-
PDF
- Split View
-
Views
-
Cite
Cite
Iacopo Muraca, Matteo Pennesi, Nazario Carrabba, Fernando Scudiero, Angela Migliorini, Niccolò Marchionni, Pierluigi Stefàno, Renato Valenti, Percutaneous left ventricular advanced support for ‘protected’ complex high-risk transcatheter mitral valve repair: a case series, European Heart Journal - Case Reports, Volume 4, Issue 3, June 2020, Pages 1–7, https://doi.org/10.1093/ehjcr/ytaa066
Close - Share Icon Share
Abstract
Currently, the use of advanced ventricular support systems during percutaneous mitral valve repair (PMVR) procedures is confined to very few selected cases in emergency or bailout situations. No cases are reported of planned use of ventricular support devices in the subgroup of high-risk patients undergoing PMVR.
We report two cases of planned and ‘protected’ procedures of PMVR with Impella CP mechanical circulatory support. No procedure-related complications occurred. At 6-month clinical follow-up evaluation, an improvement of symptoms and functional class (New York Heart Association) was reported.
In the two cases reported, PMVR with Impella CP assistance was feasible, safe and effective in the setting of severe mitral regurgitation associated with dilated and severe left ventricular dysfunction. Extending the concept of ‘complex high-risk and indicated patients/procedures’ (CHIP) from the environment of coronary intervention, a ‘protected’ approach could lead to improve technical feasibility and clinical outcome in structural interventions, as advocated for ‘protected-percutaneous coronary intervention’.
MitraClip implantation during Impella CP ventricular assistance is feasible, safe and effective in planned and ‘protected’ complex high-risk percutaneous structural interventions.
Use of Impella CP during percutaneous mitral valve repair did not affect significantly imaging guidance by transoesophageal echocardiography and the procedural/device time.
The development of a dedicated multidisciplinary ‘complex high-risk and indicated patients/procedures’ (CHIP) team for complex structural percutaneous interventions could improve technical and clinical management in this setting of patients, possibly resulting in better treatment outcomes.
Introduction
Percutaneous mitral valve repair (PMVR) is considered a relatively safe procedure in most cases even if affected by risks related to technical features and patients’ clinical profile. However, in the clinical setting, in patients with severe left ventricular (LV) dysfunction and dilated ventricles associated with functional mitral regurgitation, PMVR may result in acute haemodynamic decompensation due to alteration of LV loading conditions, mainly for an afterload mismatch. A mechanical circulatory support could be of value in this setting.1–4
Despite the risk of haemodynamic deterioration that can occur in the setting of high-risk patients during PMVR, there is no consensus in the management of LV support systems. The most frequently used mechanical circulatory support device for functional or degenerative PMVR is the intra-aortic balloon pump (IABP), despite no clear evidence in terms of clinical benefit.5,6 Moreover, the few cases reported of PMVR intervention and advanced LV support were performed in emergency or bailout situations in patients presenting with cardiogenic shock for ischaemic mitral regurgitation (MR),7 or as bridge from ischaemic cardiogenic shock to PMVR,8 or merely using the support as a bridge to surgery.9
We report two cases of planned ‘protected’ transcatheter edge-to-edge PMVR with MitraClip (Abbott, Santa Clara, CA, USA) implantation and advanced LV support with Impella CP (Abiomed, Denvers, MA, USA). In brief, the device consists of a coaxial blood pump that is positioned percutaneously across the aortic valve and works by aspirating blood from the left ventricle to expel it directly in the ascending aorta, providing LV assistance with unloading of the chamber.10
Timeline
| Time . | Events . |
|---|---|
|
|
|
|
| Time . | Events . |
|---|---|
|
|
|
|
| Time . | Events . |
|---|---|
|
|
|
|
| Time . | Events . |
|---|---|
|
|
|
|
Cases presentation
Case #1
The first patient is a 72-year-old woman with severe LV dysfunction related to idiopathic dilated cardiomyopathy leading to heart failure [New York Heart Association (NYHA) function Class IV] despite optimal medical therapy and cardiac resynchronization therapy. She was referred to our tertiary centre due to recurrent rehospitalizations for refractory heart failure. After a first phase of pharmacological treatment or haemodynamic compensation, transthoracic echocardiography (TTE) and transoesophageal echocardiography (TOE) evaluation confirmed a severely dilated ventricle (LV end-diastolic diameter: 78 mm) associated with a severe LV dysfunction [LV ejection fraction (LVEF): 0.22]. A severe functional mitral regurgitation was revealed (effective regurgitant orifice area: 0.35 cm2, vena contracta: 0.7 cm) due to tethering of the leaflets resulting in multiple jet, the major between A2 and P3 (Figure 1). The calculated Society of Thoracic Surgeon (STS) score was 8.7%. After Heart Team discussion, transcatheter intervention with MitraClip implantation was planned with advanced LV mechanical circulatory support of Impella CP. The procedure was performed as usual under TOE guidance and general anaesthesia. The Impella CP was inserted, immediately after transseptal puncture and heparinization, through left femoral artery and positioned across the aortic valve with a flow rate of 3 L/min (Figure 2 and Supplementary material online, Video S1). Despite the TOE imaging noise generated by the coaxial pump, a MitraClip XTR was successfully implanted (Figure 3). The final TTE/TOE, with Impella CP running at the lowest performance level, demonstrated an optimal result with residual mild mitral regurgitation with an average diastolic gradient of 2 mmHg and peak diastolic gradient of 5 mmHg (Figure 4). The entire procedure was performed with Impella CP assistance, which guaranteed the LV unload during the procedure. Device time including PMVR and Impella CP implantation was 38 min. Impella CP support was left in place and active. In the following hours, progressive improvement of the patient’s haemodynamic performance occurred and Impella CP support was weaned until the removal of the device. Haemostasis was guaranteed by two pre-implanted suture closure devices (Perclose Proglide; Abbott, Santa Clara, CA, USA). In-hospital course was uneventful and the patient was discharged 6 days after the index procedure with good haemodynamic compensation.
