-
PDF
- Split View
-
Views
-
Cite
Cite
Werner Budts, Owen Miller, Sonya V Babu-Narayan, Wei Li, Emanuela Valsangiacomo Buechel, Alessandra Frigiola, Annemien van den Bosch, Beatrice Bonello, Luc Mertens, Tarique Hussain, Victoria Parish, Gilbert Habib, Thor Edvardsen, Tal Geva, Jolien W Roos-Hesselink, Katarina Hanseus, Laura Dos Subira, Helmut Baumgartner, Michael Gatzoulis, Giovanni Di Salvo, Imaging the adult with simple shunt lesions: position paper from the EACVI and the ESC WG on ACHD. Endorsed by AEPC (Association for European Paediatric and Congenital Cardiology), European Heart Journal - Cardiovascular Imaging, Volume 22, Issue 6, June 2021, Pages e58–e70, https://doi.org/10.1093/ehjci/jeaa314
Close - Share Icon Share
Abstract
In 2018, the position paper ‘Imaging the adult with congenital heart disease: a multimodality imaging approach’ was published. The paper highlights, in the first part, the different imaging modalities applied in adult congenital heart disease patients. In the second part, these modalities are discussed more detailed for moderate to complex anatomical defects. Because of the length of the paper, simple lesions were not touched on. However, imaging modalities to use for simple shunt lesions are still poorly known. One is looking for structured recommendations on which they can rely when dealing with an (undiscovered) shunt lesion. This information is lacking for the initial diagnostic process, during repair and at follow-up. Therefore, this paper will focus on atrial septal defect, ventricular septal defect, and persistent arterial duct. Pre-, intra-, and post-procedural imaging techniques will be systematically discussed. This position paper will offer algorithms that might help at a glance. The document is prepared for general cardiologists, trainees, medical students, imagers/technicians to select the most appropriate imaging modality and to detect the requested information for each specific lesion. It might serve as reference to which researchers could refer when setting up a (imaging) study.
Introduction
In 2018, the position paper ‘Imaging the adult with congenital heart disease: a multimodality imaging approach’ was published.1 The paper highlights in the first part the different imaging modalities applied in adult congenital heart disease (ACHD) patients. In the second part, these modalities are discussed more detailed for moderate to complex anatomical defects. Because of the length of the paper, simple lesions were not touched on. However, imaging modalities to use for simple shunt lesions are still poorly known. One is looking for structured recommendations on which they can rely when dealing with an (undiscovered) shunt lesion. This information is lacking for the initial diagnostic process, during repair and at follow-up.
Therefore, this paper will focus on atrial septal defect (ASD), ventricular septal defect (VSD), and persistent arterial duct. Pre-, intra-, and post-procedural imaging techniques will be systematically discussed. This position paper will offer algorithms that might help at a glance. The document is prepared for general cardiologists, trainees, medical students, and imagers/technicians to select the most appropriate imaging modality and to detect the requested information for each specific lesion. It might serve as reference to which researchers could refer when setting up a (imaging) study.
Anatomy and pathophysiology
Atrial septal defect
ASD represents one of the most common lesions in ACHD and accounts for ∼8% of all congenital heart disease (CHD).
Secundum type
The secundum ASD (type II) is by far the most common type accounting for ∼80% of the ASDs. It is located within the oval fossa. These defects can vary in shape and size. The size of the ASDs ranges from several millimetres to as large as >3 cm in diameter. It can be elliptical or round. Some are fenestrated, a few have fenestrations plus aneurysmal formation. Their location can vary—e.g. more anterior, posterior, superior, or inferior locations, and may be mistaken for sinus venosus ASDs. ASD typically results in left-to-right shunting but the extent of shunt depends on right and left ventricular (LV) compliance, defects size, and left atrial (LA) or right atrial (RA) pressure. When the shunting is significant, right ventricle (RV) volume overload and pulmonary overcirculation are the consequences. Due to a lack in early symptoms and subtlety of physical findings, ASDs are often undiagnosed until adulthood.2,3
Primum type—partial atrioventricular septal defect
The primum ASD (type I) is located near the crux of the heart and accounts for ∼15% of the ASDs. Anatomically, the primum type ASD also called partial atrioventricular (AV) septal defect (AVSD) or partial AV canal belongs to the group of AVSDs. The main anatomic feature is an AV junction guarded by a common valve that has fusion between the bridging leaflets and the crest of the interventricular septum. The pathophysiology is largely dependent on the magnitude of atrial left-to-right shunting in combination with the severity of the left or/and right AV valve regurgitation.
