-
PDF
- Split View
-
Views
-
Cite
Cite
Tetsuya Nomura, Yu Sakaue, Kenshi Ono, Naotoshi Wada, Five recurrent episodes of Takotsubo syndrome provoked by various triggers over a period of 7 years: a case report, European Heart Journal - Case Reports, Volume 5, Issue 11, November 2021, ytab431, https://doi.org/10.1093/ehjcr/ytab431
Close - Share Icon Share
Abstract
Takotsubo syndrome (TTS), also known as stress cardiomyopathy or apical ballooning syndrome, presents as reversible regional left ventricular wall motion abnormalities in the absence of obstructive coronary artery disease. It is associated with a recurrence rate of approximately 4%. However, multiple recurrence episodes are rare in clinical settings, and the predictors of recurrence and preventive methods have yet to be fully elucidated.
A 69-year-old woman experienced two TTS episodes before complaining of sudden-onset epigastric pain without any particular trigger. No significant coronary lesion was observed on coronary angiography, while left ventriculography showed the typical findings of apical ballooning and a hyperkinetic wall motion at the basal level of the left ventricle. The patient was again diagnosed with recurrent TTS. On Day 5 of hospitalization, follow-up echocardiography showed mural thrombus formation in the left ventricular apex. Anticoagulant therapy with oral warfarin following intravenous heparin was effective in dissolving the thrombus. She was safely discharged on Day 16 of hospitalization. However, two additional recurrent TTS episodes provoked by emotional stress occurred afterwards. Since the final hospitalization, she has been prescribed perindopril 4 mg/day and β1-receptor-selective β-blocker bisoprolol 5 mg/day and has been able to avoid the 6th recurrence of TTS for more than 12 months at present.
Multiple recurrent TTS episodes are rare in the clinical setting. As such, the long-term follow-up of this case may provide clues on the pathophysiology of this disease and aid us in establishing effective preventive strategies.
Learning points
Takotsubo syndrome (TTS) is generally considered a benign disease and is associated with a favourable prognosis. However, knowledge regarding recurrent episodes is limited due to the scarcity of data.
A variety of factors, such as negative stressors and positive emotions, can trigger TTS events.
The predictors of recurrent TTS and its preventive methods have not yet been fully elucidated, but combination therapy (β-blocker and angiotensin-converting enzyme inhibitor/ angiotensin-II antagonist) is a promising treatment option.
Introduction
Takotsubo syndrome (TTS), also known as stress cardiomyopathy or apical ballooning syndrome, was first described in 1990 as reversible regional left ventricular wall motion abnormalities in the absence of obstructive coronary artery disease. Its clinical presentation in the acute phase mimicks acute myocardial infarction but without the involvement of plaque rupture in the coronary artery, and the wall motion abnormalities resolve completely in most cases. Postmenopausal women are predominantly affected by this pathology that is often triggered by emotional stress or medical illness. However, the detailed mechanisms and clinical features remain controversial, including the triggers of illness and ballooning patterns. In addition, TTS is associated with a recurrence rate of approximately 4% or a rate of 0.9% patients/year.1 However, multiple recurrence episodes are rare in clinical settings, and the predictors of recurrence and preventive methods have not yet been fully elucidated.2
Timeline
| Time . | Events . |
|---|---|
| 54 months previously | 1st Takotsubo syndrome (TTS) episode after laparoscopic adrenalectomy. |
| 8 months previously | 2nd TTS episode triggered by emotional stress related to her daughter’s death. |
| Day 1 | 3rd TTS onset with sudden epigastric pain, coronary angiography and left ventriculography. |
| Day 5 | Thrombus formation in the left ventricular apex confirmed by ultrasound cardiography (UCG). |
| Day 14 | Thrombus dissolution and improved wall motion abnormalities confirmed by UCG. |
| Day 16 | Discharge from hospital. |
| 4 months later | 4th TTS episode triggered by emotional stress related to her daughter’s death. |