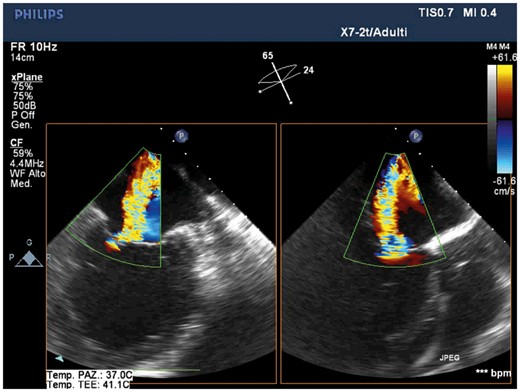
Pre-procedural transoesophageal echocardiography showing severe mitral regurgitation due to tethering of the leaflets resulting in multiple jet, the major between A2 and P3 (intercommissural and outflow tract transoesophageal views). Images show the severely dilated and spherical left ventricle.
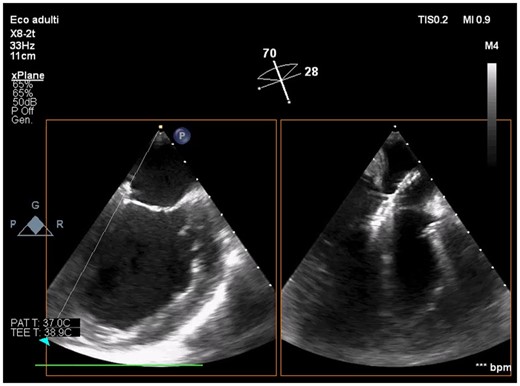
Intraprocedural transoesophageal echocardiography showing active Impella CP during mitral leaflets grasping by XTR MitraClip (intercommissural and outflow tract transoesophageal views).
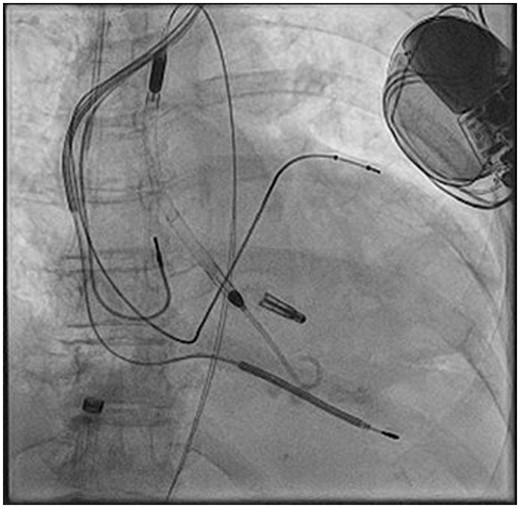
Fluoroscopic image showing the deployed XTR MitraClip and the active Impella CP. No dislocation of cardiac resynchronization defibrillator leads occurred.
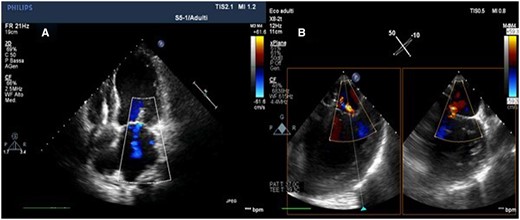
Post-procedural transthoracic echocardiography (A) and transoesophageal echocardiography (B) (intercommissural and outflow tract transoesophageal echocardiography views) showing the optimal result with residual mild mitral regurgitation.
