-
PDF
- Split View
-
Views
-
Cite
Cite
Fabian Barbieri, Ulf Landmesser, Mario Kasner, Markus Reinthaler, Combined transcatheter treatment of severe mitral regurgitation and secundum atrial septal defect in an inoperable patient: a case report, European Heart Journal - Case Reports, Volume 5, Issue 12, December 2021, ytab492, https://doi.org/10.1093/ehjcr/ytab492
Close - Share Icon Share
Abstract
Chronic mitral regurgitation (MR) is one of the most common valvular heart diseases and is associated with poor outcomes. Although other structural diseases are regularly seen in such patients, concomitant atrial septal defects (ASDs) remain a rarity in the elderly.
We report a case of an 82-year-old woman with progressive right-sided heart failure (HF) due to MR and an ASD of secundum type, despite optimal medical therapy. Combined transcatheter mitral valve repair (MVR) by utilizing a separate transseptal puncture and ASD closure was performed resulting in amelioration of symptoms.
Procedural planning for simultaneous transcatheter therapies of coupled structural heart disease entities remains complex. Our case illustrates feasibility of percutaneous edge-to-edge MVR and consecutive closure of a large secundum ASD. Different options of accessing the left atrium should be discussed on an individual basis, while additional ASD closure may be beneficial in terms of right ventricular function and symptoms of right HF.
Learning points
Transcatheter mitral valve repair (MVR) is feasible, despite presence of a large secundum type atrial septal defect (ASD). A transseptal puncture located in a posterior direction of the preformed ASD might be useful for left atrial access and should be discussed on an individual basis.
Combined MVR and ASD closure may be associated with favourable clinical outcome in patients with right-sided heart failure due to pre- and afterload reduction of the right ventricle.
Introduction
Transcatheter edge-to-edge mitral valve repair (MVR) has revolutionized therapy for patients suffering from chronic mitral regurgitation (MR).1,2 Especially in the setting of high surgical risk or inoperability, it is a valid alternative to surgical MVR and is also recommended by current guidelines.3 Although other structural diseases are regularly seen in such patients, concomitant atrial septal defect (ASD) remains a rarity in the elderly. We report on a complex case of a patient presenting with secondary MR aggravated by the presence of an ASD of secundum type.
Timeline
| Day 0 | Hospitalization due to heart failure (HF). Diagnosis of severe functional mitral regurgitation and atrial septal defect (ASD) of secundum type. Cardiac recompensation and drainage of ascites. Elective transcatheter mitral valve repair and ASD closure was planned after evaluation by the Heart Team. |
| Day 28 | Recurrent hospitalization due to right-sided HF. Cardiac recompensation and drainage of ascites. Planned procedure was brought forward. |
| Day 41 | Readmission. |
| Day 42 | Implantation of two MitraClips (Abbott Laboratories, Abbott Park, IL, USA) and a 22 mm Amplatzer occluder (Abbott Laboratories). |
| Day 48 (6 days post-procedure) | Discharge after an uneventful post-procedural observation. |
| Day 62 (20 days post-procedure) | Patient described amelioration of symptoms, no recurrence of ascites and continuously regressing peripheral oedema. |
| Day 0 | Hospitalization due to heart failure (HF). Diagnosis of severe functional mitral regurgitation and atrial septal defect (ASD) of secundum type. Cardiac recompensation and drainage of ascites. Elective transcatheter mitral valve repair and ASD closure was planned after evaluation by the Heart Team. |
| Day 28 | Recurrent hospitalization due to right-sided HF. Cardiac recompensation and drainage of ascites. Planned procedure was brought forward. |
| Day 41 | Readmission. |
| Day 42 | Implantation of two MitraClips (Abbott Laboratories, Abbott Park, IL, USA) and a 22 mm Amplatzer occluder (Abbott Laboratories). |
| Day 48 (6 days post-procedure) | Discharge after an uneventful post-procedural observation. |
| Day 62 (20 days post-procedure) | Patient described amelioration of symptoms, no recurrence of ascites and continuously regressing peripheral oedema. |
| Day 0 | Hospitalization due to heart failure (HF). Diagnosis of severe functional mitral regurgitation and atrial septal defect (ASD) of secundum type. Cardiac recompensation and drainage of ascites. Elective transcatheter mitral valve repair and ASD closure was planned after evaluation by the Heart Team. |
| Day 28 | Recurrent hospitalization due to right-sided HF. Cardiac recompensation and drainage of ascites. Planned procedure was brought forward. |