| 22 months later | 5th TTS episode triggered by emotional stress related to coronavirus disease-19 pandemic |
| Time . | Events . |
|---|---|
| 54 months previously | 1st Takotsubo syndrome (TTS) episode after laparoscopic adrenalectomy. |
| 8 months previously | 2nd TTS episode triggered by emotional stress related to her daughter’s death. |
| Day 1 | 3rd TTS onset with sudden epigastric pain, coronary angiography and left ventriculography. |
| Day 5 | Thrombus formation in the left ventricular apex confirmed by ultrasound cardiography (UCG). |
| Day 14 | Thrombus dissolution and improved wall motion abnormalities confirmed by UCG. |
| Day 16 | Discharge from hospital. |
| 4 months later | 4th TTS episode triggered by emotional stress related to her daughter’s death. |
| 22 months later | 5th TTS episode triggered by emotional stress related to coronavirus disease-19 pandemic |
| Time . | Events . |
|---|---|
| 54 months previously | 1st Takotsubo syndrome (TTS) episode after laparoscopic adrenalectomy. |
| 8 months previously | 2nd TTS episode triggered by emotional stress related to her daughter’s death. |
| Day 1 | 3rd TTS onset with sudden epigastric pain, coronary angiography and left ventriculography. |
| Day 5 | Thrombus formation in the left ventricular apex confirmed by ultrasound cardiography (UCG). |
| Day 14 | Thrombus dissolution and improved wall motion abnormalities confirmed by UCG. |
| Day 16 | Discharge from hospital. |
| 4 months later | 4th TTS episode triggered by emotional stress related to her daughter’s death. |
| 22 months later | 5th TTS episode triggered by emotional stress related to coronavirus disease-19 pandemic |
| Time . | Events . |
|---|---|
| 54 months previously | 1st Takotsubo syndrome (TTS) episode after laparoscopic adrenalectomy. |
| 8 months previously | 2nd TTS episode triggered by emotional stress related to her daughter’s death. |
| Day 1 | 3rd TTS onset with sudden epigastric pain, coronary angiography and left ventriculography. |
| Day 5 | Thrombus formation in the left ventricular apex confirmed by ultrasound cardiography (UCG). |
| Day 14 | Thrombus dissolution and improved wall motion abnormalities confirmed by UCG. |
| Day 16 | Discharge from hospital. |
| 4 months later | 4th TTS episode triggered by emotional stress related to her daughter’s death. |
| 22 months later | 5th TTS episode triggered by emotional stress related to coronavirus disease-19 pandemic |
Case presentation
On the day of onset, the 69-year-old woman was eating lunch with her friends in the afternoon. However, in the evening, she complained of sudden-onset epigastric pain, and consulted our emergency room. She had two TTS previous episodes with the first episode occurring just after undergoing laparoscopic adrenalectomy for Cushing’s syndrome, and the second episode being provoked by the reminiscence of her daughter’s death.
Laboratory examinations revealed negative troponin T, and slightly increased brain natriuretic peptide (45.6 pg/mL; cut-off value 18.4 pg/mL). The electrocardiogram (ECG) showed sinus rhythm with no remarkable ST-T wave changes (Figure 1A). On the other hand, the echocardiogram [ultrasound cardiography (UCG)] demonstrated an abnormal anterior-apical wall motion, but neither notable valvular dysfunction nor shunt flow was observed. Her InterTAK Diagnostic Score was calculated to be 54 points (female sex, no ST-segment depression, psychiatric disorders, and QTc prolongation).3 Following the diagnostic algorithm of TTS, we conducted left ventriculography, which showed the typical findings of apical ballooning, and hyperkinetic wall motion at the basal level of the left ventricle. The ejection fraction was 40.3% (Figure 2A and B). Coronary angiography ruled out obstructive coronary disease (Figure 2C and D). Cardiovascular magnetic resonance imaging showed the typical regional wall motion abnormality and a high-intensity signal in the short tau inversion recovery image (Figure 2E). Furthermore, there was no late gadolinium enhancement. The patient did not show any signs or symptoms that were suspicious for viral infections. The serum C-reactive protein level was within normal limits, and no pericardial effusion was detected in any imaging modality. Based on these findings, we ruled out the possibility of myocarditis and diagnosed with recurrent TTS.