Sinus venosus type
Sinus venosus defects (SVDs) comprise ∼4–11% of ASDs.4 They are not per se a defect in the inter-atrial septum but there is an interatrial communication involving the wall between RA, superior vena cava (SVC), and right pulmonary veins (superior SVD) or the wall between the right lower pulmonary veins and the RA or at the inferior caval-right atrial junction (inferior SVD).5,6 SVDs are, therefore, usually associated with partial anomalous pulmonary venous return. The defect in general results in a left-to-right shunt with volume overload of the right heart and pulmonary circulation.
Sinus coronarius type
A coronary sinus defect is rare. It is often associated with persistent left SVC and sometimes with complex CHD. It is characterized by the absence of at least a portion of the common wall that separates the coronary sinus and the LA.2 Interatrial shunting occurs through the defect in the wall on the LA side, which is continuous with the orifice of the coronary sinus opening on the RA side. When the defect is large, venous blood gets into the left atrium (LA) and desaturation can occur.
Ventricular septal defect
A perimembranous VSD is defined by fibrous continuity between the tricuspid and aortic valves. It often extends to the muscular parts of the septum either towards the inlet, outlet, or trabecular parts of the septum. A muscular VSD has muscular borders and can be situated anywhere within the muscular septum. Based on the location, muscular VSDs can be further described as inlet, outlet, and trabecular defects. A doubly committed VSD is an outlet type VSD below the aortic and pulmonary valves with fibrous continuity between the two valves. A VSD can be single or there can be multiple VSDs coexisting. The apical septum can have multiple muscular VSDs (Swiss cheese type).3,7
Usually a VSD shunts left-to-right. Right-to-left shunting occurs in the presence of increased pulmonary vascular resistance or right outflow obstruction. Large left-right shunting can result in LV volume loading with LV dilatation and secondary mitral valve regurgitation. If the VSD is not restrictive to pressure, chronic increase in pulmonary pressure results in irreversible increase in pulmonary vascular resistance with reversal of the shunt direction (Eisenmenger syndrome).
Persistent arterial duct
The ductus arteriosus (DA) or arterial duct is a connection, located few mm distal to the origin of the left subclavian artery, between the main pulmonary trunk and descending aorta. Persistent DA (PDA) accounts for 5–10% of all CHD. After birth, pulmonary artery pressures decrease progressively so that the duct needs to close to avoid upcoming left-to-right (aorta > pulmonary artery) shunt. If this process does not happen the duct persists and causes volume overload of the pulmonary artery, the pulmonary vascular bed, and the left heart. The haemodynamic consequences of PDA depend on the magnitude of left-to-right shunt, the size of the PDA, the systemic and the pulmonary vascular resistances. If left untreated this might lead to left heart failure, the development of pulmonary arterial hypertension (PAH), and right heart failure. A persistent duct is frequently associated with other cardiovascular anomalies (e.g. coarctation of the aorta) and might have a broad spectrum of anatomical variation.8
(Preprocedural) imaging of the native defect
This paragraph describes the imaging of the native defect. For the indications to intervene is referred to the 2020 ESC guidelines of the management of Adult Congenital Heart Disease.9
Atrial septal defect
Echocardiography
Transthoracic echocardiography (TTE) has a central role in establishing the diagnosis and the haemodynamic relevance of the defect. Shunting at atrial level must be suspected in the presence of RV dilatation. Optimal echocardiographic imaging of the ASDs requires imaging from multiple windows, planes, and sweeps as well as the use of colour Doppler. Figures 1 and 2 show a transthoracic apical view of a secundum and a primum type ASD, respectively. The echocardiographic evaluation includes quantification of the size and shape, the rims of tissue surrounding, the degree and direction of shunting, and the remodelling and changes in size and function of the cardiac chambers and pulmonary circulation. The 3D visualization with 3D echocardiography, especially with transoesophageal echocardiography (TOE), might provide incremental information (Figure 3).
