior repair, evaluating the number of defects and their geometry, and anatomical relationship with surrounding structures. Patch closure techniques with Teflon, Dacron, or autologous pericardium are preferred over direct closure, especially for larger defects, to minimize risk of suture dehiscence and recurrence. While direct closure or a direct mattress suture reinforced with Teflon felt may be considered in some cases of blow-out types of rupture, suturing through friable necrotic tissue is challenging and increases the risk of recurrence.23
In a small series of nine patients who underwent percutaneous PsA closure, all were successfully closed with a fluoroscopy time of 36 min (range: 12–76 min) with significant overall improvement in heart failure class during follow-up.24 Thus, this approach may be a viable option for those considered high risk for surgical intervention.
A comprehensive approach is required to decrease the risk of recurrent PsA. Prompt diagnosis and treatment of predisposing conditions, such as recurrent TTS, subclinical myocarditis, or myocardial ischaemia, are essential. During surgical repair, complete excision of the necrotic tissue, reinforcement with supportive patches, and ensuring adequate haemostasis decrease the risk of recurrence. Furthermore, close follow-up and optimal medical management of underlying cardiovascular risk factors, including heart failure and hypertension, play a vital role in preventing recurrence.
Conclusion
Left ventricular PsA is a rare but serious and potentially fatal complication of TTS. Awareness, early diagnosis using multimodality imaging, and prompt treatment with close follow-up are critical to minimizing the risk of serious complications. Additionally, it is important to note that steroid use can increase the risk of LV wall rupture in acute myocardial injury syndromes.
Lead author biography

Dr Umer is an imaging fellow at the University of Louisville and has interests in multimodality imaging of cardiomyopathies.
Supplementary material
Supplementary material is available at European Heart Journal – Case Reports online.
Consent: The authors confirm that written consent for the submission and publication of this case report, including images and associated text, has been obtained from the patient in line with the COPE guidelines.
Funding: None declared.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
Author notes
Conflict of interest: None declared.




Comments