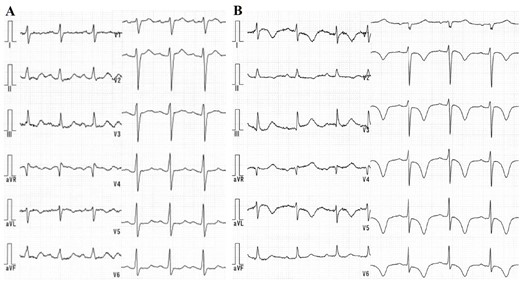
(A) The electrocardiogram on arrival showing a sinus rhythm and no remarkable ST-T change. (B) T-wave inversion in the broad range of the limb and chest leads on Day 2 of hospitalization.
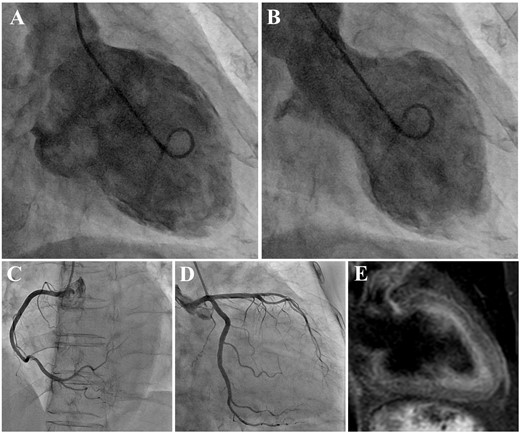
(A) Left ventriculography in diastolic phase. (B) Left ventriculography in systolic phase demonstrating the typical findings of apical ballooning and a hyperkinetic wall motion at the basal level of the left ventricle. The coronary angiogram showing intact coronary arteries. Right coronary artery (C). Left coronary artery from right anterior oblique caudal view (D). (E) A short tau inversion recovery image of cardiovascular magnetic resonance imaging showing high intensity at the area of regional wall motion abnormality.
On the following day, T-wave inversion in the broad range of limb and chest leads emerged on the ECG (Figure 1B). On Day 5 of hospitalization, she underwent follow-up UCG, which revealed mural thrombus formation in the left ventricular apex (Figure 3A and B). Anticoagulant therapy with oral warfarin following intravenous heparin effectively dissolved the thrombus, which was confirmed by UCG on Day 14 of hospitalization.
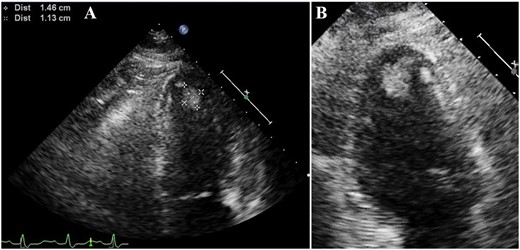
(A) Ultrasound cardiography showing mural thrombus formation (1.46 × 1.13 cm) in the left ventricular apex on Day 5 of hospitalization. (B) Magnified image of the apex.
The blood chemical concentrations of each catecholamine were within normal limits (Figure 4A). On the other hand, 123I-metaiodobenzylguanidine (123I-MIBG) scintigraphy showed reduced H/M ratios of 1.59 in the early phase and 1.24 in the delayed phase, respectively. The washout rate was increased to 49.08%, suggesting decreased sympathetic function (Figure 4B).
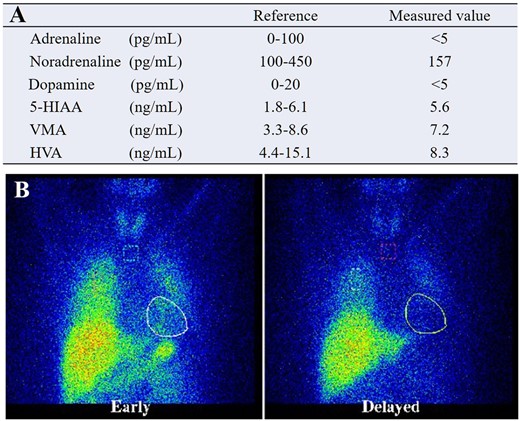
(A) Blood chemical concentrations of each kind of catecholamine, all within normal limits. (B) 123I-metaiodobenzylguanidine scintigraphy suggesting a decreased sympathetic function.
