-
PDF
- Split View
-
Views
-
Cite
Cite
Kara Morton, Brittain Heindl, Samuel K McElwee, Silvio Litovsky, Mustafa I Ahmed, Stephen Clarkson, Percutaneous debulking of tricuspid valve endocarditis in severe COVID-19 pneumonia after prolonged venovenous extracorporeal membrane oxygenation with right-ventricular support: a case series, European Heart Journal - Case Reports, Volume 7, Issue 2, February 2023, ytac409, https://doi.org/10.1093/ehjcr/ytac409
Close - Share Icon Share
Abstract
Over the past 2 years, the utilization of venovenous extracorporeal membrane oxygenation (VV-ECMO) for the treatment of coronavirus disease 2019 (COVID-19) acute respiratory distress syndrome (ARDS) has increased. While supporting respiratory function, VV-ECMO requires large-bore indwelling venous cannulas, which risk bleeding and infections, including endocarditis.
We describe two adults hospitalized for COVID-19 pneumonia who developed ARDS and right-ventricular failure, requiring VV-ECMO and ProtekDuo cannulation. After over 100 days with these devices, both patients developed tricuspid valve vegetations. Our first patient was decannulated from ECMO and discharged, but re-presented with a segmental pulmonary embolism and tricuspid mass. The Inari FlowTriver system was chosen to percutaneously remove both the tricuspid mass and pulmonary thromboembolism. Pathological examination of the mass demonstrated Candida albicans endocarditis in the setting of Candida fungemia. Our second patient developed a tricuspid valve vegetation which was also removed with the FlowTriever system. Pathological examination demonstrated endocarditis consistent with Pseudomonas aeruginosa in the setting of Pseudomonas bacteremia. Both patients experienced resolution of fungemia and bacteremia after percutaneous vegetation removal. After ECMO decannulation and percutaneous debulking, both patients experienced prolonged hospital stays for ventilator weaning and were eventually discharged with supplemental oxygen.
VV-ECMO and right-ventricular support devices are invasive and create various risks, including bloodstream infection and infective endocarditis. Percutaneous debulking of valvular vegetations associated with these right-sided indwelling devices may be an effective means of infection source control. It is unclear whether prolonged use of VV-ECMO provides a mortality benefit in COVID-19 ARDS.
Prolonged VV-ECMO and ProtekDuo right-ventricular cannulation increase the risk of device-associated bloodstream infection and may also increase the risk of developing tricuspid valve endocarditis.
The Inari FlowTriever system, approved for the percutaneous removal of pulmonary thromboembolism, may show promise for debulking large valvular vegetations as a method of infection source control, and to reduce the need for surgical intervention.
It is unclear whether the utilization of VV-ECMO in cases of COVID-19-associated ARDS provides a significant mortality benefit.
Introduction
The coronavirus disease 2019 (COVID-19) pandemic has profoundly affected the US healthcare system. Based on a 2021 analysis of data from the US National COVID Cohort Collaborative, 20.2% of adults hospitalized for COVID-19 experienced a severe clinical course involving either invasive ventilatory support, extracorporeal membrane oxygenation (ECMO), or death.1 Of those 6565 severe cases, 2790 (42.5%) required invasive ventilatory support or ECMO. We present two patients with similar hospital courses: both patients were not fully vaccinated and developed COVID-19 acute respiratory distress syndrome (ARDS) requiring mechanical ventilation and venovenous ECMO (VV-ECMO) initiation. Each patient then had right-ventricular failure requiring reconfiguration to a ProtekDuo right-ventricular support system (LivaNova, London, UK). Both patients developed tricuspid valve vegetations and underwent percutaneous debulking with the FlowTriever system (Inari Medical, Irvine, CA, USA).