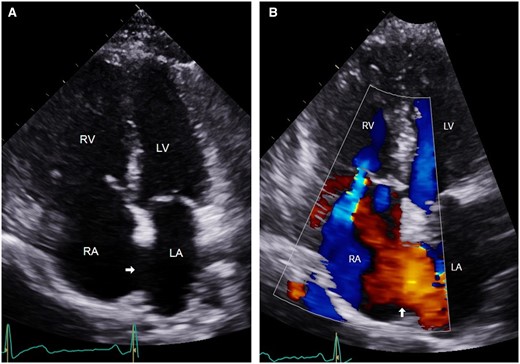
(A) Apical four-chamber view of transthoracic echocardiogram showing large secundum ASD (arrow). (B) Colour Doppler showing left to right shunt across the ASD (arrow). LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle.
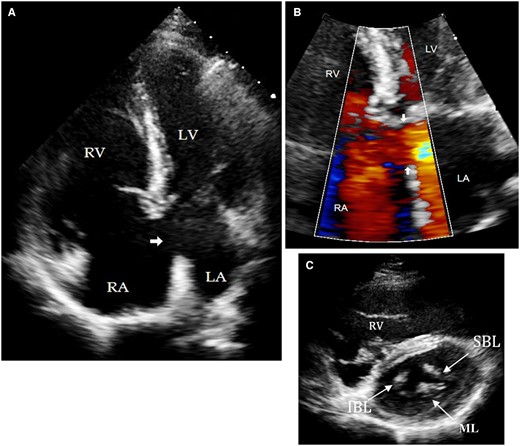
(A) Transthoracic echocardiogram of apical four chamber view showing a primum ASD (arrow). (B) Focus view of atrioventricular junction colour Doppler showing left-to-right shunt across primum ASD (arrow). (C) Short-axis view of same patient showing the tri-leaflet left AV valve. IBL, inferior bridging leaflet; LA, left atrium; LV, left ventricle; ML, mural leaflet; RA, right atrium; RV, right ventricle; SBL, superior bridging leaflet.
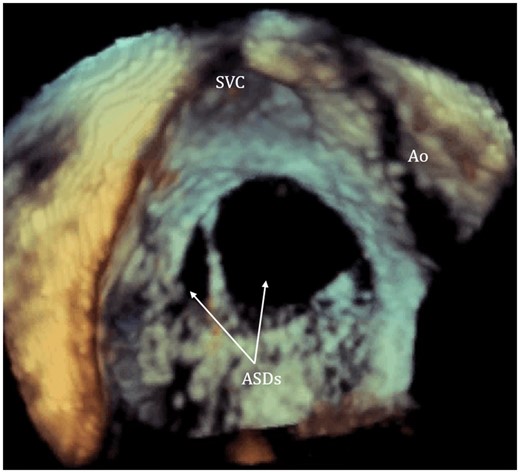
The 3D visualization of intra-atrial septum from right atrium using transoesophageal echocardiogram demonstrating two separate secundum ASDs with irregular shape (arrow pointed). ASDs, atrial septal defects; Ao, aorta; SVC, superior vena cava.