She was haemodynamically stable, and no arrhythmic events were observed during hospitalization. She was safely discharged on Day 16 of hospitalization. However, two additional recurrent TTS episodes provoked by emotional stress occurred afterwards (Table 1). Since the final hospitalization, she has been prescribed perindopril 4 mg/day and β1-receptor-selective β-blocker bisoprolol 5 mg/day (changed from non-selective β-blocker carvedilol after the 5th recurrent episode) and has been able to avoid the 6th recurrence of TTS for more than 12 months at present.
Clinical presentation of a 69-year-old female with five recurrent episodes of TTS
| Event of hospitalization . | 1st . | 2nd . | 3rd . | 4th . | 5th . |
|---|---|---|---|---|---|
| Duration from initial episode (months) | 46 | 54 | 58 | 76 | |
| Symptoms | Dyspnoea | Chest squeezing | Epigastric pain | Anterior chest pain | Chest squeezing |
| Trigger | Laparoscopic adrenalectomy | Emotional stress related to family death | No trigger | Emotional stress related to family death | Emotional stress related to COVID-19 pandemic |
| ACE-I/ARB/β-blocker at onset | Candesartan 8 mg | Perindopril 4 mg | Perindopril 4 mg | Perindopril 4 mg | Perindopril 4 mg + Carvedilol 20 mg |
| ECG on admission | T-wave inversion ST-segment depression QTc prolongation | QTc prolongation | QTc prolongation | QTc prolongation | T-wave inversion ST-segment depression |
| InterTAK diagostic score (points) | 55 | 78 | 54 | 78 | 60 |
| Qualitative troponin T | Negative | Negative | Negative | Negative | Negative |
| BNP (pg/mL) | 118.8 | 78.2 | 45.6 | 41.4 | 58.6 |
| Wall motion abnormality | Hypokinesis at the apex | Apical ballooning | Apical ballooning | Apical ballooning | Hypokinesis at the apex |
| EF (%) | 52.4 | 40.9 | 40.3 | 39.9 | 50.0 |
| Timing of confirmed wall motion recovery (days) | 6 | 6 | 14 | 14 | 4 |
| Hospitalization (days) | 16 | 7 | 16 | 23 | 5 |
| Complication | None | None | Ventricular thrombus | None | None |
| Event of hospitalization . | 1st . | 2nd . | 3rd . | 4th . | 5th . |
|---|---|---|---|---|---|
| Duration from initial episode (months) | 46 | 54 | 58 | 76 | |
| Symptoms | Dyspnoea | Chest squeezing | Epigastric pain | Anterior chest pain | Chest squeezing |
| Trigger | Laparoscopic adrenalectomy | Emotional stress related to family death | No trigger | Emotional stress related to family death | Emotional stress related to COVID-19 pandemic |
| ACE-I/ARB/β-blocker at onset | Candesartan 8 mg | Perindopril 4 mg | Perindopril 4 mg | Perindopril 4 mg | Perindopril 4 mg + Carvedilol 20 mg |
| ECG on admission | T-wave inversion ST-segment depression QTc prolongation | QTc prolongation | QTc prolongation | QTc prolongation | T-wave inversion ST-segment depression |
| InterTAK diagostic score (points) | 55 | 78 | 54 | 78 | 60 |
| Qualitative troponin T | Negative | Negative | Negative | Negative | Negative |
| BNP (pg/mL) | 118.8 | 78.2 | 45.6 | 41.4 | 58.6 |
| Wall motion abnormality | Hypokinesis at the apex | Apical ballooning | Apical ballooning | Apical ballooning | Hypokinesis at the apex |
| EF (%) | 52.4 | 40.9 | 40.3 | 39.9 | 50.0 |
| Timing of confirmed wall motion recovery (days) | 6 | 6 | 14 | 14 | 4 |
| Hospitalization (days) | 16 | 7 | 16 | 23 | 5 |
| Complication | None | None | Ventricular thrombus | None | None |
Clinical presentation of a 69-year-old female with five recurrent episodes of TTS
| Event of hospitalization . | 1st . | 2nd . | 3rd . | 4th . | 5th . |
|---|---|---|---|---|---|
| Duration from initial episode (months) | 46 | 54 | 58 | 76 | |