Timeline
| . | Time . | Event . |
|---|---|---|
| Patient 1 | Presentation | Admitted to an outside hospital Tested positive for COVID-19 |
| Day 3 | Transferred to our institution Intubated and mechanically ventilated Cannulated for VV-ECMO | |
| Day 8 | Tracheostomy placed | |
| Day 20 | Blood cultures positive for Enterococcus faecalis; treated with vancomycin | |
| Day 39 | ProtekDuo cannula connected to VV-ECMO circuit | |
| Day 52 | Occlusive deep vein thrombosis in the right common femoral vein and saphenous junction to right posterior tibial vein | |
| Day 100 | Blood cultures positive for Candida albicans; treated with micafungin | |
| Day 123 | Decannulated from VV-ECMO Decannulated from tracheostomy | |
| Day 149 | Discharged home with oral apixaban and supplemental oxygen | |
| Day 174 | Re-presented to our institution with dyspnoea and increased oxygen requirement Computed tomography angiogram with right pulmonary artery thromboembolism Taken for thrombectomy of pulmonary thromboembolism (PTE) with Inari FlowTriever Tricuspid valve mass debulked with Inari FlowTriever, consistent with Candida endocarditis | |
| Day 175 | Intubated; pressors initiated for haemodynamic collapse Blood cultures, drawn on admission, returned positive for C. albicans; treated with amphotericin B | |
| Day 185 | Surveillance blood cultures negative | |
| Day 186 | Tracheostomy placed and transferred to Pulmonary Special Care Unit | |
| Day 248 | Decannulated from tracheostomy | |
| Day 255 | Discharged to inpatient rehabilitation facility on supplemental oxygen | |
| Patient 2 | Presentation | Tested positive for COVID-19 Admitted to acute care hospital room |
| Day 3 | Oxygen requirement escalates to high-flow nasal cannula at 100% fraction of inspired oxygen Transferred to intensive care unit Normal transthoracic echocardiogram | |
| Day 9 | Intubated and mechanically ventilated | |
| Day 15 | Continuous renal replacement therapy initiated for acute kidney failure | |
| Day 16 | Cannulated for VV-ECMO | |
| Day 29 | Atrial fibrillation with a rapid ventricular response Direct current cardioversion, reverted to sinus rhythm Repeat transthoracic echocardiogram with reduced left-ventricular ejection fraction to 40–45% and severe right-ventricular dilation | |
| Day 51 | ProtekDuo cannula placed due to hypotension and persistent right-ventricular failure | |
| Day 70 | Blood cultures positive for E. faecalis; treated with ampicillin and ceftriaxone | |
| Day 157 | Blood cultures positive for Candida parapsilosis; treated with micafungin | |
| Day 160 | Blood cultures positive for Candida glabrata; micafungin escalated to amphotericin B | |
| Day 167 | Transthoracic echocardiogram demonstrates new tricuspid valve vegetations and ProtekDuo-associated vegetations ProtekDuo cannula removed, VV-ECMO reconfigured | |
| Day 171 | Transthoracic echocardiogram demonstrates persistent tricuspid valve vegetation | |
| Day 173 | Inari FlowTriever used to debulk tricuspid valve vegetation | |
| Day 174 | Blood cultures positive for Pseudomonas aeruginosa; treated with amikacin | |
| Day 181 | Negative blood cultures | |
| Day 193 | Decannulated from VV-ECMO | |
| Day 213 | Discharged home | |
| Day 217 | Re-presented to our institution with dyspnoea and hypercapnic respiratory failure | |
| Day 221 | Intubated and mechanically ventilated | |
| Day 223 | Tracheostomy placed, continued ventilator weaning | |
| Day 233 | Patient discharged home with tracheostomy in place |
| . | Time . | Event . |
|---|---|---|
| Patient 1 | Presentation | Admitted to an outside hospital Tested positive for COVID-19 |
| Day 3 | Transferred to our institution Intubated and mechanically ventilated Cannulated for VV-ECMO | |
| Day 8 | Tracheostomy placed | |
| Day 20 | Blood cultures positive for Enterococcus faecalis; treated with vancomycin | |
| Day 39 | ProtekDuo cannula connected to VV-ECMO circuit | |
| Day 52 | Occlusive deep vein thrombosis in the right common femoral vein and saphenous junction to right posterior tibial vein | |
| Day 100 | Blood cultures positive for Candida albicans; treated with micafungin | |
| Day 123 | Decannulated from VV-ECMO Decannulated from tracheostomy | |
| Day 149 | Discharged home with oral apixaban and supplemental oxygen | |