Cardiovascular magnetic resonance and computed tomography
Cardiovascular magnetic resonance (CMR) complements unique haemodynamic and quantitative data.10 Shunt degree is quantified by measuring the ratio of the pulmonary flow over the systemic flow (Qp/Qs)11,12 and cine images enable exact quantification of the volume and function of the dilated right-sided cardiac structures. For multiple and unusually located atrial defects, such as superior (Figure 4A) and inferior sinus venosus type, cine images in orthogonal planes to each other can be very helpful for anatomical delineation of the defect.13 Finally, partial anomalous pulmonary venous connections (PAPVCs) are commonly associated with atrial shunts of all types, and typically in the superior SVD; these anomalies can be easily detected by CMR (Figure 4B).14 Cross-sectional imaging is helpful for planning surgical repair.15
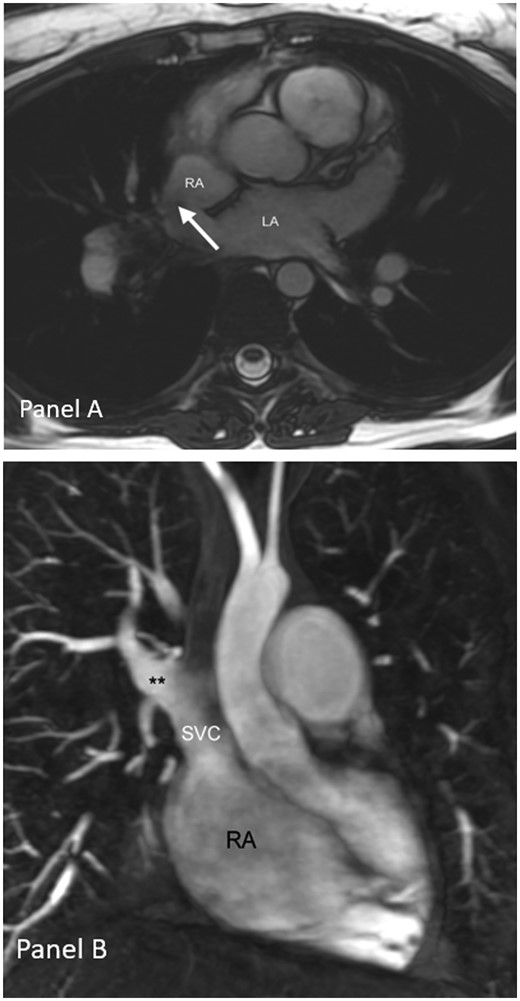
(A) Atrial septal defect of sinus venosus type visualized on axial CMR cine images and (B) CMR of partial anomalous pulmonary venous connection of the right upper pulmonary vein**. LA, left atrium; RA, right atrium; SVC, superior vena cava.
Computed tomography (CT) provides clear visualization of the pulmonary veins and is, therefore, helpful for ruling out PAPVC. CT angiography of the coronary arteries might be helpful in the preoperative setting to exclude non-invasively significant coronary atherosclerosis. However, CMR remains clearly superior to CT if flow measurements are required.16
Catheterization
For ASD diagnosis, catheterization and angiography are not the first-choice imaging modalities. However, catheterization remains the golden standard to measure pressures and to calculate pulmonary vascular resistance, particularly for diagnosing pulmonary (arterial) hypertension.17 In patients with non-invasive signs of pulmonary artery pressure elevation, invasive measurement of pulmonary vascular resistance is even mandatory. In patients with LV disease and elevated filling pressure, careful haemodynamic evaluation including balloon testing is required to weigh benefit and risk of closure. Contrast injection in the right upper pulmonary vein visualizes the left-sided septum and the left-to-right shunt in a secundum and primum type defect (left anterior oblique and cranial camera position). Direct angiography in a pulmonary vein or a pulmonary artery angiogram with focus on the levophase of contrast helps to localize PAPVC in SVD.
Figure 5 summarizes the diagnostic algorithm in ASD patients.
