-
PDF
- Split View
-
Views
-
Cite
Cite
Irvan Adenin, Rachmat Dediat Kapnosa Hasani, Multiple direct fetal amiodarone administration for supraventricular tachycardia with hydrops fetalis: a case report, European Heart Journal - Case Reports, Volume 7, Issue 4, April 2023, ytad128, https://doi.org/10.1093/ehjcr/ytad128
Close - Share Icon Share
Abstract
Fetal supraventricular tachycardia (SVT) is the most common fetal tachyarrhythmia and can cause fetal heart failure and intrauterine death. The management varies within institution and usually based on published case series, institutional experience.
Fetal SVT with hydrops (ascites and subcutaneous edema) was diagnosed at 26 weeks of gestational age. The first direct injection of fetal amiodarone into the umbilical vein resulted in temporary cardioversion to the sinus rhythm and mild transient maternal adverse event. The second direct fetal amiodarone to the fetal peritoneal cavity resulted in conversion to sinus rhythm, resolution of fetal hydrops, and normal fetal growth until delivery at 39 weeks gestational age.
Treatment of fetal SVT often requires prolonged maternal antiarrhythmic treatment and carries a significant risk of maternal adverse events. Direct fetal antiarrhythmic treatment often requires achieving adequate therapeutic drugs, especially in hydropic fetus. Amiodarone is one of drugs options for fetal SVT with hydrops because it has been shown to be highly effective with low fetal mortality. Continuous vital sign and ECG monitoring should be performed during direct fetal antiarrhythmic administration.
Direct fetal amiodarone administration by an experienced operator may be considered on Fetal SVT with hydrops when effective and rapid response transplacental drugs were not available
Continuous vital sign and ECG monitoring should be performed during direct fetal antiarrhythmic administration.
Introduction
Fetal supraventricular tachycardia (SVT) is a cardiac rhythm anomaly complicating ∼1:1.000 pregnancies and accounting for up to 90% of all fetal tachyarrhythmias.1 It is characterized by a 1:1 atrioventricular conduction with heart rates >200 beats per minute (bpm).2 Untreated sustained fetal SVT may cause low cardiac output resulting in ascites, pleural and/or pericardial effusions, and skin edema and can cause intrauterine fetal demise in 9% to 17% of cases.3 Fetal tachycardia with subsequent hydrops is a known risk factor for neurological abnormalities.4 The management of fetal tachycardias varies by institution and is usually based on published case series, institutional experience, the presence of fetal hydrops, and gestational age.2 In this manuscript, we present our unique experience in treating fetal SVT with hydrops fetalis using multiple routes of direct fetal amiodarone administration.
Timeline of intrauterine fetal intervention
| Gestational Age (Days after 1st Fetal Therapy) . | Transplacental Medication . | Direct Fetal Medication . | Result/Effect . | |
|---|---|---|---|---|
| Maternal . | Fetal . | |||
| 26 + 2 weeks (Day 0) | Amiodarone 1200 mg/d PO | — |
|
|
| 26 + 3 weeks (Day 1) | Amiodarone 1200 mg/d PO | Amiodarone 2.5 mg Intraumbilical vein |
|
|
| 26 + 5 weeks (Day 3) | Amiodarone 1200 mg/d PO | Amiodarone 5 mg Intraperitoneal cavity |
|
|
| 26 + 6 weeks (Day 4) | Amiodarone 600 mg/d PO | — |
|
|
| 27 + 2 weeks (Day 7) | Amiodarone 400 mg/d PO | — |
|
|
| 28 + 2 weeks (Day 14) | Amiodarone 200 mg/d PO | — |
|
|
| 29 + 1 weeks until delivery at 39 weeks | Amiodarone 100 mg/d PO | — |
|
|
| Gestational Age (Days after 1st Fetal Therapy) . | Transplacental Medication . | Direct Fetal Medication . | Result/Effect . | |
|---|---|---|---|---|
| Maternal . | Fetal . | |||
| 26 + 2 weeks (Day 0) | Amiodarone 1200 mg/d PO | — |
