-
PDF
- Split View
-
Views
-
Cite
Cite
Nan Nan, Lili Pan, Ran Dong, Xiantao Song, Mismatch of systematic and local inflammatory activity in Takayasu arteritis with coronary involvement: a case report, European Heart Journal - Case Reports, Volume 7, Issue 8, August 2023, ytad346, https://doi.org/10.1093/ehjcr/ytad346
Close - Share Icon Share
Abstract
Accurate evaluation of the activity stage in Takayasu arteritis (TA) is important for the revascularization of TA with coronary artery involvement (TA-CAD). Here, we report the case of a patient with a mismatch of systemic and local inflammatory activity, leading to 13 times the need for recurrent coronary revascularization.
A 31-year-old woman with a family history of coronary artery disease underwent percutaneous coronary intervention (PCI) for critical ostial lesions. This patient was identified with Numano Type V TA and she underwent optimal medical therapy and PCIs. Her clinical inflammatory markers were quickly normalized. However, in-stent restenosis events recurred every 3 months. Virtual-histology intravascular ultrasound (VH-IVUS) and 18F-fluorodeoxyglucose (FDG)-positron emission tomography (PET)/computed tomography (CT) confirmed local vascular inflammation. A coronary artery bypass graft (CABG) was also conducted. Before this procedure, both the CABG grafts and the anastomotic areas were accurately assessed with 18FDG-PET/CT. Eventually, this patient remained both angina- and event-free for 2 years post-CABG.
The persistence of TA activity despite normal clinical inflammatory markers is uncommon as is the need for recurrent revascularization after appropriate PCI management. Intracoronary imaging and 18FDG-PET/CT play a critical role in assessing TA activity as well as precisely guiding CABG grafts and anastomosis sites to prevent graft failure.
Refractory Takayasu arteritis-coronary artery disease may be due to a mismatch between systemic and local inflammatory activity.
18F-fluorodeoxyglucose-positron emission tomography/computed tomography can guide decisions regarding the coronary artery bypass graft technique and graft preference according to the affected vascular locations in order to prevent graft failure.
Introduction
Takayasu arteritis (TA) often involves the coronary arteries (TA-CAD). It most commonly affects the ostium and is sometimes fatal.1 However, the effects of coronary revascularization for coronary arterial stenosis in TA have not been well studied. Percutaneous coronary intervention (PCI) is associated with a high risk of restenosis.2,3 Coronary artery bypass graft (CABG) is considered to result in better prognoses in severe cases of coronary stenosis.4,5 However, during the stable stage, PCI and CABG yield comparable results. Coronary artery bypass graft is recommended if TA patients are in the active stage and urgent revascularization is needed.6
Anti-inflammatory treatment is also fundamentally essential in TA-CAD patients. Glucocorticoids, immunosuppressive treatment, and tocilizumab have all been reported to be successful in treating TA-CAD.7,8 Anti-inflammatory therapy can also reduce cardiac events in patients with recurrent coronary revascularization before a TA diagnosis.5,9 As a result, early and precise diagnosis and treatment plans seem to be crucial for achieving successful clinical outcomes in TA patients.9
In this study, we discuss the case of a young woman who experienced recurrent revascularization despite an early TA diagnosis, early therapy, and normal inflammatory markers.
Summary figure
Case presentation
In August 2017, a 31-year-old woman was first hospitalized for typical exertional angina. At the age of 55, her mother was diagnosed with coronary artery disease. She gave premature birth to a daughter at 29 due to pre-eclampsia. She had no history of hypertension, diabetes, dyslipidaemia, cigarette smoking, or alcohol abuse. She had a weak bilateral radial artery pulse and elevated serum inflammatory markers, including a high-sensitive C-reactive protein level of 6.61 mg/L, an erythrocyte sedimentation rate of 46 mm/h, and tumour necrosis factor-α 99 pg/mL. White blood cell count was normal, while the neutrophil ratio was 92.8%, which is higher than normal. Electrocardiogram at anginal attack showed ST-T changes. Echocardiology showed mild aortic regurgitation with normal function. Coronary angiogram showed right coronary artery (RCA) and left main (LM) ostial 90% stenoses. Sirolimus-eluting stents (SES) 4.0 × 15 and 3.5 × 20 mm were implanted in the LM and RCA ostia (see Supplementary material online). Inflammatory indicators in her serum were also increased. Computed tomography angiography (CTA) revealed a stenosis of the left subclavian artery, abdominal aorta, and common iliac artery in addition to coronary ostial stenosis. No familial hypercholesterolemia gene mutations were discovered.