| Day 174 | Re-presented to our institution with dyspnoea and increased oxygen requirement Computed tomography angiogram with right pulmonary artery thromboembolism Taken for thrombectomy of pulmonary thromboembolism (PTE) with Inari FlowTriever Tricuspid valve mass debulked with Inari FlowTriever, consistent with Candida endocarditis | |
| Day 175 | Intubated; pressors initiated for haemodynamic collapse Blood cultures, drawn on admission, returned positive for C. albicans; treated with amphotericin B | |
| Day 185 | Surveillance blood cultures negative | |
| Day 186 | Tracheostomy placed and transferred to Pulmonary Special Care Unit | |
| Day 248 | Decannulated from tracheostomy | |
| Day 255 | Discharged to inpatient rehabilitation facility on supplemental oxygen | |
| Patient 2 | Presentation | Tested positive for COVID-19 Admitted to acute care hospital room |
| Day 3 | Oxygen requirement escalates to high-flow nasal cannula at 100% fraction of inspired oxygen Transferred to intensive care unit Normal transthoracic echocardiogram | |
| Day 9 | Intubated and mechanically ventilated | |
| Day 15 | Continuous renal replacement therapy initiated for acute kidney failure | |
| Day 16 | Cannulated for VV-ECMO | |
| Day 29 | Atrial fibrillation with a rapid ventricular response Direct current cardioversion, reverted to sinus rhythm Repeat transthoracic echocardiogram with reduced left-ventricular ejection fraction to 40–45% and severe right-ventricular dilation | |
| Day 51 | ProtekDuo cannula placed due to hypotension and persistent right-ventricular failure | |
| Day 70 | Blood cultures positive for E. faecalis; treated with ampicillin and ceftriaxone | |
| Day 157 | Blood cultures positive for Candida parapsilosis; treated with micafungin | |
| Day 160 | Blood cultures positive for Candida glabrata; micafungin escalated to amphotericin B | |
| Day 167 | Transthoracic echocardiogram demonstrates new tricuspid valve vegetations and ProtekDuo-associated vegetations ProtekDuo cannula removed, VV-ECMO reconfigured | |
| Day 171 | Transthoracic echocardiogram demonstrates persistent tricuspid valve vegetation | |
| Day 173 | Inari FlowTriever used to debulk tricuspid valve vegetation | |
| Day 174 | Blood cultures positive for Pseudomonas aeruginosa; treated with amikacin | |
| Day 181 | Negative blood cultures | |
| Day 193 | Decannulated from VV-ECMO | |
| Day 213 | Discharged home | |
| Day 217 | Re-presented to our institution with dyspnoea and hypercapnic respiratory failure | |
| Day 221 | Intubated and mechanically ventilated | |
| Day 223 | Tracheostomy placed, continued ventilator weaning | |
| Day 233 | Patient discharged home with tracheostomy in place |
| . | Time . | Event . |
|---|---|---|
| Patient 1 | Presentation | Admitted to an outside hospital Tested positive for COVID-19 |
| Day 3 | Transferred to our institution Intubated and mechanically ventilated Cannulated for VV-ECMO | |
| Day 8 | Tracheostomy placed | |
| Day 20 | Blood cultures positive for Enterococcus faecalis; treated with vancomycin | |
| Day 39 | ProtekDuo cannula connected to VV-ECMO circuit | |
| Day 52 | Occlusive deep vein thrombosis in the right common femoral vein and saphenous junction to right posterior tibial vein | |
| Day 100 | Blood cultures positive for Candida albicans; treated with micafungin | |
| Day 123 | Decannulated from VV-ECMO Decannulated from tracheostomy | |
| Day 149 | Discharged home with oral apixaban and supplemental oxygen | |
| Day 174 | Re-presented to our institution with dyspnoea and increased oxygen requirement Computed tomography angiogram with right pulmonary artery thromboembolism Taken for thrombectomy of pulmonary thromboembolism (PTE) with Inari FlowTriever Tricuspid valve mass debulked with Inari FlowTriever, consistent with Candida endocarditis | |
| Day 175 | Intubated; pressors initiated for haemodynamic collapse Blood cultures, drawn on admission, returned positive for C. albicans; treated with amphotericin B | |
| Day 185 | Surveillance blood cultures negative | |
| Day 186 | Tracheostomy placed and transferred to Pulmonary Special Care Unit | |
| Day 248 | Decannulated from tracheostomy | |
| Day 255 | Discharged to inpatient rehabilitation facility on supplemental oxygen | |
| Patient 2 | Presentation | Tested positive for COVID-19 Admitted to acute care hospital room |
| Day 3 | Oxygen requirement escalates to high-flow nasal cannula at 100% fraction of inspired oxygen Transferred to intensive care unit Normal transthoracic echocardiogram | |