At 1- and 6-month follow-up, the patient showed improved symptoms and functional class (NYHA functional Class II). The TTE evaluation showed an improvement of LV systolic function (LVEF 0.27) and confirmed the post-procedural result showing a residual mild mitral regurgitation (regurgitant volume of 12 mL).
Case #2
The second patient was a 50-year-old woman affected by refractory congestive heart failure due to a post-ischaemic adverse LV remodelling leading to severe functional mitral valve regurgitation with a tethering of posterior mitral leaflet. Six months before, the patient was treated with primary percutaneous coronary intervention (PCI) of left anterior descending artery for a late presentation myocardial infarction with concomitant chronic total occlusion of the right coronary artery. An arrhythmic storm and haemodynamic instability occurred during primary PCI requiring mechanical circulatory support with IABP. At 1 month, after cardiac magnetic resonance imaging showed no residual myocardial viability with an LVEF of 0.18, the patient received an implantable cardioverter-defibrillator. In the following months, the patient was severely symptomatic for dyspnoea (NYHA functional Class IV). Considering the high risk for surgical mitral repair (STS score 7.5%), no need for further revascularization or ventriculoplasty as patient was not considered a candidate for open-heart surgery/heart transplantation, after Heart Team discussion, the patient was planned for MitraClip implantation. The pre-intervention TTE/TOE evaluation confirmed a severe systolic dysfunction (LVEF 0.18), atrial enlargement, diastolic dysfunction, and pulmonary hypertension. Transoesophageal echocardiography showed degenerative leaflets and tethering of the posterior mitral valve leaflet, no evidence of flail leaflets and multiple MR jets between A2-P1 and A2-P3 scallops (Figure 5). After multidisciplinary discussion, the procedure of PMVR was planned with advanced LV assistance by Impella CP. Similar to previous case, Impella CP implantation was performed with a transfemoral approach, after transseptal puncture and heparinization. A single MitraClip XTR was successfully delivered. Device time including PMVR and Impella CP implantation was 47 min (Figures 6 and 7 and Supplementary material online, Video S2). Impella CP was weaned at the end of the procedure and the haemostasis performed by two pre-implanted suture devices (Perclose Proglide; Abbott, Santa Clara, CA, USA). The final TOE/TTE demonstrated an optimal result with residual mild mitral regurgitation (Figure 8). No complications occurred. In-hospital course was uneventful and the patient was discharged 5 days after index procedure. At 1- and 6-month follow-up, the patient showed an optimal clinical compensation, improvement of symptoms, and functional class (NYHA functional Class II). Transthoracic echocardiography evaluation showed an LVEF of 0.31 (end-diastolic volume: 178 mL; end-systolic volume: 122 mL); a trivial residual mitral regurgitation with an average diastolic gradient of 2 mmHg; an increase stroke volume from 30 to 40 mL; a persistent severe tricuspid regurgitation without pulmonary hypertension evaluated by pulmonary artery acceleration time.
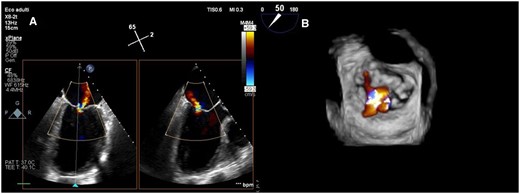
Pre-procedural transoesophageal echocardiography (intercommissural and outflow tract views) showing degenerated leaflets and tethering of posterior mitral leaflets determining severe eccentric mitral regurgitation (A). Three-dimensional surgical visualization showing multiple mitral regurgitation jets between A2-P1 and A2-P3 scallops (B).
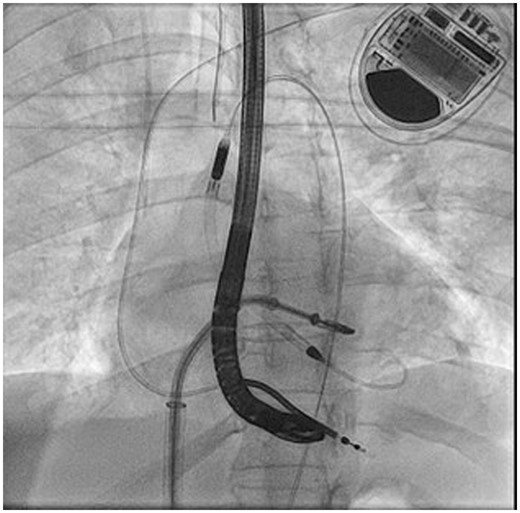
Fluoroscopic guidance during XTR MitraClip deployment with Impella CP active across the aortic valve.