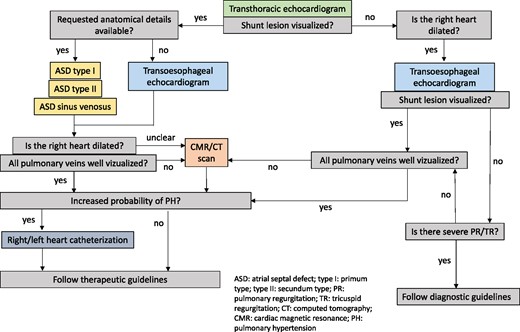
Diagnostic algorithm in suspected ASD. ASD, atrial septal defect; CT, computed tomography; CMR, cardiac magnetic resonance; PH, pulmonary hypertension; PR, pulmonary regurgitation; TR, tricuspid regurgitation.
Ventricular septal defect
Echocardiography
TTE almost always permits accurate identification of the location, size, and the number of VSDs, their haemodynamic impact, and delineation of associated anatomic features. Optimal echocardiographic imaging of the VSDs in the different areas (membranous septum, outlet, inlet, and muscular trabecular parts) requires imaging from multiple windows, planes, and sweeps as well as the use of colour Doppler.18,19 An example of a perimembranous VSD is illustrated in Figure 6. Echocardiography also provides haemodynamic information on VSD physiology including shunt direction (left-right or right-left), presence of restriction to pressure represented by pressure gradient, assessment of RV pressures, and LV and RV dimensions.20 3D echocardiography can provide additional information on the VSD morphology and LV volumes and ejection fraction.21 TOE plays a minor role in the assessment of VSDs but may be useful in patients with poor imaging windows.
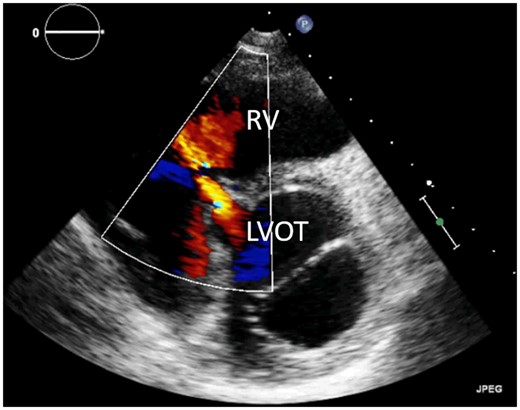
Transthoracic echocardiogram of perimembranous ventricular septal defect, parasternal short-axis view. LVOT, left ventricular outflow tract; RV, right ventricle.
Cardiovascular magnetic resonance and computed tomography
For VSD, CMR enables quantification of shunt by measuring the pulmonary flow and the systemic flow (Qp/Qs)10 and accurate quantification of associated increased LV chamber size. Double chambered RV, a form of RV outflow obstruction, is an unusual complication of a small VSD which is easily evaluated with CMR. CMR short-axis cines and subsequent orthogonal RV oblique planes can delineate the double chamber RV jet. A transaxial cine stack may be useful for multiple VSDs. For large VSDs, non-invasive features of pulmonary hypertension provided by CMR include degree of pulmonary artery dilatation, ventricular septal flattening in systole, and accurate measurement of RV mass.
CT may provide clear visualization but CMR remains clearly superior to CT if flow measurements are required. Also, CT angiography of the coronary arteries might be helpful in the preoperative setting to exclude non-invasively significant coronary atherosclerosis.
Catheterization
For VSD diagnosis, catheterization and angiography are rarely indicated. Pressure measurements and shunt and resistance calculations can be performed for a better insight into the haemodynamic repercussion of the VSD. Similar to ASD patients, in VSD patients with non-invasive signs of pulmonary artery pressure elevation, invasive measurement of pulmonary vascular resistance is even mandatory. Angiography in the LV helps in detailing the anatomical position of the defect and in estimating the degree of shunt. This might be useful for appropriate device selection in case of percutaneous closure.22 Standard angiographic views are left anterior oblique for muscular defects and left anterior oblique and cranial for perimembranous and doubly committed lesions.
Figure 7 summarizes the diagnostic algorithm in VSD patients.