| Day 9 | Intubated and mechanically ventilated | |
| Day 15 | Continuous renal replacement therapy initiated for acute kidney failure | |
| Day 16 | Cannulated for VV-ECMO | |
| Day 29 | Atrial fibrillation with a rapid ventricular response Direct current cardioversion, reverted to sinus rhythm Repeat transthoracic echocardiogram with reduced left-ventricular ejection fraction to 40–45% and severe right-ventricular dilation | |
| Day 51 | ProtekDuo cannula placed due to hypotension and persistent right-ventricular failure | |
| Day 70 | Blood cultures positive for E. faecalis; treated with ampicillin and ceftriaxone | |
| Day 157 | Blood cultures positive for Candida parapsilosis; treated with micafungin | |
| Day 160 | Blood cultures positive for Candida glabrata; micafungin escalated to amphotericin B | |
| Day 167 | Transthoracic echocardiogram demonstrates new tricuspid valve vegetations and ProtekDuo-associated vegetations ProtekDuo cannula removed, VV-ECMO reconfigured | |
| Day 171 | Transthoracic echocardiogram demonstrates persistent tricuspid valve vegetation | |
| Day 173 | Inari FlowTriever used to debulk tricuspid valve vegetation | |
| Day 174 | Blood cultures positive for Pseudomonas aeruginosa; treated with amikacin | |
| Day 181 | Negative blood cultures | |
| Day 193 | Decannulated from VV-ECMO | |
| Day 213 | Discharged home | |
| Day 217 | Re-presented to our institution with dyspnoea and hypercapnic respiratory failure | |
| Day 221 | Intubated and mechanically ventilated | |
| Day 223 | Tracheostomy placed, continued ventilator weaning | |
| Day 233 | Patient discharged home with tracheostomy in place |
| . | Time . | Event . |
|---|---|---|
| Patient 1 | Presentation | Admitted to an outside hospital Tested positive for COVID-19 |
| Day 3 | Transferred to our institution Intubated and mechanically ventilated Cannulated for VV-ECMO | |
| Day 8 | Tracheostomy placed | |
| Day 20 | Blood cultures positive for Enterococcus faecalis; treated with vancomycin | |
| Day 39 | ProtekDuo cannula connected to VV-ECMO circuit | |
| Day 52 | Occlusive deep vein thrombosis in the right common femoral vein and saphenous junction to right posterior tibial vein | |
| Day 100 | Blood cultures positive for Candida albicans; treated with micafungin | |
| Day 123 | Decannulated from VV-ECMO Decannulated from tracheostomy | |
| Day 149 | Discharged home with oral apixaban and supplemental oxygen | |
| Day 174 | Re-presented to our institution with dyspnoea and increased oxygen requirement Computed tomography angiogram with right pulmonary artery thromboembolism Taken for thrombectomy of pulmonary thromboembolism (PTE) with Inari FlowTriever Tricuspid valve mass debulked with Inari FlowTriever, consistent with Candida endocarditis | |
| Day 175 | Intubated; pressors initiated for haemodynamic collapse Blood cultures, drawn on admission, returned positive for C. albicans; treated with amphotericin B | |
| Day 185 | Surveillance blood cultures negative | |
| Day 186 | Tracheostomy placed and transferred to Pulmonary Special Care Unit | |
| Day 248 | Decannulated from tracheostomy | |
| Day 255 | Discharged to inpatient rehabilitation facility on supplemental oxygen | |
| Patient 2 | Presentation | Tested positive for COVID-19 Admitted to acute care hospital room |
| Day 3 | Oxygen requirement escalates to high-flow nasal cannula at 100% fraction of inspired oxygen Transferred to intensive care unit Normal transthoracic echocardiogram | |
| Day 9 | Intubated and mechanically ventilated | |
| Day 15 | Continuous renal replacement therapy initiated for acute kidney failure | |
| Day 16 | Cannulated for VV-ECMO | |
| Day 29 | Atrial fibrillation with a rapid ventricular response Direct current cardioversion, reverted to sinus rhythm Repeat transthoracic echocardiogram with reduced left-ventricular ejection fraction to 40–45% and severe right-ventricular dilation | |
| Day 51 | ProtekDuo cannula placed due to hypotension and persistent right-ventricular failure | |
| Day 70 | Blood cultures positive for E. faecalis; treated with ampicillin and ceftriaxone | |
| Day 157 | Blood cultures positive for Candida parapsilosis; treated with micafungin | |
| Day 160 | Blood cultures positive for Candida glabrata; micafungin escalated to amphotericin B | |