She was subsequently diagnosed with TA-CAD and Numano Type V and was started on prednisolone (PSL) therapy at 1 mg/kg/day. Cyclophosphamide (CTX) was given for 6 months and was then replaced by methotrexate (MTX) due to the patient developing severe gastrointestinal reactions. The patient's inflammatory biomarkers were quickly declined to normal. Unfortunately, the combined application of PSL, MTX and even tocilizumab (TCZ, 8 mg/kg q.4.w.) did not prevent the occurrence of in-stent restenosis (ISR) either. She underwent five coronary revascularizations at intervals of 2–9 months due to recurrent ISR. The inflammatory biomarkersremained normal. Virtual-histology intravascular ultrasound (VH-IVUS) that was used to detect the lumen in December 2018 demonstrated rapid (within 2 months since the last incident) and homogeneous in-stent fibrosis (Figure 1B and C), but the extra-stent vessels were generally normal (Figure 1A and D). Drug-coated balloons (DCBs) were utilized for ISR; even that patient’s longest time period without a cardiac episode was 9 months, but was still short. Since then, she has experienced six ISR occurrences, including occurrences of myocardial infarction and heart failure. Another SES 3.5 × 18 mm stent was implanted in the left anterior descending artery (LAD). Aspirin, ticagrelor, atorvastatin, and bisoprolol were routinely administered for 3 years. The disease progression, therapies, and inflammatory markers are illustrated in Figure 2.
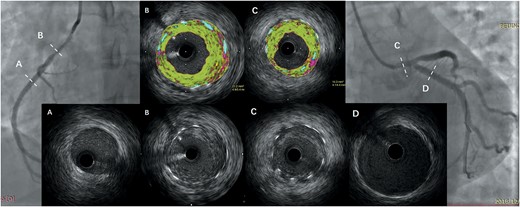
Virtual-histology intravascular ultrasound showing a rapid (within 2 months) and homogeneous in-stent fibrosis (B and C) but normal extra-stent vessels (A and D).
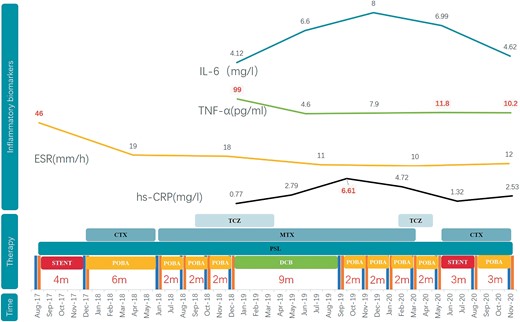
Cardiac events and corresponding therapies and inflammatory markers.
The patient eventually underwent a CABG procedure on 26 November 2020. 18F-fluorodeoxyglucose (FDG)-positron emission tomography (PET)/computed tomography (CT) was conducted before the surgery. Except for high 18FDG uptake in the left margin of the ascending aorta (Figure 3A) and brachiocephalic trunk (Figure 3B), there were no signs of active TA, including in the region of the left internal mammary artery (LIMA) (Figure 3C). The inflammatory markersremained normal. The immunosuppressive medications comprisedTCZ 8 mg/kg/week, CTX 50 mg/day, and PSL 10 mg/day. Left internal mammary artery to the distal LAD, saphenous vein graft (SVG) to OM, and SVG to the distal PDA were used in CABG. An SVG anastomosis with the ascending aorta avoided the high 18FDG uptake area. The anastomotic location did not exhibit any inflammatory activity, according to the post-operative pathological data (Figure 4). This patient remained both event- and angina-free after 2 years of CABG under PSL (10 mg/day) and CTX (50 mg/day) treatment. A CTA that was performed after 1 year revealed that the LM and LAD stents had been completely obstructed (Figure 5A and B). However, the LIMA and SVG grafts had been unobstructed (Figure 5D–F). The patency of the RCA ostial stent may be the cause of the proximal anastomotic stenosis of the PDA SVG graft (Figure 5C and F).
