-
PDF
- Split View
-
Views
-
Cite
Cite
Hiroshi Fujita, Tsuyoshi Ito, Shohei Kikuchi, Yoshihiro Seo, Postprocedural ascending aortic dissection after transcatheter aortic valve implantation: a case report, European Heart Journal - Case Reports, Volume 7, Issue 1, January 2023, ytac486, https://doi.org/10.1093/ehjcr/ytac486
Close - Share Icon Share
Abstract
Transcatheter aortic valve implantation (TAVI) has been established as an effective and safe treatment for patients with severe aortic stenosis (AS). It is reported that vascular complications, especially aortic dissection, are rare. However, aortic dissection may be a serious consequence if it occurs. We experienced a case of delayed onset of ascending aortic dissection after TAVI.
An 82-year-old woman presented with dyspnoea and general fatigue. Echocardiography revealed severe AS and she was diagnosed with heart failure associated with AS. She had difficulty controlling heart failure and required the intervention of the aortic valve. We evaluated the aortic valve and access routes with contrast-enhanced computed tomography (CT), which showed marked dilatation of the ascending aorta. Transcatheter aortic valve implantation was performed and the procedure was completed without major complications. Transoesophageal echocardiography during the procedure did not detect any obvious arterial injury. However, on the second postoperative day, the patient suddenly became unconscious and a CT indicated an ascending aortic dissection. Unfortunately, she passed away. An autopsy revealed the fragility of the ascending aorta.
Patients with AS and aortic root dilatation may develop delayed onset of ascending aortic dissection after TAVI.
The arterial wall of the dilated aorta is fragile.
In cases of severe aortic stenosis with dilatation of the ascending aorta, we should carefully perform transcatheter aortic valve implantation and carefully manage postoperatively.
Introduction
Transcatheter aortic valve implantation (TAVI) is established as a useful and safe treatment for patients with severe aortic stenosis (AS).1 Complications associated with TAVI, such as annulus rupture and aortic injury, are relatively rare. However, when these complications do occur, they can lead to serious consequences, especially ascending aortic dissection (AD), which is one of the fatal complications. The mechanism for the development of ascending AD is thought to be due to aortic injury caused by devices, and it has been reported that most cases occur during procedures.2 In addition, it has been reported that some cases of ascending AD associated with aortic injury due to the edge of the self-expanding valve occurred several months after TAVI.3–5 We reported a case of delayed onset of ascending AD on the second day after TAVI without suspected device association.
Timeline
Case summary
An 82-year-old woman with no known past medical history presented with dyspnoea. Blood pressure was 92/56 mmHg, pulse 87/min, irregular. Oxygen saturation was 92% (room air). Her weight, body mass index, and body surface area: 57 kg, 25 kg/m2, and 1.5 m2. The crescendo–decrescendo systolic murmur was noted in the aortic area. The bibasilar crackles were heard. No jugular venous distension and leg oedema were noted. Chest X-ray showed pulmonary congestion. An electrocardiogram indicated atrial fibrillation rhythm. Brain natriuretic peptide was high. The transthoracic echocardiogram showed reduced left ventricular contractility and moderate to severe AS [ejection fraction (EF): 27%, aortic valve area: 0.5 cm2, peak velocity: 3.3 m/s, mean pressure gradient: 27 mmHg]. She was admitted with a diagnosis of heart failure due to left ventricular dysfunction and AS. She had low output syndrome so we have started dobutamine continuously at the cardiac care unit. However, her symptoms did not improve. Echocardiography showed suggested classical low-flow low-gradient severe AS (Figure 1). Dobutamine stress echocardiography was performed to assess the severity of AS. However, the stroke volume index (SVi) did not increase, making it difficult to evaluate the severity of AS. Therefore, we measured the aortic valve calcium score using electrocardiogram-synchronized cardiac computed tomography (CT), following the American College of Cardiology Foundation guidelines.6 Aortic valve calcium score was as high as 1372 and we diagnosed her as likely to have severe AS. In this case, the Society of Thoracic Surgeons Predicted Risk of mortality was as high, so we decided to perform TAVI. We evaluated the aortic valve and access route with contrast-enhanced CT (Figure 2). We found a remarkable ascending aorta dilatation (57 × 58 mm) (Figure 2). We performed TAVI with a trans-femoral approach under general anaesthesia. The prosthetic valve (SAPIEN3 26 mm: Edwards Lifesciences, Irvine, CA, USA) was carefully delivered and implanted without balloon aortic valvuloplasty. The prosthetic valve was implanted with the implantation pressure at nominal −2 cc (Figure 3). Transoesophageal echocardiography (TEE) was performed to carefully check the ascending and descending aorta for injuries, but there were no abnormal findings (Figure 3). Postoperatively, blood pressure was elevated over 200 mmHg and she required an antihypertensive drug (nicardipine: 1.0 µg/kg/min). She required continuation of dobutamine because echocardiography showed only slight improvement in EF and SVi. On the second day after the TAVI, she suddenly developed a loss of consciousness and the CT showed ascending AD (Figure 4). We considered AD difficult to treat with percutaneous intervention due to the need to revascularize the cerebral vessels, so we suggested surgery. Her family did not want to have surgery due to the high risk and unfortunately, she passed away. On autopsy, the macroscopic finding showed a sharp dissection entry 5 cm distal to the prosthetic valve level (Figure 5). Microscopic examination showed that the elastic laminar (EL) of the ascending aorta was thin compared with the control vessel and fragmentation of EL was observed (Figure 5).
