-
PDF
- Split View
-
Views
-
Cite
Cite
Pierre Rossignon, Sofia Morra, Quentin de Hemptinne, Didier de Cannière, Philippe Unger, Papillary muscle rupture without severe mitral regurgitation following percutaneous balloon mitral commissurotomy: a case report, European Heart Journal - Case Reports, Volume 8, Issue 3, March 2024, ytae114, https://doi.org/10.1093/ehjcr/ytae114
Close - Share Icon Share
Abstract
Percutaneous transvenous mitral commissurotomy (PTMC) is the first-line therapy of clinically significant rheumatic mitral stenosis. While the procedure is generally safe, new onset or aggravation of mitral regurgitation (MR) may occur, mainly due to commissural splitting and, less frequently, to leaflet tear and chordal rupture. Papillary muscle rupture (PMR) is exceedingly rare in this setting.
A 74-year-old woman with a history of aortic valve replacement and prior rheumatic mitral commissurotomy presented for worsening exercise intolerance and exertional dyspnoea. Transthoracic echocardiography showed a mean pressure gradient of 10 mmHg and a mitral valve area of 1.0 cm², consistent with clinically significant mitral stenosis. Subsequent PTMC was complicated by anterolateral PMR. However, the resulting MR was unexpectedly only of mild-to-moderate severity. Because of residual mitral stenosis and persisting symptoms, surgical mechanical mitral valve replacement and tricuspid annuloplasty were performed 6 weeks after PTMC. Papillary muscle rupture was confirmed during surgery.
We herein describe the occurrence of PMR induced by PTMC; the resulting MR was unexpectedly of mild-to-moderate severity, as a result of extensive rheumatic lesions limiting valve mobility. This case challenges the dogma according to which PMR invariably leads to severe MR. This might not be necessarily the case when it occurs following PTMC.
Papillary muscle rupture following PTMC in the context of rheumatic mitral valve disease can result in non-severe MR.
This underscores the impact of extensive rheumatic lesions on valve mobility and challenges the dogma according to which PMR invariably leads to severe MR.
Careful peri- and post-PTMC echocardiographic evaluations are crucial to detect potential complications of the procedure.
Introduction
Percutaneous transvenous mitral commissurotomy (PTMC) and mitral valve surgery are established therapeutic options for patients with clinically significant rheumatic mitral stenosis.1 While PTMC is generally considered safe and effective, it is not devoid of complications, the two more common being mitral regurgitation (MR) and cardiac tamponade.2 Among these, papillary muscle rupture (PMR) remains exceedingly rare.
Whereas PMR is considered to almost universally result in severe MR, often with acute life-threatening cardiogenic shock and pulmonary oedema, we herein present a case of anterolateral PMR occurring during PTMC, which unexpectedly resulted in mild-to-moderate MR.
Case presentation
A 74-year-old woman was admitted with worsening heart failure. She reported a history of rheumatic heart disease with surgical mitral commissurotomy performed 54 years ago and aortic valve replacement with a mechanical prosthesis 15 years ago. Additionally, she had persistent atrial fibrillation (AF) for the past 17 years; the left atrial appendage had been excised during the previous surgery.
The patient’s medical history was generally unremarkable, except for systemic arterial hypertension. Medications included loop diuretics, angiotensin-converting enzyme inhibitors, low-dose beta-blockers, dihydropyridine calcium channel blockers, and acenocoumarol.
On clinical examination, there were bilateral lower limb oedema and bibasilar lung crackles, indicative of decompensated heart failure. The electrocardiogram showed AF with a ventricular rate of 90/min. Laboratory investigations at admission showed the following: haemoglobin 12.8 g/dL (12–16 g/dL), white blood count 7380 × 103/µL (3.50–11 × 103/µL), international normalized ratio (INR) 3.3 (target INR 3.0), and normal renal and hepatic function. Electrolytes were within normal range. NT-proBNP level was 1505 ng/L (<125 ng/L). The chest X-ray revealed interstitial syndrome.