|
|
| 26 + 3 weeks (Day 1) | Amiodarone 1200 mg/d PO | Amiodarone 2.5 mg Intraumbilical vein |
|
|
| 26 + 5 weeks (Day 3) | Amiodarone 1200 mg/d PO | Amiodarone 5 mg Intraperitoneal cavity |
|
|
| 26 + 6 weeks (Day 4) | Amiodarone 600 mg/d PO | — |
|
|
| 27 + 2 weeks (Day 7) | Amiodarone 400 mg/d PO | — |
|
|
| 28 + 2 weeks (Day 14) | Amiodarone 200 mg/d PO | — |
|
|
| 29 + 1 weeks until delivery at 39 weeks | Amiodarone 100 mg/d PO | — |
|
|
| Gestational Age (Days after 1st Fetal Therapy) . | Transplacental Medication . | Direct Fetal Medication . | Result/Effect . | |
|---|---|---|---|---|
| Maternal . | Fetal . | |||
| 26 + 2 weeks (Day 0) | Amiodarone 1200 mg/d PO | — |
|
|
| 26 + 3 weeks (Day 1) | Amiodarone 1200 mg/d PO | Amiodarone 2.5 mg Intraumbilical vein |
|
|
| 26 + 5 weeks (Day 3) | Amiodarone 1200 mg/d PO | Amiodarone 5 mg Intraperitoneal cavity |
|
|
| 26 + 6 weeks (Day 4) | Amiodarone 600 mg/d PO | — |
|
|
| 27 + 2 weeks (Day 7) | Amiodarone 400 mg/d PO | — |
|
|
| 28 + 2 weeks (Day 14) | Amiodarone 200 mg/d PO | — |
|
|
| 29 + 1 weeks until delivery at 39 weeks | Amiodarone 100 mg/d PO | — |
|
|
| Gestational Age (Days after 1st Fetal Therapy) . | Transplacental Medication . | Direct Fetal Medication . | Result/Effect . | |
|---|---|---|---|---|
| Maternal . | Fetal . | |||
| 26 + 2 weeks (Day 0) | Amiodarone 1200 mg/d PO | — |
|
|
| 26 + 3 weeks (Day 1) | Amiodarone 1200 mg/d PO | Amiodarone 2.5 mg Intraumbilical vein |
|
|
| 26 + 5 weeks (Day 3) | Amiodarone 1200 mg/d PO | Amiodarone 5 mg Intraperitoneal cavity |
|
|
| 26 + 6 weeks (Day 4) | Amiodarone 600 mg/d PO | — |
|
|
| 27 + 2 weeks (Day 7) | Amiodarone 400 mg/d PO | — |
|
|
| 28 + 2 weeks (Day 14) | Amiodarone 200 mg/d PO | — |
|
|
| 29 + 1 weeks until delivery at 39 weeks | Amiodarone 100 mg/d PO | — |
|
|
Case report
A 31-year-old nulliparous 26 weeks’ pregnant woman was referred to our hospital. She had no notable previous illness or medical condition. Ultrasound and fetal echocardiography revealed fetal SVT with a suspected short ventriculoatrial (VA) interval, fetal heart rate (FHR) of 216 bpm, and 1:1 conduction. We also found ascites and subcutaneous edema as signs of fetal heart failure (Figure 1). Maternal biochemistry for thyroid function and infection was normal.
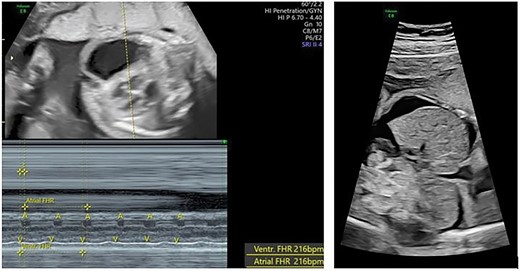
Fetal supraventricular tachycardia (short VA and 1:1 atrial-ventricular conduction) with signs of heart failure (ascites).
Direct fetal antiarrhythmic therapy with amiodarone was planned after the patient’s consent was received. We administered 1200 mg of oral amiodarone 24 h before the procedure to prevent redistribution to the maternal compartment after performing maternal electrocardiogram (ECG) and consulting the cardiologist.
At the beginning of the procedure, we planned to use the fetal intravenous route (dose 2.5 mg/kg) and to continue with the intraperitoneal (IP) route (dose 2.5 mg/kg). With ultrasound guidance, we identified the injection site and administered 1% lidocaine as local anesthesia. With the freehand technique, the amniocentesis needle was inserted and slowly advanced to the umbilical cord. We performed a small aspiration to ensure the needle was in the umbilical vein and continued with a slow injection of 2.5 mg of amiodarone (Figure 2). Before we administered the IP dose, the patient suddenly complained of shortness of breath. We stopped the procedure and gave her oxygen (3 L/min) via a nasal cannula, and subsequent maternal vital signs were within normal limits. Ultrasound showed an FHR of 107 bpm. We decided not to continue with the IP dose.
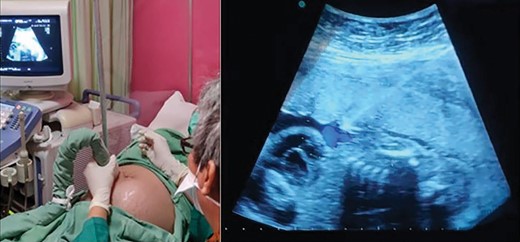
First direct fetal intervention: amiodarone injection into the fetal umbilical vein.