| Day 167 | Transthoracic echocardiogram demonstrates new tricuspid valve vegetations and ProtekDuo-associated vegetations ProtekDuo cannula removed, VV-ECMO reconfigured | |
| Day 171 | Transthoracic echocardiogram demonstrates persistent tricuspid valve vegetation | |
| Day 173 | Inari FlowTriever used to debulk tricuspid valve vegetation | |
| Day 174 | Blood cultures positive for Pseudomonas aeruginosa; treated with amikacin | |
| Day 181 | Negative blood cultures | |
| Day 193 | Decannulated from VV-ECMO | |
| Day 213 | Discharged home | |
| Day 217 | Re-presented to our institution with dyspnoea and hypercapnic respiratory failure | |
| Day 221 | Intubated and mechanically ventilated | |
| Day 223 | Tracheostomy placed, continued ventilator weaning | |
| Day 233 | Patient discharged home with tracheostomy in place |
Case presentation
Case 1
A 48-year-old female with obesity and anxiety presented to the emergency department with worsening shortness of breath. She was discharged 1 month earlier, after a hospitalization for COVID-19 ARDS and right-ventricular failure requiring 4 months of VV-ECMO via ProtekDuo configuration. Her hospitalization was complicated by a pneumothorax, candidaemia, a right femoral deep vein thrombosis, and chronic hypoxic respiratory failure requiring 3 L of home oxygen at discharge. She had received one dose of the mRNA COVID-19 vaccine series. The patient reported worsening exertional dyspnoea for 3 days prior to presentation, which prompted her return. On presentation, she was tachycardic, tachypnoeic, and her oxygen saturation levels were below 80% despite 5 L of supplemental oxygen via nasal cannula. Initial laboratory values returned with a lactic acid of 1.1 mmol/L, creatinine of 1.2 mg/dL, white cell count of 53.0 × 109 cells/L, venous blood pH of 7.1, and venous blood CO2 of 76.6 mmHg. A computed tomographic pulmonary angiogram demonstrated a new occlusive PTE in the right main pulmonary artery (Figure 1). A bedside transthoracic echocardiogram demonstrated mild dilation of her right ventricle and masses in the right atrium and on the tricuspid valve, believed to be a thrombus in transit. She was started on a heparin infusion and admitted to the cardiac critical care unit. She was administered vancomycin, cefepime, and micafungin.
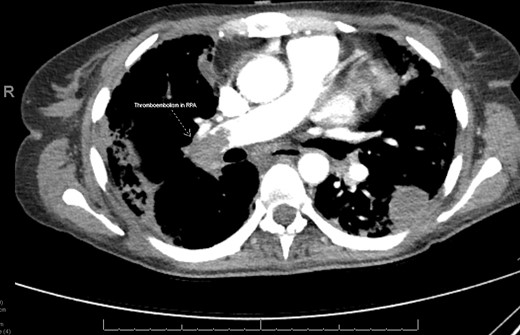
Computed tomographic pulmonary angiogram demonstrating an occlusive thromboembolism in the right pulmonary artery in Patient 1. RPA, right pulmonary artery.
Given the large amount of thrombus, and the patient’s respiratory failure, mechanical thrombectomy of her PTE with the FlowTriever system was performed under fluoroscopic and transoesophageal echocardiographic guidance. The same system was used to first debulk the right atrial and tricuspid valve masses (Figure 2, Supplementary material online, Video S1), followed by the removal of a large quantity of embolic material from the right pulmonary artery. Multiple tan-brown fragments were retrieved from the tricuspid valve, in aggregate measuring 6.4 × 5.5 × 0.5 cm. Pathology of the fragments demonstrated filamentous organisms suggestive of fungal endocarditis (Figures 3 and 4). Her blood cultures, drawn on admission, returned positive for C. albicans. The following night, the patient became unresponsive and hypotensive requiring intubation and the addition of three vasopressors. Repeat transthoracic echocardiogram demonstrated normal right- and left-ventricular systolic function without vegetation or thrombus. She was continued on the heparin infusion to clear the remaining clot burden, and her haemodynamics gradually improved without additional interventions. She required tracheostomy placement for chronic respiratory failure 12 days following re-presentation to the hospital and was transferred to the ventilator weaning unit. She was decannulated from her tracheostomy 248 days following her original presentation and was discharged to a rehabilitation facility 1 week later, requiring 1 L of supplemental oxygen. She required 2 months of inpatient rehabilitation and subsequently underwent a tricuspid valve replacement. She continues to follow with our cardiothoracic surgery clinic.
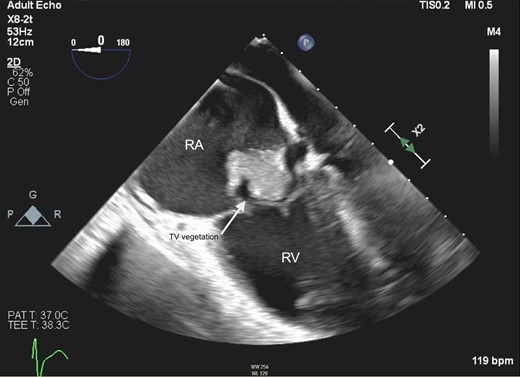
Mid-oesophageal four-chamber view on transoesophageal echocardiography demonstrating mobile tricuspid valve vegetation in Patient 1. RA, right atrium; RV, right ventricle; TV, tricuspid valve.