On transthoracic echocardiography, mitral valve area (MVA) was 1.0 cm² (as measured by both planimetry and continuity equation), and mean transmitral pressure gradient was 10 mmHg; these findings were deemed consistent with clinically significant mitral stenosis. Trace MR was also observed. The Wilkins score—a grading system describing echocardiographic appearance of the mitral valve and used to select patients suitable for PTMC1—was 9 (mobility 3, thickening 2, calcifications 3, and subvalvular thickening 1). Noticeably, the subvalvular apparatus exhibited only mild calcifications of the subvalvular chordae and of the head of papillary muscle. There were severe left and right atrial dilatation, and tricuspid annulus was dilated (end-diastolic diameter 40 mm), with visually estimated moderate secondary tricuspid regurgitation. Estimated systolic pulmonary pressure was 48 mmHg, and indexed stroke volume was 28 mL/m2. Transoesophageal echocardiography (TEE) confirmed thickened, poorly mobile, and moderately calcified mitral valve leaflets (Figure 1A and B; see Supplementary online, video 1), and there was no atrial thrombus. Due to a EuroSCORE II–predicted surgical mortality of 5.84%, PTMC was offered as first-line treatment, despite unfavourable patient characteristics, including advanced age, history of commissurotomy, persistent AF, and a Wilkins score >8 indicating suboptimal suitability for the procedure.1 Transoesophageal echocardiography–guided PTMC was performed using a 26 mm Inoue balloon catheter (Toray Medical®). A stepwise incremental dilatation technique, calculated based on reference balloon size minus 2 mm as per the formula by Hung et al.,3 was employed. After the second inflation at 25 mm, a 16 × 8 mm left ventricular mass was noticed, swinging in systole towards the outflow tract, which remained attached to the anterior mitral leaflet via one or several chordae (Figure 1C and D; see Supplementary online, videos 2–4). This mobile mass was consistent with a ruptured anterolateral PMR, and the procedure was immediately aborted. Unexpectedly, despite the papillary muscle disruption, the resulting MR was only of mild-to-moderate severity (Figure 1E; see Supplementary online, video 2). There was good clinical and haemodynamic tolerance, favouring initially a watchful waiting strategy. On repeated echocardiography, performed at Days 4 and 10 after PTMC, MR severity remained mild to moderate, with an effective mitral regurgitant orifice area of 0.10 cm2 and a regurgitant volume of 21 mL (Figure 1F and G). The mean mitral gradient was 7 mmHg, and MVA was 1.4 cm2 by planimetry. During outpatient follow-up visits, the patient reported limited but stable exercise capacity (New York Heart Association III). As a result, the decision was made to proceed with surgery. Due to patient’s hesitancy, the operation was only performed 6 weeks later and involved removal of the ruptured papillary muscle (Figure 1H), placement of a mechanical mitral valve prosthesis, and tricuspid annuloplasty. The procedure was uncomplicated and resulted in a marked symptomatic improvement, with the patient’s length of stay being 16 days, followed by rehabilitation. Her last follow-up visit, conducted 6 months after the intervention, demonstrated sustained and significant functional improvement.
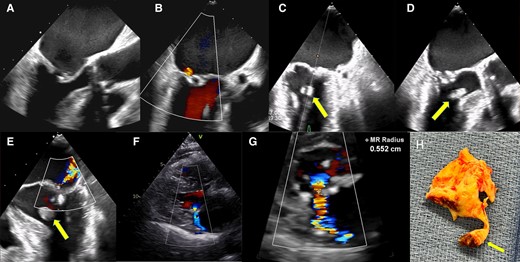
(A) Preprocedural transoesophageal echocardiography mid-oesophageal diastolic still frame view (120°) showing rheumatic mitral stenosis. (B) Preprocedural transoesophageal echocardiography systolic still frame (100°) showing trace of mitral regurgitation. (C) Per-procedural transoesophageal echocardiography (45°): after the second balloon inflation, a rounded echogenic mass is seen during systole in the left ventricular outflow tract (arrow). (D) Per-procedural transoesophageal echocardiography (135°): same echogenic mass. (E) Per-procedural transoesophageal echocardiography (0°): colour flow Doppler showing an eccentric jet consistent with moderate mitral regurgitation. (F) Post-procedure transthoracic parasternal long-axis view showing moderate mitral regurgitation. (G) The proximal isovelocity surface area (PISA) radius is 0.55 cm, and the mitral effective regurgitant volume and surface area are 21 mL and 10 mm2, respectively. (H) Surgical specimen showing valve remnants connected by a chorda to ruptured papillary muscle with necrotic tissue at its base (arrow).
Discussion
Our 74-year-old patient presented with severe mitral stenosis and a history of prior commissurotomy for rheumatic heart disease. Despite challenging characteristics, including age and a high Wilkins score, the decision to proceed with PTMC was made. This led to a ruptured anterolateral papillary muscle, a rare periprocedural complication. Unexpectedly, despite PMR, the resulting MR was only of mild-to-moderate severity.
Mitral regurgitation commonly occurs following PTMC and is often attributed to commissural splitting, which typically leads to a mild increase in MR and yields a favourable outcome.4 Other mechanisms of MR such as leaflet tearing and subvalvular damage tend to result in severe MR with haemodynamic impairment, necessitating urgent surgery and leading to a less favourable prognosis.4 Other complications following PTMC include cardiac tamponade, embolic events, and bleeding.4 The occurrence of PMR in this setting is exceedingly rare.