| Day 41 | Readmission. |
| Day 42 | Implantation of two MitraClips (Abbott Laboratories, Abbott Park, IL, USA) and a 22 mm Amplatzer occluder (Abbott Laboratories). |
| Day 48 (6 days post-procedure) | Discharge after an uneventful post-procedural observation. |
| Day 62 (20 days post-procedure) | Patient described amelioration of symptoms, no recurrence of ascites and continuously regressing peripheral oedema. |
| Day 0 | Hospitalization due to heart failure (HF). Diagnosis of severe functional mitral regurgitation and atrial septal defect (ASD) of secundum type. Cardiac recompensation and drainage of ascites. Elective transcatheter mitral valve repair and ASD closure was planned after evaluation by the Heart Team. |
| Day 28 | Recurrent hospitalization due to right-sided HF. Cardiac recompensation and drainage of ascites. Planned procedure was brought forward. |
| Day 41 | Readmission. |
| Day 42 | Implantation of two MitraClips (Abbott Laboratories, Abbott Park, IL, USA) and a 22 mm Amplatzer occluder (Abbott Laboratories). |
| Day 48 (6 days post-procedure) | Discharge after an uneventful post-procedural observation. |
| Day 62 (20 days post-procedure) | Patient described amelioration of symptoms, no recurrence of ascites and continuously regressing peripheral oedema. |
Case presentation
An 82-year-old Caucasian woman presented at our emergency department because of progressive dyspnoea and concomitant weight gain over the last 10 days. Upon request, she denied any form of angina, a history of syncope or fever. At primary evaluation, peripheral oedema and painless bulging flanks were found. Further physical examination revealed a holosystolic murmur with its punctum maximum over Erb’s point (3rd intercostal space, leftsided, parasternal), while chest radiography confirmed cardiomegaly with pulmonary congestion without any signs of infection or pleural effusion. Known comorbidities were permanent atrial fibrillation, hypercholesterolaemia, arterial hypertension, and chronic kidney disease (CKD, Stage 4). Cardiovascular pharmacological therapy included oral anticoagulation, a beta-blocker, and diuretics, which were halted for intravenous (IV) treatment with furosemide upon admission. Furthermore, drainage of ascites was conducted to relieve symptoms of the patient. Echocardiography confirmed the suspected diagnosis of acute heart failure (HF) showing left atrial functional MR [effective regurgitation orifice area (EROA): 0.7 cm2 (cut-off for severe MR: ≥0.4 cm2); vena contracta (VC): 9 mm (severe MR: ≥7 mm); regurgitation volume: 90 mL (severe MR: ≥60 mL)] and a secundum type ASD with significant left-to-right shunt (15 × 10 mm; Figure 1, Supplemental Videos 1–5). Besides, both atria and the right ventricle were substantially dilated, whereas left ventricular end-diastolic diameter was in normal range. Right ventricular (RV) function [tricuspid annular plane systolic excursion (TAPSE): 15 mm (normal value: ≥17 mm)] was moderately impaired, on the contrary left ventricular function was still preserved (ejection fraction 67%). Furthermore, she was found to have massive secondary tricuspid regurgitation (TR) (VC: 16 mm). Right-heart catheterization revealed mainly post-capillary pulmonary hypertension [mean pulmonary artery pressure: 40 mmHg (normal value: <25 mmHg), wedge pressure: 25 mmHg (≤15 mmHg), 3.3 WU (<3.0 WU), Qp:Qs = 2.5]. Invasive coronary angiography was deferred due to the complete lack of anginal symptoms and the presence of severe CKD.
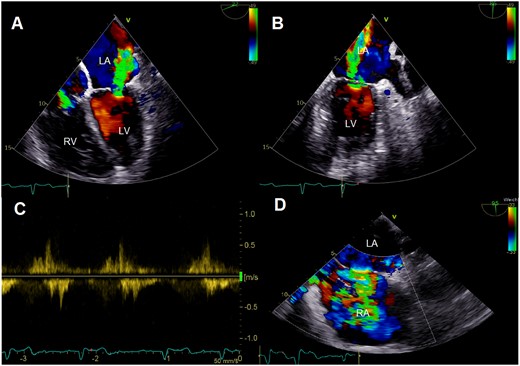
Colour Doppler images of preprocedural transoesophageal echocardiography showing severe mitral regurgitation in four-chamber (A) and two-chamber view (B) with systolic reversal of pulmonary vein flow (C). Furthermore, significance of left-to-right shunt via an atrial septal defect is displayed (D). LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle.
Potential treatment options were discussed in the interdisciplinary Heart Team meeting and due to the high-risk constellation of the patient, transcatheter MVR and ASD was recommended in a single session. Consequently, the patient was further stabilized by continuously administering IV diuretics and discharged on intensified oral diuretic therapy with an appointment made for the procedure. Unfortunately, she returned to our emergency department with similar symptoms requiring hospitalization approximately 2 weeks later. Again, drainage of ascites and IV diuretics were administered to improve symptoms. In respect of repetitive HF hospitalizations, the date of planned procedure was brought forward. Meanwhile, the patient was referred to a secondary hospital to prevent further deterioration by continuing IV diuretics.