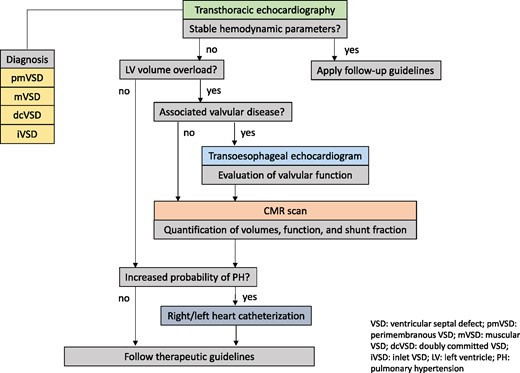
Diagnostic algorithm in suspected VSD. dcVSD, doubly committed VSD; iVSD, inlet VSD; LV, left ventricle; mVSD, muscular VSD; PH, pulmonary hypertension; pmVSD, perimembranous VSD; VSD, ventricular septal defect.
Persistent arterial duct
Echocardiography
TTE remains the first choice for PDA diagnosis and to evaluate the haemodynamic consequences and detect associated lesions. The best imaging windows in adults are the parasternal short axis and the suprasternal view. A continuous colour flow from the pulmonary artery to the pulmonary valve is very suggestive for a PDA (Figure 8A). Direct visualization of the duct through this axis is rare. In patients with at least moderate size PDA the left cardiac chambers are dilated. In suprasternal view, a PDA causes continuous colour flow from the distal part of the aortic arch to the pulmonary artery (Figure 8B). In both windows, a continuous Doppler flow signal aligns with the colour flow.23 In the presence of elevated pulmonary vascular resistance, the shunt maybe bidirectional or right-to-left. Contrast echo may help to visualize the right-to-left-shunt across the PDA.
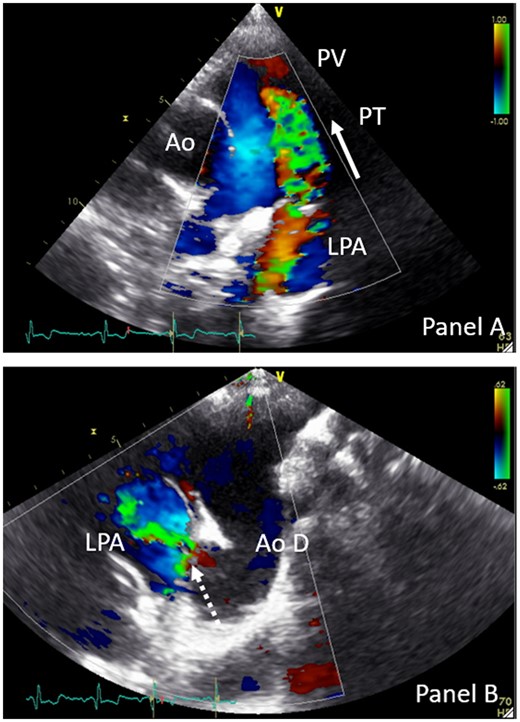
TTE parasternal short-axis colour Doppler view (A) and suprasternal colour Doppler view of PDA (B). Dotted arrow indicates PDA; full arrow indicates flow direction of the systolic and diastolic colour flow. Ao, aorta; Ao D, aorta descendens; LPA, left pulmonary artery; PT, pulmonary trunk; PV, pulmonary valve.
Cardiac magnetic resonance and computed tomography
Cross-sectional imaging is rarely required for imaging PDA. PDA can be found during assessment of dilated left-sided cardiac chambers but is frequently an incidental finding as a jet is seen on cine images between the aortic arch and the pulmonary artery. This can be verified by visualization of a connection between both vessels. The advantage of CMR is the combination of clear anatomical images with flow measurements enabling calculation of the extracardiac shunt.10,11,24 CT can be indicated to exclude coronary atherosclerosis in a preprocedural setting.