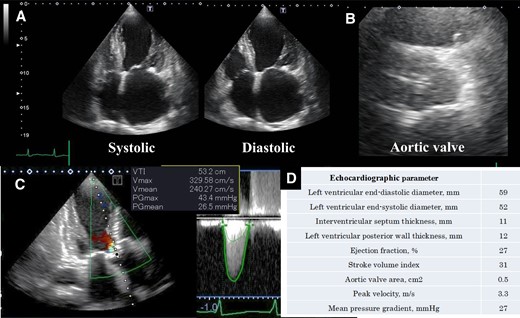
Echocardiographic assessment. (A) Echocardiographic showed left ventricular contractility, (B) aortic valve at axial view, (C) echocardiography continuous-wave Doppler across the aortic valve, and (D) echocardiographic parameters.
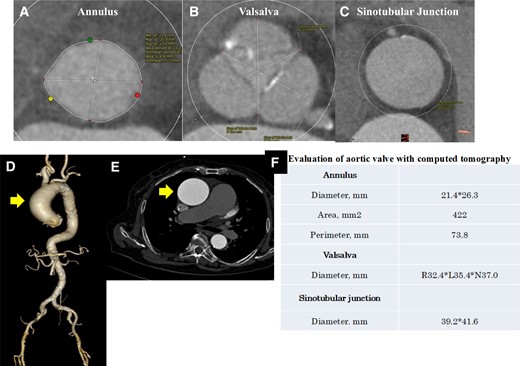
Evaluation of the aortic valve and access route with computed tomography. (A–C) The aortic valve at axial view. (D) The aorta in three-dimensional view. (E) The ascending aorta at axial view. Ascending aortic dilatation of 57 × 58 mm was observed. (F) Evaluation of the aortic valve with computed tomography.
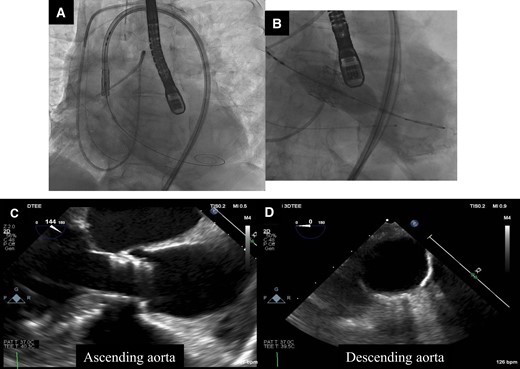
Valve delivery and implantation and aortic evaluation with transoesophageal echocardiography during transcatheter aortic valve implantation. (A) The prosthetic valve was delivered carefully. (B) The prosthetic valve was implanted at pressure of nominal −2 cc. (C and D) There were no injuries to the ascending and descending aorta.
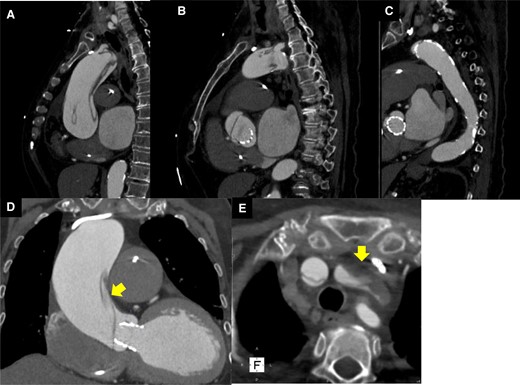
Computed tomography on the second day after transcatheter aortic valve implantation. (A–C) Aortic dissection was observed in the ascending aorta. (D) The entry of aortic dissection. (E) Left internal carotid artery occlusion.