One day after the procedure, the FHR returned to 220 bpm. The patient agreed to undergo another direct fetal antiarrhythmic therapy via the fetal IP route in the operation room with continuous maternal vital sign and ECG monitoring. With ultrasound guidance and the freehand technique, we administered 5 mg (dose 5 mg/kg) amiodarone to the fetal peritoneal cavity (Figure 3). This time, the procedure was uneventful, but the FHR was still 220 bpm immediately after the procedure.
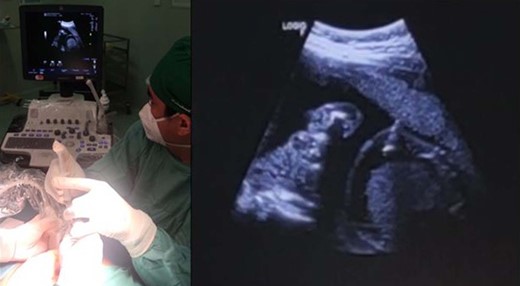
Second direct fetal intervention: amiodarone injection into the fetal peritoneal cavity.
The morning after the procedure, the FHR had decreased to 120 bpm with sinus rhythm (Figure 4A). During 4 days of observation, the FHR was continuously normal (∼120–130 bpm), and we decided to reduce the maternal amiodarone to 400 mg/day and discharged the patient. We performed a weekly follow-up, and the fetal hydrops (ascites and subcutaneous edema) had resolved 2 weeks after the procedure (Figure 4B). Further follow-up was uneventful, and we gradually decreased the maternal amiodarone dose to 100 mg/day. At 39+5 weeks of gestation, we performed a cesarean section, and a baby girl weighing 3400 g with an APGAR score of 9/10 was delivered. Neonatal ECG and thyroid screening were within normal limits. Echocardiography revealed a small patent oval foramen with a left-to-right shunt. The baby was discharged 3 days after delivery and exhibited normal growth until 3 months of follow-up (latest follow-up).
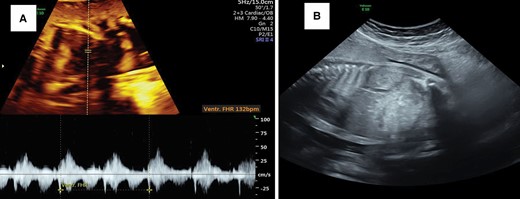
(A) Cardioversion to a sinus rhythm 12 h after intraperitoneal amiodarone. (B) Resolution of hydrops (ascites) 2 weeks after the procedure.
Discussion
Fetal SVT is a treatable arrhythmia but can lead to fetal hydrops with a significant risk of fetal demise if untreated.1,5 Robust protocols are lacking to guide the treatment of fetal SVT. Although several antiarrhythmic options exist in the literature, management is often difficult and requires prolonged maternal antiarrhythmic treatment. However, the nonresolution of hydrops despite rate reversion remains a therapeutic challenge.1 Most cases of SVT diagnosed before birth have a shorter V–A interval than an A–V interval (short V–A tachycardia). This results from an accessory pathway (atrioventricular reentry tachycardia) where there is rapid conduction from the ventricle back into the atrium.2 Several drugs can be used as initial treatment for fetal SVT with short VA, such as digoxin, flecainide, sotalol, and amiodarone.2,6 In the presence of fetal hydrops, the transplacental transfer of most antiarrhythmic medications is hindered, and effective drug levels may be difficult to obtain.7 Digoxin is the most common first-line drug but has limited utility in hydrops.6 Flecainide and sotalol can cross the placenta better than digoxin in fetal hydrops but are unavailable in our region.4,6 Amiodarone, which is available in our region, has promising efficacy in fetal SVT with hydrops fetalis.