One week later, the patient was retransferred in stable condition to our department. Procedure was conducted by using a right femoral vein access in general anaesthesia. Transseptal puncture was performed under transoesophageal echocardiography guidance posterior to the immanent ASD (Figure 2). Due to a relatively short posterior leaflet (7 mm), an NTR MitraClip device was selected and implanted in medio-central position. Although MR reduction was present, a significant jet lateral to the first clip remained. Further reduction of MR was accomplished by implantation of a second clip (Figure 3A–C, Video 1, Supplemental Videos 6–14). After confirmation of MR reduction, echocardiographic re-evaluation of the ASD still demonstrated a significant left-to-right shunt (Figure 3D). Preselected strategy of ASD closure was therefore maintained and stretched ASD diameter (17 mm) was elaborated via a 34 mm compliant sizing balloon. To ensure complete closure of both defects, the pre-existing and the iatrogenic ASD, with one device and avoid embolization in the context of a floppy primary septum, a 22 mm Amplatzer ASD occluder was implanted without any notable complications (Figure 4, Videos 2 and 3, Supplemental Videos 15–17). Post-procedural transthoracic echocardiography (TTE) showed a mitral transvalvular mean gradient of 5 mmHg, pericardial effusion was excluded. At the first post-procedural day, the patient was transferred to the ward and discharged 6 days after the procedure.
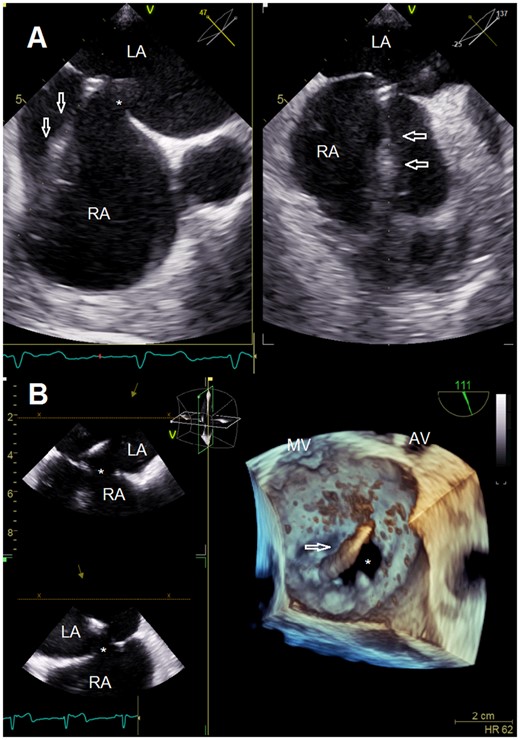
Transseptal puncture guided by multi-plane transesophageal echocardiography (A). Clip delivery system introduced into the left atrium via a separate transseptal puncture posterior to the immanent atrial septal defect (B). AV, aortic valve; LA, left atrium; MV, mitral valve; RA, right atrium; *, atrial septal defect; arrowheads, transseptal puncture stiletto (A) and clip delivery system (B).
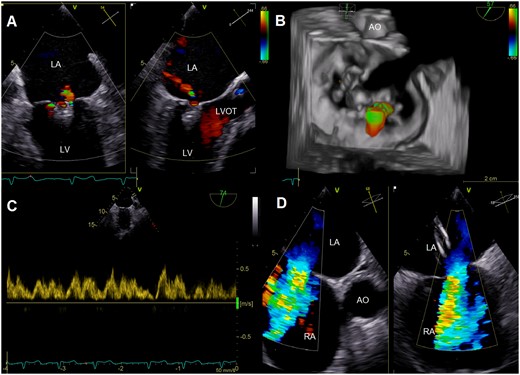
Multi-plane colour Doppler images (A) and three-dimensional reconstruction with colour Doppler (B) after deployment of two MitraClips showing significant reduction of mitral regurgitation. Systolic pulmonary vein flow reversal was eliminated (C), while colour Doppler images illustrated persistence of significant left-to-right shunt via the atrial septal defect (D). AO, aorta; LA, left atrium; LV, left ventricle; LVOT, left ventricular outflow tract; RA, right atrium.