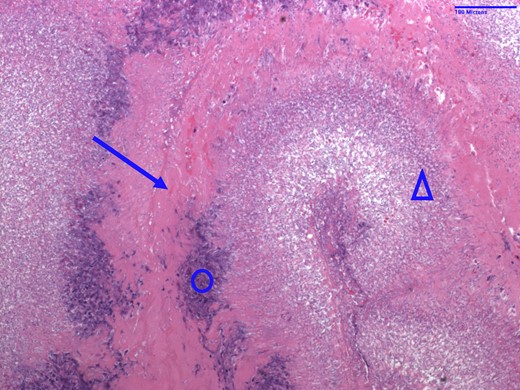
Haematoxylin and eosin stain of tricuspid valve vegetation specimen from Patient 1 indicative of fungal endocarditis. Arrow = fibrin; circle = acute inflammation; triangle = fungal colonies consistent with Candida albicans.
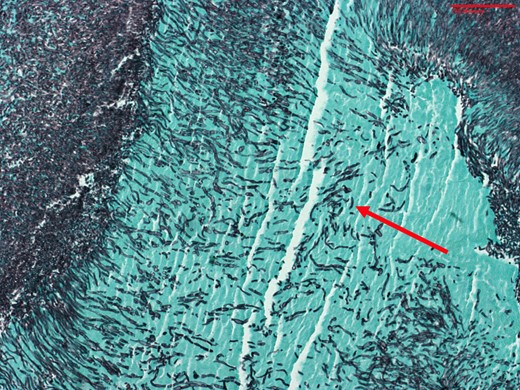
Grocott methenamine silver stain of the tricuspid valve vegetation specimen from Patient 1 demonstrating fungal organisms consistent with Candida albicans pseudohyphae. Arrow = fungal organism.
Case 2
A 39-year-old male with obesity and no other past medical history presented with symptoms of COVID-19 after a recent exposure. He was unvaccinated. He was tachycardic, tachypnoeic, and required 4 L via nasal cannula to maintain an oxygen saturation of 95%. Subsequent COVID-19 polymerase chain reaction test was positive, and he was admitted to an acute care bed for acute hypoxemic respiratory failure and was treated with dexamethasone and remdesivir. His respiratory status continued to worsen, and he was eventually transferred to the intensive care unit and intubated. Despite continued supportive care, he progressed to severe ARDS and was subsequently cannulated for VV-ECMO via a right femoral vein to right internal jugular configuration. His ECMO course was further complicated by ventilator-associated P. aeruginosa pneumonia, pneumothorax, acute kidney failure requiring dialysis, and atrial fibrillation with a rapid ventricular response requiring mechanical cardioversion. On ECMO Day 35, he developed progressive shock and was diagnosed with right-ventricular failure, for which he was reconfigured to a ProtekDuo cannula for additional support. On ECMO Day 124, a transthoracic echocardiogram was performed due to C. glabrata and C. parapsilosis fungemia, demonstrating new vegetations on the tricuspid valve and the ProtekDuo cannula. The largest vegetation measured 1.4 × 1.1 cm (Figure 5, Supplementary material online, Video S2). An area of non-mobile thickening was also noted on the right atrial wall, adjacent to the tricuspid valve, measuring 2.1 × 1.3 cm. Also present was moderate-to-severe tricuspid regurgitation and a flattened interventricular septum consistent with right-ventricular pressure overload. The right ventricle was severely dilated with preserved systolic function, and the left-ventricular ejection fraction was 55–60%.
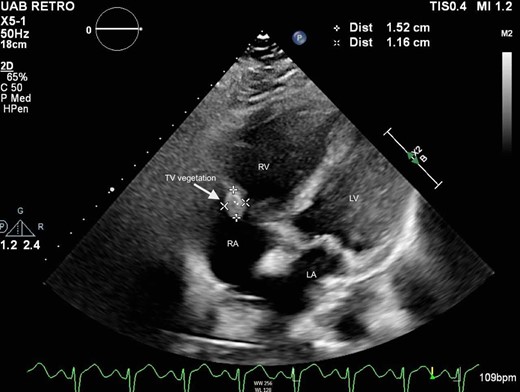
Subcostal four-chamber view on transthoracic echocardiogram demonstrating tricuspid valve vegetation in Patient 2. LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle; TV, tricuspid valve.