When PMR occurs as a complication of myocardial infarction,5 it almost universally results into severe MR, leading to acute heart failure and haemodynamic compromise. Less frequently, PMR can also result from other conditions, including infectious causes, such as syphilis, vegetating valvulitis, and myocardial abscesses, and non-infectious ones, such as mitral annular calcification, periarteritis nodosa, takotsubo cardiomyopathy, myocarditis, and Ehlers–Danlos syndrome.5,6
Very rarely, it may be the consequence of a traumatic mechanical injury, sometimes iatrogenic,7 as in the present case. A comprehensive review of the English literature allowed us to identify only seven cases of complete PMR in the setting of PTMC.8–14 Importantly, no echocardiographic or procedure-related factor can predict subvalvular rupture during PTMC.4
A striking feature of the present case is that the resulting MR was only of mild-to-moderate severity. This finding is in line with a previous case of anterolateral PMR occurring during PTMC, resulting in mild MR.8 Thus, two of the eight—including ours—reported cases (25%) of PTMC-induced PMR resulted in only mild–moderate MR, challenging the dogma according to which PMR invariably leads to severe and poorly tolerated MR. In the specific setting of rheumatic mitral valve stenosis, restricted valve mobility through commissural fusion may have prevented the development of severe MR by limiting the extent of leaflet protrusion.
One can also speculate on the embolic potential of the ruptured papillary muscle, given its highly mobile appearance in the left ventricular outflow tract (Figure 1C; Supplementary online, video 3). There is no existing literature on the short-to-mid-term embolic risk of PMR, as this situation virtually always requires urgent cardiac surgery. In the present case, there was no clinical evidence of systemic embolization during the 6-week follow-up before surgery. Additionally, surgical examination revealed robust, calcified chordae firmly attached to the ruptured papillary muscle, a finding which would argue for a low systemic embolic potential, at least in the short term.
In this patient, mitral valve restenosis occurred ∼54 years after the initial surgical commissurotomy. Due to progressive symptoms and hospitalization for decompensated heart failure, reintervention became mandatory. While mitral valve surgery is generally the preferred therapeutic choice for patients with a history of commissurotomy, PTMC can be considered in selected cases.1 The heart team initially proposed PTMC, driven by considerations of high-risk surgery, commissural fusion on echocardiography, and patient’s reluctance to undergo a third cardiac surgery.
While mitral valve remodelling resulting from the previous commissurotomy might have contributed to subvalvular fragility, it is unclear whether it decisively caused the PMR. Indeed, there is no available literature on the specific risks associated with PMR in this setting. The commissurotomy occurred 54 years ago, and surgical examination revealed severe papillary muscle calcification, suggesting progressive rheumatic disease, not surgery-induced remodelling, as the primary cause.
After a successful PTMC, surgical reintervention is frequently needed, even decades later,15 primarily due to symptomatic restenosis.1,15 Such reintervention typically entails either valve replacement or repair.15 In our case, immediate PTMC results were suboptimal, as the procedure had to be prematurely halted due to iatrogenic PMR. Consequently, the mitral valve orifice remained <1.5 cm2, and the patient remained symptomatic. Subsequent surgical reintervention was collaboratively decided with the patient to enhance functional status and quality of life. As repair seemed unlikely, we selected a mechanical mitral valve, considering the patient’s need for anticoagulation due to a mechanical aortic valve and AF.
Conclusion
We herein present the very rare occurrence of an iatrogenic traumatic PMR induced by balloon commissurotomy. In this rheumatic setting, PMR unexpectedly resulted in only mild-to-moderate MR severity. This unique scenario underscores the impact of extensive rheumatic lesions on valve mobility and challenges the dogma according to which PMR invariably leads to severe MR.
Lead author biography

Pierre Rossignon, MD, is a physician in the Department of Cardiology at CHU Saint-Pierre in Brussels, Belgium. Dr Rossignon is a cardiology assistant at the Université Libre de Bruxelles (ULB) and has a particular interest in cardiac intensive care and heart failure.
Supplementary material
Supplementary material is available at European Heart Journal – Case Reports online.
Acknowledgements
We extend our gratitude to Pr Vandenbossche JL, former Head of the Cardiology Department at CHU St-Pierre, Brussels, for his valuable contributions to this case report. His extensive expertise and guidance significantly enhanced the quality of this manuscript. We are truly grateful for his dedicated involvement in the care of the patient discussed in this report.
Consent: The authors confirm that written consent for the submission and publication of this case report, including images and associated text, has been obtained from the patient in line with COPE guidance.
Funding: This research was self-funded and supported by the authors. No external sources of funding were received for this publication.
Data availability
The data used to support the findings of this case are available from the corresponding author upon reasonable request.
References
Author notes
Conflict of interest: None declared.




Comments