Transplacental amiodarone alone or combined with digoxin or flecainide successfully achieved cardioversion of fetal SVT with hydrops or ventricular dysfunction in 14 of 15 cases (93%). When using combined amiodarone and digoxin, which are the only drugs available in our region, the successful cardioversion rate decreased to 73%. This method also had a long cardioversion time of up to 26 days in successful cases.8 The long cardioversion time can be attributed to the poor placental transfer of amiodarone (10–40%), which is worse in hydrops. Amiodarone can accumulate in fetal compartments due to a long half-life.7 However, the rapid and persistent control of FHR is essential for preventing cerebral complications in SVT. Prolonged hemodynamic compromise predisposes the fetus to cerebral ischemia due to the narrow autoregulatory range of systemic blood pressure and immature cerebral autoregulation.4
Direct fetal treatment may be needed to attain the optimal drug concentration in the fetus and achieve rapid cardioversion. Several modes of direct fetal treatment have been reported in the literature, including intra-amniotic, intravascular (IV), IP, intramuscular, and intracardiac, to rapidly achieve high drug concentrations in the fetal compartment.4,9 The IP route is technically easier and provides a depot for sustained drug release but has a longer response.10 The IV route provides direct access to the fetal circulation and allows quicker response but has the risk of cord injury and a shorter drug effect.4,10 Combined direct intraumbilical and IP application results in immediate cardioversion and a longer drug effect. Cord injury during IV drug administration can lead to hematoma, blood flow occlusion, and fetal death.4 The risk of cord injury during the direct administration of fetal IV drugs can be minimized when performed by a highly experienced operator. The reported rate of cordocentesis-associated fetal loss in highly experienced operators was only 0.6%11 The IP route is technically more straightforward than other methods of direct fetal treatment. A needle is inserted under ultrasound into the fetal peritoneal cavity, which is easily accessible when marked ascites occurs. Because of its simplicity, direct fetal treatment via the IP route should be safer than the IV route. In one series of fetal SVF with hydrops, one fetus received 10 doses of IP amiodarone for 3 weeks and had no complications from the procedure.4 Because of these reasons, we decided to directly perform the quicker direct fetal treatment instead of the slower transplacental treatment.
In the beginning, we planned to use the combined IV–IP route because it has a quicker response and a more prolonged drug effect.4 Amiodarone was chosen because of its efficiency and long half-life, limiting the number of invasive fetal procedures required to maintain therapeutic levels.7 Amiodarone did not negatively affect cardiac contractility compared with other agents. Before the direct fetal procedure, the mother was loaded with the drug to prevent any drug administered directly into the fetal circulation from simply redistributing into the maternal circulation.4 During direct fetal amiodarone IV administration, the patient complained of shortness of breath, and we did not continue with the IP dose. This procedure provides direct cardioversion of fetal SVT but is only sustained for 12 h. The second direct fetal treatment by the IP route had a longer response time (12 h) but lasting cardioversion to sinus rhythm until delivery and resolution of hydrops without adverse events.
Antiarrhythmic therapy can have profound maternal adverse effects.12 Fetal SVF often requires prolonged maternal antiarrhythmic treatment to achieve adequate therapeutic drug levels in the fetus.1 During the intraumbilical administration of amiodarone, the patient complained of sudden shortness of breath with subsequent normal physical and ECG examinations. The second direct administration via the IP route and continuation of maternal amiodarone therapy did not result in another maternal adverse event. Based on the available data, we cannot conclude that the transient maternal adverse event was due to amiodarone administration. We suggest that continuously monitoring the vital signs and ECG should be considered during this procedure. The 23% risk of neonatal hypothyroidism due to chronic amiodarone use is also a concern. However, most of the cases are transient, requiring only short-term treatment.4 In the current case, the neonatal thyroid function test was within normal limits.
Conclusions
The management of fetal ventricular tachycardias varies with each center, and drug therapy is typically based on published case series, institutional experience, and the presence of fetal hydrops. Direct fetal amiodarone administration by an experienced operator may be considered for fetal SVT with hydrops when effective and rapid-response transplacental drugs are unavailable. Continuous vital sign and ECG monitoring should be performed during direct fetal antiarrhythmic administration.
Lead author biography
 Irvan Adenin, MD, OBGYN, MFM, PHD is a senior obstetrician and fetomaternal consultant at the Indonesian National Center for Maternal and Children Health, Harapan Kita Maternal & Children Hospital. He has completed his medical degree and obstetric & gynecology specialist from the Sumatra Utara University. He has completed fetomaternal subspecialty training from Padjajaran University. He also has doctoral degree from University of Indonesia. His interests include fetal echocardiography and intrauterine fetal intervention.
Irvan Adenin, MD, OBGYN, MFM, PHD is a senior obstetrician and fetomaternal consultant at the Indonesian National Center for Maternal and Children Health, Harapan Kita Maternal & Children Hospital. He has completed his medical degree and obstetric & gynecology specialist from the Sumatra Utara University. He has completed fetomaternal subspecialty training from Padjajaran University. He also has doctoral degree from University of Indonesia. His interests include fetal echocardiography and intrauterine fetal intervention.
Supplementary material
Supplementary material is available at European Heart Journal—Case Reports.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The authors confirm that written consent for the submission of the case report, including images and associated text, has been obtained from the patient according to the COPE guidance.
Funding: None declared.
Data availability
The data underlying this article are available in the article and in its online supplementary material.
References
Author notes
Conflict of interest: None declared.




Comments