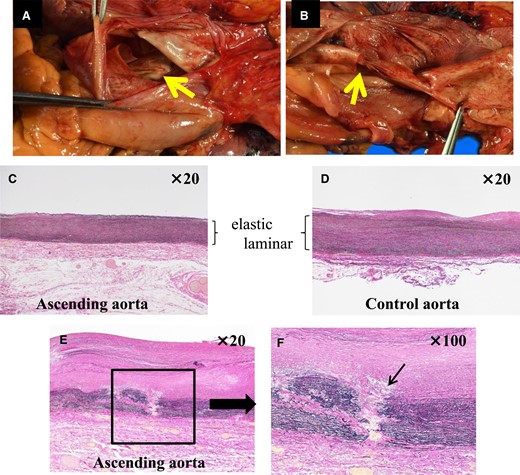
Macroscopic findings and microscopic findings at autopsy (Elastica Van Gieson stain). (A and B) There was a dissection entry 5 cm distal to the prosthetic valve position. (C) Ascending aorta. (D) Control aorta. The elastic laminar was thinning in the ascending aorta compared with the control aorta. (E and F) The arrow indicated fragmentation to the elastic laminar.
Discussion
We experienced a case of development of ascending AD 2 days after TAVI. Transcatheter aortic valve implantation has been established as an effective and safe treatment.1 It has been reported that vascular complications associated with TAVI are ∼5%.7 In particular, the incidence of AD was ∼0.12%.8 Rylski et al.9 reported that TAVI was performed on 98 severe AS patients who had ascending aortic dilatation (40–50 mm) and the incidence of complications during TAVI was low at 1%, with no occurrence of AD. It is reported that AD rarely occurs during TAVI and TAVI can be performed safely with dilated ascending aorta. In contrast, it has been reported that the arterial wall becomes more fragile with aortic dilatation. Furthermore, it has been reported that smooth muscle cell apoptosis correlates with the size of the aorta and fibrous collagen decreases with aortic dilatation, especially the arterial wall over 50 mm is highly fragile.10 However, there are no case reports of TAVI in patients with ascending aorta >50 mm. In this case, the ascending aorta was dilated to 58 mm and the arterial wall was considered to be remarkably fragile. Furthermore, microscopic findings showed thinning and fragmentation of the EL (Figure 5). This finding indicates arterial wall fragility and is a frequently observed in cases of spontaneous acute AD.11,12 Generally, the improvement of obstruction of the aortic valve after TAVI leads to the intensification of load transmission, such as acute pressure and volume changes, towards the arterial system. It has been reported that the EL of the aortic wall responds to this load and maintains aortic compliance.13 In this case, SVi was improved and blood pressure was elevated remarkably, postoperatively. The fragile arterial wall gradually became unable to adapt to these haemodynamic changes, and AD may have occurred on the second day after TAVI.
It has been reported that AD can occur as a result of procedures and devices.3–5,14,15 We discussed the possibility of complications associated with the devices. However, we thought AD might be associated less with the devices because the entrance of AD was away from the prosthetic valve and TEE did not detect AD immediately after TAVI.
Transcatheter aortic valve implantation is a safe treatment, but we considered the fragility of the aortic wall resulting from aortic dilatation to be one of the risk factors for delayed-onset AD. When we perform TAVI on patients with aortic dilatation, we need to be careful about postoperative haemodynamic changes, including blood pressure management.
Conclusion
In this case, the arterial wall was fragile due to significant ascending aorta dilatation. Therefore, the haemodynamic changes associated with AS improvement may have affected the occurrence of AD.
Lead author biography
 Hiroshi Fujita is an interventional cardiologist and assistant professor in the Department of Cardiology at Nagoya City University Graduate School of Medical Sciences. He had interventional training at Toyohashi Heart Center, Toyohashi, Japan, from 2005 to 2009.
Hiroshi Fujita is an interventional cardiologist and assistant professor in the Department of Cardiology at Nagoya City University Graduate School of Medical Sciences. He had interventional training at Toyohashi Heart Center, Toyohashi, Japan, from 2005 to 2009.
Supplementary material
Supplementary material is available at European Heart Journal – Case Reports online.
Acknowledgements
None.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The authors confirm that written consent for submission and publication of this case report including image(s) and associated text has been obtained from the family of the patient in line with COPE guidance.
Funding: None declared.
References
Author notes
Conflict of interest: None declared.





Comments