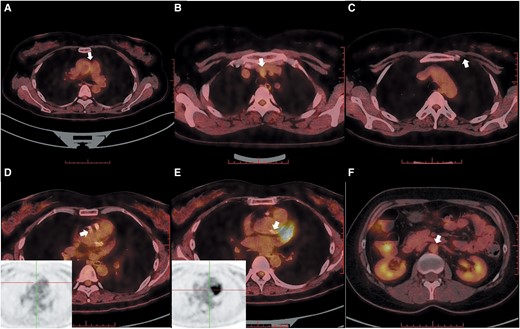
18F-fluorodeoxyglucose-positron emission tomography/computed tomography of large vessels. (A) High 18F-fluorodeoxyglucose uptake in the left margin of the ascending aorta. (B) High 18F-fluorodeoxyglucose uptake in the brachiocephalic trunk. (C) No significant uptake in the left internal mammary artery area. (D) No significant uptake in the ostial left main. (E) No significant uptake in the ostial right coronary artery. (F) No significant uptake in the abdominal aorta.
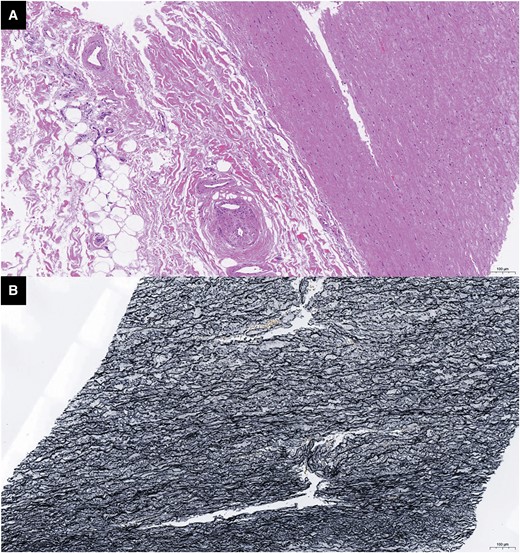
Pathology of the ascending aorta after coronary artery bypass graft. (A) H&E dyeing: there was no obvious intimal hyperplasia or inflammatory cell infiltration in the ascending aorta media layer. (B) Elastic fibre dyeing: the elastic fibres were aligned with no necrosis in anastomosis of asending aorta.
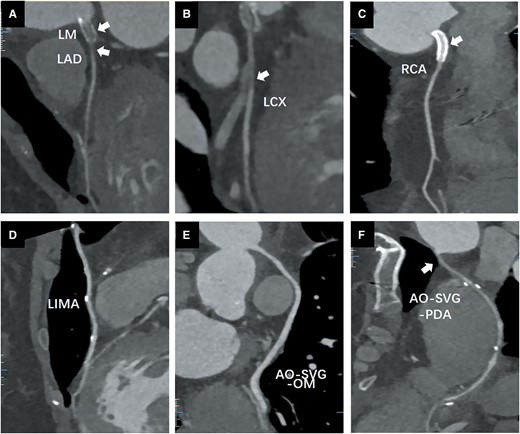
Coronary computed tomography angiography after 1 year of coronary artery bypass graft. (A) Left main and left anterior descending artery stent occlusion; (B) left circumflex (LCX) proximal segment occlusion; (C) right coronary artery ostial stent was not stenosed. (D) Left internal mammary artery was not stenosed. (E) Aorta (AO)-saphenous vein graft-LCX was not stenosed. (F) Proximal AO-saphenous vein graft-OM anastomosis was stenosed.
Discussion
Takayasu arteritis is a rare granulomatous vasculitis, but coronary artery involvement is not rare in TA, especially in Numano Type V.10 A significant proportion of patients receive revascularization before anti-inflammatory therapy and experience recurrent restenosis.5,9,11 Fortunately, subsequent anti-inflammatory medication reduces cardiac events in most patients.2,5,7–9,11 However, our patient had 13 reported cardiac events, the most common in any reported TA cases to date, despite the early diagnosis and normalization of inflammatory markers after the timely initiation of treatment. As a result, this instance provided us with some crucial information on TA with coronary artery involvement.
First, an early and accurate diagnosis of TA and an optimal anti-inflammatory therapy do not necessarily imply a good clinical outcome. Although past studies have shown that TA regresses following immunosuppressive therapies, glucocorticoids, immunosuppressive therapies, and tocilizumab,7,8 unfortunately, the progress of the disease in this young woman could not be stopped by these standard therapies.