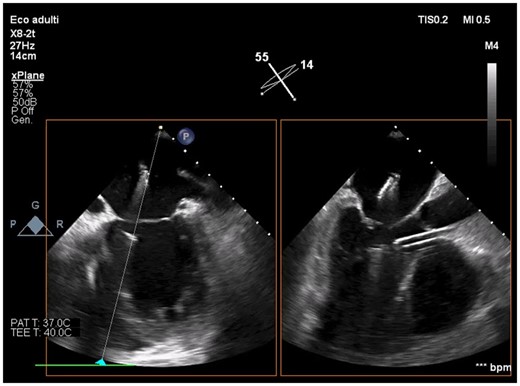
Intraprocedural transoesophageal echocardiography showing active Impella CP before mitral leaflets grasping by XTR MitraClip (intercommissural and outflow tract transoesophageal views).
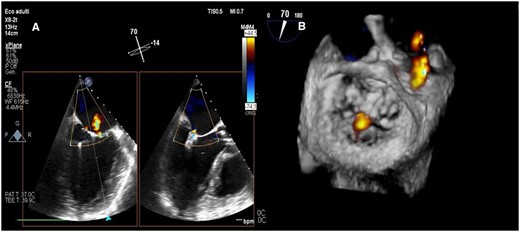
Final transoesophageal echocardiography demonstrating trivial/mild mitral regurgitation after XTR MitraClip delivery and Impella CP removal in intercommissural and outflow tract views (A) and three-dimensional surgical visualization (B).
Discussion
The highlights of this report can be summarized as follows: (i) in the two cases, a planned and ‘protected’ procedures of PMVR with Impella CP mechanical circulatory support was feasible, safe and effective in the setting of severe mitral regurgitation associated with dilated and severe left ventricle dysfunction. (ii) Extending the concept of ‘complex high-risk and indicated patients/procedures’ (CHIP)11 from the environment of coronary intervention, a ‘protected’ approach could lead to improve technical feasibility and clinical outcome in structural interventions, as advocated for ‘protected-PCI’.12,13
There is currently no consensus in current guidelines advocating the use of advanced LV support devices in patients undergoing percutaneous structural heart disease intervention.14 The use of advanced LV support devices such as Impella CP could be considered in selected cases as shown by this case series. Potential advantages of Impella CP support for percutaneous mitral valve edge-to-edge repair with MitraClip system are mainly due to:
Mechanical circulatory support in patients at risk of sudden and severe haemodynamic instability, during a complex and time-consuming procedure.
Left ventricular unloading, reducing volumes and likely the mitral leaflets tethering, may favour the mitral leaflets coaptation with improvement of grasping manoeuvres.6
There are only few reports in literature describing the use of LV advance support in patients undergoing PMVR6,15–17 and all reports describe the use of Impella CP in emergency setting for refractory acute heart failure or cardiogenic shock. We planned the use of Impella CP in substantially stable patients but at high risk of haemodynamic deterioration. To our knowledge, these are the only cases in which the use of the Impella CP device for the PMVR intervention was planned for a ‘protected’ procedure. Furthermore, all the steps of PMVR were performed, while the Impella CP device was maintained in place and active, differently from the other cases reported. The presence of the active Impella CP catheter in working position did not affect TOE imaging quality and did not lengthen the device time.
The case series we are reporting, expand the concept of CHIP to the environment of structural intervention, in particular to patients with associated: (i) several comorbidities, (ii) concomitant severe LV dysfunction and decompensation, and (iii) complex and high-risk percutaneous interventions for structural heart disease.
This setting has received growing interest in the field of interventional cardiology and is focused on complex coronary percutaneous revascularization with advanced LV support. Protected revascularization in CHIP patients are rapidly increasing and will be even more in the future.12,13 The development of specific programmes and dedicated operators, working in a multidisciplinary CHIP team, should improve cultural competencies, technical skills and consequently the clinical outcome of this high-risk patients. Moreover, the complexity of this population requires expert figures in Heart Teams, including advanced heart failure specialists, multimodality imaging experts, structural heart interventionists, cardiologist–intensivists, and cardiothoracic surgeons.
Conclusions
In the two cases reported, the advanced LV support use in planned and ‘protected’ complex PMVR was feasible, safe and effective in the structural CHIP setting. Finally, the concept of well-defined protected-PCI should be extended and translated to ‘protected’ percutaneous structural interventions.
Lead author biography
Dr Iacopo Muraca is a resident in Cardiology at the University Hospital of Careggi, Florence, from which he graduated with honour in 2015. Currently performing an advanced interventional cardiology training, he participates in the invasive cardiology unit activities. He is actively working to the diagnostic and therapeutic process of patients with ischaemic heart disease and structural heart disease subjected to percutaneous treatment.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Acknowledgements
The authors are indebted to the Cath Lab and CCU staffs for their precious help. They are also very grateful to Fabio Torrini and Paola Baldini (A.R. Card Onlus Foundation, Florence, Italy) for their secretarial assistance.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The authors confirm that written consent for submission and publication of this case series including images and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: none declared.





Comments