His endocarditis was presumed to be catheter associated; therefore, the ProtekDuo cannula was removed and his ECMO cannulation strategy was reconfigured to a right femoral vein to left internal jugular approach. On ECMO Day 141, the tricuspid valve and right atrial vegetations were aspirated with the Inari FlowTriever system under transoesophageal echocardiographic and fluoroscopic guidance. A large vegetation was present above the posterior leaflet of the tricuspid valve, comprised of two mobile components with a central portion adherent to the posterior right atrial wall. Multiple tan-grey specimens were retrieved, aggregating to 3.0 × 2.8 × 0.5 cm. Pathological examination demonstrated infective endocarditis with bacterial colonies consistent with P. aeruginosa bacilli (Figure 6). Blood cultures drawn the following day were positive for multi-drug resistant P. aeruginosa.
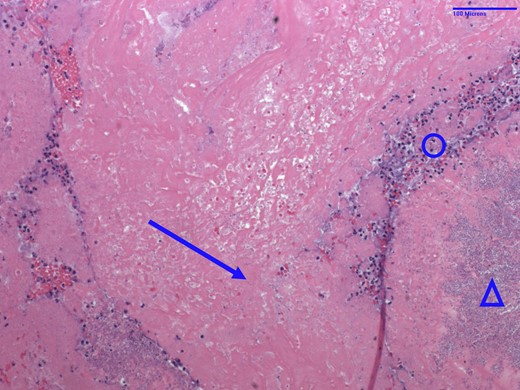
Haematoxylin and eosin stain of tricuspid valve vegetation specimen from Patient 2. Arrow = fibrin; circle = acute inflammation; triangle = bacterial bacilli colonies consistent with Pseudomonas aeruginosa.
On transoesophageal echocardiogram, the post-procedure tricuspid regurgitation remained severe but had improved compared with pre-procedure. Repeat blood cultures 4 days post-debulking demonstrated no growth and remained clear thereafter. The patient was gradually weaned from mechanical ventilation and was decannulated from VV-ECMO on hospitalization Day 193. He was discharged home on hospitalization Day 213; however, he returned 4 days later with worsening hypoxemia and hypercapnia due to volume overload from an inadequate oral diuretic regimen. He required re-intubation followed by tracheostomy placement for chronic respiratory failure. He was eventually weaned to a tracheal collar and discharged home with a continued need for respiratory support via tracheal collar and portable ventilator. He continues to follow with our outpatient pulmonary clinic and is no longer tracheostomy dependent.
Discussion
We present two cases, one incompletely vaccinated patient and one unvaccinated patient, hospitalized with severe COVID-19 pneumonia requiring VV-ECMO and ProtekDuo cannulation who subsequently developed tricuspid valve vegetations, removed with the Inari FlowTriever system. We would like to highlight three main points that arise from these cases. First, we observed an increased risk of right-sided endocarditis secondary to nosocomial bloodstream infections as a likely consequence of VV-ECMO and ProtekDuo support, although there is insufficient data to support this association currently. Our cases also propose a potential utility in percutaneous debulking of right-sided vegetations, specifically using the Inari FlowTriever, as a means of source control for endocarditis and bacteraemia in poor surgical candidates. Lastly, we question whether there a significant benefit of long-term ECMO with right-ventricular support in cases of ARDS caused by COVID-19.
The ProtekDuo is a dual lumen catheter inserted into the internal jugular vein that drains from the right atrium into the pulmonary artery, bypassing the right ventricle. This form of support is particularly useful in cases of right-ventricular dysfunction. The addition of a membrane oxygenator also allows the device to function in a VV-ECMO capacity. Potential disadvantages of the ProtekDuo include greater technical expertise for its utilization, as well as indwelling device-associated infection. The most common microbes among ECMO-associated bloodstream infections are coagulase negative Staphylococcal species followed by the Candida species, Enterococcus, and Pseudomonas.2 The incidence of Candida fungemia has been associated with prolonged intensive care unit stays, procedures, and broad-spectrum antibiotic use among others.3,4 Right-sided native valve endocarditis, seen in both cases, makes up only 5–10% of endocarditis cases.5 Of these, ∼9% are related to intracardiac devices, while over 10% are associated with intravenous drug use.6 We were unable to find data describing the incidence of VV-ECMO or ProtekDuo-associated bloodstream infection or endocarditis. Nonetheless, these cannulas were likely the cause of the bloodstream infections and tricuspid endocarditis in our patients, especially given that the ProtekDuo has direct contact with the tricuspid valve.