Second, coronary revascularization of TA is still considered difficult to perform. Some reports suggest that medical therapy alone can be used to regress or even reverse coronary lesions in TA.7 However, ostial coronary lesions and symptoms of heart failure, as in our patient’s case, have been linked to high death rates.12 Thus, revascularization is required for treating severe coronary lesions, particularly during the stable stage of TA.6 However, neither the use of SES nor of DCB was able to prevent ISR in our patient, even though prior evidence suggests that both SES and DCB led to favourable clinical results in TA.2,13
Third, in our patient, inflammatory markers were not associated with TA activity. Restenosis still frequently occurred, even though early anti-inflammatory therapy quickly normalized the inflammatory markers. Virtual-histology intravascular ultrasound accurately identified the characteristics of local inflammatory activity. 18F-fluorodeoxyglucose-positron emission tomography/computed tomography demonstrated high sensitivity and specificity in inflammatory diseases,1 which we employed to identify high levels of local vascular uptake and to evaluate CABG grafts and distal anastomosis sites. As a result, intracoronary imaging9 and 18FDG-PET/CT14 are suggested for determining TA activity locations even when clinical inflammatory markers are normal. 18F-fluorodeoxyglucose-positron emission tomography/computed tomography can guide decisions regarding the CABG technique and graft preference according to the affected vascular locations in order to prevent graft failure.
Conclusion
It is uncommon to see local TA activity with normal systematic inflammatory markers and optimal medical and PCI treatment. Refractory TA with coronary involvement results from this mismatch. Intracoronary imaging and 18FDG-PET/CT play a crucial role in the evaluation of the TA activity stage as well as precisely guiding CABG grafts and anastomosis sites to prevent graft failure.
Lead author biography
 Dr Xiantao Song, MD, is Director of Cardiology of Beijing Anzhen Hospital and Chief of Beijing Engineering Research Center of Cardiovascular Wisdom Diagnosis and Treatment. He is engaged in the interventions of complex coronary artery disease. He established the Multi-Disciplinary Treatment (MDT) Department of Cardiology, Rheumatology and Immunology.
Dr Xiantao Song, MD, is Director of Cardiology of Beijing Anzhen Hospital and Chief of Beijing Engineering Research Center of Cardiovascular Wisdom Diagnosis and Treatment. He is engaged in the interventions of complex coronary artery disease. He established the Multi-Disciplinary Treatment (MDT) Department of Cardiology, Rheumatology and Immunology.
Supplementary material
Supplementary material is available at European Heart Journal – Case Reports.
Acknowledgements
We thank Min Zhang, Yuan Zhou, Mingduo Zhang, and Yanlong Ren from the Department of Cardiology and Keming Peng from the Zibo District Hospital of Traditional Chinese Medicine, Zibo City, Shandong Province, for their PCIs. Hua Liao, Weiping Ci, and Na Gao from the Department of Rheumatology contributed to the patient’s anti-inflammatory treatment regimen and follow-up. Together with Ran Dong, Jubing Zheng and Kui Zhang from the Department of Cardiac Surgery performed the CABG surgery. Hongzhi Mi and Wei Dong from the Department of Nuclear Medicine contributed to the scan and analysis of 18FDG-PET/CT. Lei Xu and Lijun Zhang from the Department of Radiology contributed to the scan and analysis of coronary CT angiography. Wen Fu and Dong Chen from the Department of Pathology analysed the post-operative pathology of the patient.
Consent: The authors confirm that written consent for submission and publication of this case report including images and associated text has been obtained from the patient in line with COPE guidance.
Funding: This work was supported by the Chinese Cardiovascular Association-Access fund (grant number 2019-CCA-ACCESS-077), ‘DengFeng’ Talent Training Program (grant number DFL20220603), and Beijing Engineering Research Center of Cardiovascular Wisdom Diagnosis and Treatment.
Data availability
The data underlying this article are available in the article and in its online Supplementary material and will be shared on reasonable request to the corresponding author.
References
Author notes
Conflict of interest: None declared.
- angina pectoris
- percutaneous coronary intervention
- coronary artery bypass surgery
- coronary arteriosclerosis
- coronary artery
- inflammatory markers
- takayasu's arteritis
- computed tomography
- coronary revascularization
- intravascular ultrasonography
- anastomosis, surgical
- tissue transplants
- diagnostic imaging
- histology
- positron
- coronary arteritis
- revascularization
- fluorodeoxyglucose positron emission tomography
- intracoronary route
- mismatch





Comments