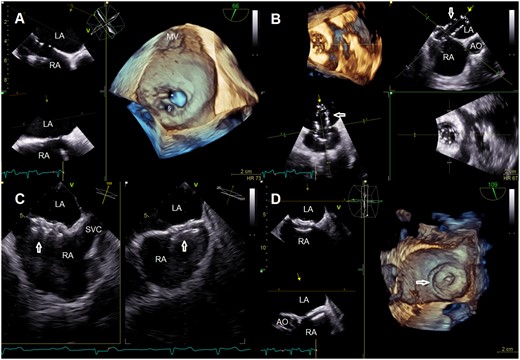
Multi-plane view with three-dimensional reconstruction showing the pre-existing and the artificial atrial septal defect (A), its sizing with balloon (B) and after deployment of an Amplatzer occluder (C, D). AO, aorta; LA, left atrium; MV, mitral valve; RA, right atrium; SVC, superior vena cava; *, atrial septal defect; §, artificial atrial septal defect; arrowheads, sizing ballon (B) and Amplatzer occluder (C, D).
Multi-plane view showing implantation of the second MitraClip.
Multi-plane view showing implantation of the Amplatzer occluder.
Three-dimensional reconstruction showing final results after implantation of two clips significant reduction of mitral regurgitation and elimination of left-to-right shunt.
Two weeks later, she returned for short-term ambulatory follow-up showing further amelioration of symptoms, no recurrence of ascites, and continuously regressing peripheral oedema. Furthermore, confirmation of trace MR with improvements in TAPSE (19 mm) and TR [EROA: 0.5 cm2 (cut-off for severe TR: ≥0.4 cm2); VC: 12 mm (severe TR: ≥7 mm); regurgitation volume: 43 mL (severe TR: ≥45 mL)] were observed in TTE.
Discussion
Transcatheter MVR is a continuously evolving form of therapy for MR, which allows treatment of patients deemed as high risk or inoperable.1–3 Although transcatheter MVR has become a standard of therapy in most tertiary hospitals, MR may also occur in combination with other structural diseases increasing the complexity of procedural planning. Generally, the presence of a secundum ASD is very tempting to be used for left atrial access as the preformed defect may easily be passed with the guiding catheter, but impaired manoeuvrability and lack of stability of the clip delivery system (CDS) may complicate the procedure. In terms of manoeuvrability, the proximity of the line of coaptation to the ASD ostium causes suboptimal angulation of the CDS. This is especially of importance in primary MR due to prolapse or flail leaflet, as the line of coaptation is usually located in or above the plane of the mitral annulus.4 A similar situation was found in our patient, who presented with left atrial functional MR and coaptation line close to the annular plane. We therefore performed a transseptal puncture posterior to the ASD, which improved the deliverability of the devices due to a higher position of the CDS, besides the already mentioned optimization in stability compared to a free access through the ASD. However, transseptal puncture may not always be possible due to differences in anatomy of the defect. Fixation of the transseptal guide, for example by using a snare from the right jugular vein to grasp the CDS, might improve stability.
Overall, it remains debatable, which interventions were necessary for treatment of our patient. TR was interpreted as an expression of right-sided pressure and volume overload. Therefore, the aim of chosen therapy was to offer best possible RV relief by reducing afterload via MVR and preload via ASD closure.5,6 Although treatment of TR was initially deferred, it remained as option for supportive therapy in case of persisting HF symptoms. There are also certain situations in which ASD closure should be deferred. In patients with ongoing left ventricular disease, the benefit of shunt elimination always needs to be weighed against potential harm because of increase in left ventricular filling pressure. Furthermore, ASD closure is contraindicated in Eisenmenger syndrome and severe pulmonary hypertension (≥5 WU), despite pharmacological treatment. Repetition of right-heart catheterization might be considered after MVR, whenever there is doubt about benefits of ASD closure.5
This case presentation illustrates feasibility of transcatheter MVR in patient with a large secundum ASD. Different options of accessing the left atrium should be discussed on an individual basis. Additional ASD closure may be beneficial in terms of RV function and symptoms of right HF.
Lead author biography
Fabian Barbieri graduated at the Medical University of Innsbruck (Austria), where he has also started to work as resident in 2018 and finished his PhD thesis. He continued his career at the Charité – Universitätsmedizin Berlin, Campus Benjamin Franklin (Germany) in 2021.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The authors confirm that written consent for submission and publication of this case report including images and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: F.B. received grant support from Abbott Laboratories and Boston Scientific as well as consulting fees from Boston Scientific. U.L. reports personal fees from Abbott Laboratories, Biotronik and Boston Scientific.
Funding: We acknowledge support from the German Research Foundation (DFG) and the Open Access Publication Fund of Charité – Universitätsmedizin Berlin.
References
Author notes
Mario Kasner, Markus Reinthaler contributed equally to this work.
- heart failure, right-sided
- mitral valve insufficiency
- mitral valve insufficiency, chronic
- mitral valve repair
- left atrium
- atrial septal defect
- heart failure
- heart valve diseases
- patent foramen ovale
- ventricular function, right
- structural disorder of heart
- older adult
- autism spectrum disorder
- closure of defect of interatrial septum
- medical management
- transcatheter mitral valve repair
- mitral valve clip





Comments