| Symptoms | Dyspnoea | Chest squeezing | Epigastric pain | Anterior chest pain | Chest squeezing |
| Trigger | Laparoscopic adrenalectomy | Emotional stress related to family death | No trigger | Emotional stress related to family death | Emotional stress related to COVID-19 pandemic |
| ACE-I/ARB/β-blocker at onset | Candesartan 8 mg | Perindopril 4 mg | Perindopril 4 mg | Perindopril 4 mg | Perindopril 4 mg + Carvedilol 20 mg |
| ECG on admission | T-wave inversion ST-segment depression QTc prolongation | QTc prolongation | QTc prolongation | QTc prolongation | T-wave inversion ST-segment depression |
| InterTAK diagostic score (points) | 55 | 78 | 54 | 78 | 60 |
| Qualitative troponin T | Negative | Negative | Negative | Negative | Negative |
| BNP (pg/mL) | 118.8 | 78.2 | 45.6 | 41.4 | 58.6 |
| Wall motion abnormality | Hypokinesis at the apex | Apical ballooning | Apical ballooning | Apical ballooning | Hypokinesis at the apex |
| EF (%) | 52.4 | 40.9 | 40.3 | 39.9 | 50.0 |
| Timing of confirmed wall motion recovery (days) | 6 | 6 | 14 | 14 | 4 |
| Hospitalization (days) | 16 | 7 | 16 | 23 | 5 |
| Complication | None | None | Ventricular thrombus | None | None |
| Event of hospitalization . | 1st . | 2nd . | 3rd . | 4th . | 5th . |
|---|---|---|---|---|---|
| Duration from initial episode (months) | 46 | 54 | 58 | 76 | |
| Symptoms | Dyspnoea | Chest squeezing | Epigastric pain | Anterior chest pain | Chest squeezing |
| Trigger | Laparoscopic adrenalectomy | Emotional stress related to family death | No trigger | Emotional stress related to family death | Emotional stress related to COVID-19 pandemic |
| ACE-I/ARB/β-blocker at onset | Candesartan 8 mg | Perindopril 4 mg | Perindopril 4 mg | Perindopril 4 mg | Perindopril 4 mg + Carvedilol 20 mg |
| ECG on admission | T-wave inversion ST-segment depression QTc prolongation | QTc prolongation | QTc prolongation | QTc prolongation | T-wave inversion ST-segment depression |
| InterTAK diagostic score (points) | 55 | 78 | 54 | 78 | 60 |
| Qualitative troponin T | Negative | Negative | Negative | Negative | Negative |
| BNP (pg/mL) | 118.8 | 78.2 | 45.6 | 41.4 | 58.6 |
| Wall motion abnormality | Hypokinesis at the apex | Apical ballooning | Apical ballooning | Apical ballooning | Hypokinesis at the apex |
| EF (%) | 52.4 | 40.9 | 40.3 | 39.9 | 50.0 |
| Timing of confirmed wall motion recovery (days) | 6 | 6 | 14 | 14 | 4 |
| Hospitalization (days) | 16 | 7 | 16 | 23 | 5 |
| Complication | None | None | Ventricular thrombus | None | None |
Discussion
The recurrence of TTS is defined as a TTS event that occurs after the complete recovery of the wall motion abnormalities related to a previous TTS event. All five TTS episodes that occurred in our case satisfied the said criteria. In the International Takotsubo Registry, recurrent TTS episodes occurred in 66 patients (4.7%) of 1402 patients at time intervals of 30 days to 9.9 years after the first episode.4
Considering the frequency of TTS recurrence, we have rarely encountered multiple recurrent episodes of TTS in one patient. Accordingly, there are only a few reports about cases with more than three recurrences of TTS.5,6 Takotsubo syndrome is usually associated with myocardial necrosis to some extent. An elevated admission troponin level is said to be a predictor of worse in-hospital outcomes. The lesser extent of cardiac damage in our case, as indicated by the negative troponin level, might have contributed to the quick recovery from TTS. In my opinion, we can make a hypothesis that these conditions might have been involved in the patient’s five TTS recurrences.