In cases of right-sided native valve infective endocarditis where medical management alone is insufficient or the valve is considerably damaged, guidelines recommend an operative approach via median sternotomy followed by either valvular repair or prosthetic replacement.7 However, case reports have described successful percutaneous debulking of large valvular vegetations with the AngioVac system (AngioDynamics, Latham, NY, USA), which was designed for the removal of intravascular thrombi and emboli.8 Such an approach may decrease right heart strain and reduce the risk of pulmonary embolism, persistent bacteraemia, and mortality.9 The AngioVac system, however, utilizes extracorporeal circulation, requiring two venous access sites: one for the AngioVac cannula and a second for the reinfusion cannula. The FlowTriever system is a catheter-based device designed for the removal of PTE.10 It requires one venous access site, introduced over a single guidewire, and does not require extracorporeal support (Figure 7). Both of our patients experienced resolution of persistent candidaemia and bacteraemia after tricuspid valvular debulking with the FlowTriever system. This device may show promise as a potentially safe and effective method of removing right-sided infective valvular vegetations, but data demonstrating its efficacy for this purpose are not available.
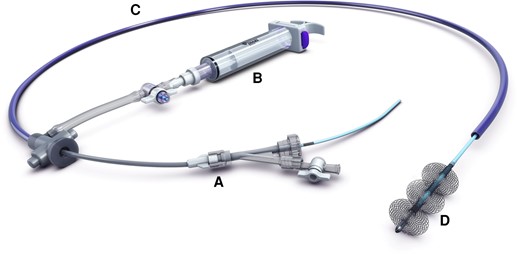
The Inari Medical FlowTriever system, comprised of a vascular introducer (A), a large-bore syringe for aspiration (B), a single trackable catheter (C), and nitinol mesh discs at the terminal end (D) to directly engage the clot or vegetation. Permission was obtained from Inari Medical for the reproduction of the schematic image of the FlowTriever® System for publication in this manuscript. Inari Medical retains the copyright to the above image per USC Title 17.
Despite the risks of VV-ECMO noted above, its implementation in severe COVID-19 disease is increasing. Thousands of adults hospitalized for COVID-19 have developed severe disease leading to invasive ventilatory support, ECMO, or death.1 Though approximate numbers have not been reported, over 90% of COVID-19 ARDS cases requiring pulmonary support have employed VV-ECMO as opposed to venoarterial ECMO.11 The 90-day mortality associated with VV-ECMO utilization in COVID-19 ARDS is between 39 and 55%, which is similar to pre-pandemic mortality rates of ECMO for ARDS.11–13 We could not find randomized controlled trials demonstrating a mortality benefit with VV-ECMO in cases of COVID-19 ARDS, though the EOLIA trial demonstrated no short-term mortality benefit in viral ARDS treated with early VV-ECMO compared with invasive mechanical ventilation with VV-ECMO as a rescue therapy.14 An observational study reported improved mortality at 6 months among patients with COVID-19 ARDS compared with patients with viral ARDS treated with VV-ECMO.15 However, the patients included in this study were on VV-ECMO for 8–30 days, which is considerably shorter than the duration of ECMO in our patients. Prolonged use of VV-ECMO may be complicated by bleeding, kidney injury, nosocomial bloodstream infections, and thromboembolic events, limiting its benefits.16 There may be hesitancy among researchers towards designing trials for ECMO support in COVID-19 ARDS given ethical concerns.
Conclusion
In this study, we reported two patients with COVID-19 ARDS requiring invasive cardiopulmonary support, an increasingly common scenario. These cases are unique, however, in that both patients developed nosocomial bloodstream infections and tricuspid valve endocarditis, likely a result of the prolonged placement of the intravascular VV-ECMO cannulas and intracardiac ProtekDuo devices. The utilization of the minimally invasive FlowTriever system to debulk the tricuspid vegetations allowed for infectious source control in both patients while reducing their vegetation burdens. Further investigation is needed to examine the benefits of percutaneous debulking of valvular endocarditis with this device in patients who may be poor surgical candidates. At the time of the drafting of this manuscript, both patients have been discharged from the hospital requiring some degree of respiratory support and significant physical rehabilitation; therefore, the question of whether prolonged ECMO support provides a mortality benefit in severe COVID-19 ARDS remains to be answered.
Lead author biography
 Dr Kara Morton obtained her medical degree from the University of Louisville School of Medicine. She is a resident physician in Internal Medicine at the University of Alabama at Birmingham, AL, USA. She plans to pursue cardiology fellowship.
Dr Kara Morton obtained her medical degree from the University of Louisville School of Medicine. She is a resident physician in Internal Medicine at the University of Alabama at Birmingham, AL, USA. She plans to pursue cardiology fellowship.
Supplementary material
Supplementary material is available at European Heart Journal – Case Reports online.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The authors confirm that written consent for submission and publication of this case series, including images and associated text, have been obtained from each patient in line with COPE guidance.
Funding: None.
References
Author notes
Conflict of interest: The authors declare no conflicts of interest.




Comments