During these recurrent episodes, a variety of triggering events and different patterns of ventricular involvement was reported in the same patient. A multicentre TTS registry showed that up to 20% of cases had a variable TTS pattern at recurrence.7 Neurogenic myocardial stunning is one of the proposed mechanisms of TTS.8 The myocardial uptake of 123I-MIBG reflects myocardial sympathetic innervation. As such, it is reduced for months in dysfunctional segments despite the normal perfusion, consistent with regional disturbance of sympathetic neuronal activity. In our case, the affected region was always the left ventricular apex, the most typically affected region, which corresponds to the lesion with abnormal findings on 123I-MIBG scintigraphy.
Regarding the triggers (physical and/or emotional) of TTS recurrence, almost half of recurrent episodes were reported to be triggered by a new stressor compared with the first TTS event. The International Takotsubo Registry showed that comorbidities such as psychiatric and neurologic disorders were independent predictors of TTS recurrence.4 The triggers in each recurrent episode in our case showed varying patterns (Table 1). Notably, besides emotional and physical stressors, enjoyable episodes may also trigger recurrent TTS.9 In our case, she had a comorbidity of depression for several years, which might have affected her multiple recurrent TTS episodes.
Although patients with TTS are considered to have favourable prognoses, the recent international expert consensus document reported that the rates of serious adverse in-hospital events such as cardiogenic shock and death are comparable to acute coronary syndrome.2 Common in-hospital complications include thromboembolic events, cardiogenic shock, pulmonary oedema, and arrhythmias. In our case, intraventricular thrombus formation occurred in the 3rd recurrence, which was safely resolved with anticoagulation therapy without causing any embolic events. Although serious complications caused by a single TTS episode are infrequent, repetitive TTS events will increase the risk of serious in-hospital complications. Therefore, it is important to manage these patients appropriately to avoid recurrent TTS events.
However, the prevention of TTS recurrence has not yet been established. Because TTS is associated with hyperadrenergic stimulation, patients with TTS are often prescribed β-blockers for daily use. On the other hand, it was reported that an angiotensin-converting enzyme inhibitor or AT-II antagonist was more effective to reduce TTS recurrence than β-blockers.10 Moreover, combination therapy might be more effective than stand-alone therapy.11 Possibly, the mechanisms of effective combination therapy involve a reduction in sympathetic activity or the suppression of inflammatory reactions through interactions with the renin–angiotensin system.12 However, a recent study demonstrated that combination therapy lacked efficacy for TTS recurrence.1 Our case is worthwhile as the heterogeneous TTS recurrences occurred in the same individual. By continuously following up on this patient, we can compare the effect of medicines under identical backgrounds, allowing for the identification of effective preventative strategies.
Conclusion
Over 7 years, the patient experienced five recurrent episodes of TTS that were provoked by a variety of triggers. Multiple recurrent TTS episodes are clinically rare, and the long-term follow-up of this case may provide clues on the pathophysiology of this disease and aid us in establishing effective preventive strategies.
Lead author biography
Tetsuya Nomura graduated from Kyoto Prefectural University of Medicine in 1999 and completed doctoral course in medicine in 2007. Currently, he is a director in the Department of Cardiovascular Medicine in Kyoto Chubu Medical Center. He specializes in interventional cardiology and endovascular treatment.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Acknowledgements
We would like to thank Editage (www.Editage.com) for English language editing.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The authors confirm that written consent for submission and publication of this case report, including the images and associated text, has been obtained from the patient in line with COPE guidance.
Conflict of interest: None declared.
Funding: None declared.





Comments