Catheterization
For PDA diagnosis, catheterization and angiography are rarely done. Pressure measurements and shunt and resistance calculations can be performed for a better insight in the haemodynamic repercussion of the duct. In patients with non-invasive signs of pulmonary artery pressure elevation, invasive measurement of pulmonary vascular resistance is even mandatory. Angiography at the aortic orifice of the arterial duct helps in detailing the anatomical variation and shunt estimation. This might be useful for appropriate device selection in percutaneous closure. Standard angiographic views are left lateral and right anterior oblique.25
Figure 9 summarizes the diagnostic algorithm in PDA patients.
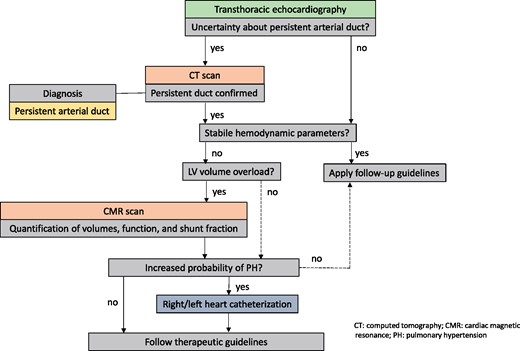
Diagnostic algorithm in suspected PDA. CT, computed tomography; CMR, cardiac magnetic resonance; PDA, persistent arterial duct; PH, pulmonary hypertension.
Intraprocedural imaging
Atrial septal defect
Imaging modalities used during closure include fluoroscopy accompanied by either TOE or intracardiac echocardiography (ICE).26–28 ICE is preferred by some operators29 as the interventionist can control both device and imaging and general anaesthesia is avoided. Some operators employ TTE or avoid fluoroscopy entirely.30,31 3D echocardiography may be used for defect sizing, multiple defects, device on device placement, and to reduce radiation exposure.26,32–35 Fusion of echocardiographic and fluoroscopic images is applied at some centres.36 Role of echocardiographic imaging:
Guidance of the guidewire/catheter through the correct defect and into the left pulmonary veins (avoidance of left atrial appendage).
Monitoring of balloon sizing, where performed, measurement of defect size, shape and number, exclusion of residual shunt, and impingement on adjacent structures.
Device deployment: most occlusion devices have a ‘double disc’ configuration with a central waist. Residual flow around device should be assessed, as well as ‘rocking’ which can indicate an unstable position. Impingement on adjacent structures including the mitral valve, tricuspid valve, and aortic root needs to be assessed.
Imaging after release: device configuration may change following release. Imaging of device in relation to all rims, including colour flow Doppler for residual leaks, impingement on adjacent structures including the atrial roof.
Exclusion of immediate complications, such as pericardial effusion or thrombus formation.
Ventricular septal defect
The imaging modalities used during closure include fluoroscopy accompanied by TOE (and TTE).37–40 Role of echocardiographic imaging:
Monitoring of deployment: most operators close VSDs by forming a catheter circuit through the left and right heart. Device deployment is done via sheaths entering the defect from the right ventricle. The left side of the VSD occlusion device is deployed and the device is approximated to the ventricular septum. The right-sided disc is then deployed and imaged for residual defects and impingement on adjacent structures, including the aortic and tricuspid valves.
Assessment after release (residual shunt, impingement on adjacent structures).
Persistent arterial duct
While PDA closure is generally performed under fluoroscopic guidance in adults, TTE can be very helpful to guide percutaneous PDA closure particularly in children and newborns.41–43 With the ultrasound probe placed on the suprasternal notch to show the long-axis view of the aortic arch it is possible to visualize the catheter inserted into the aortic arch heading the PDA.41–43 From the parasternal short-axis view, echo can visualize the position of the guide wire into the main pulmonary artery. Combining these views TTE can be used to guide a percutaneous closure of the PDA.
TOE is helpful confirming complete closure of the PDA after surgical ligation/operation.41–43
Live 3D TOE44 has been proposed for monitoring a transcatheter procedure since it cuts down the fluoroscopy time. It allows delineation of the anatomical details or its variations, confirms the position of the device after closure and rules out any residual shunts.
Post-procedural imaging
Atrial septal defect
Secundum type
TTE is the first-choice modality; TOE or CMR may be performed when image quality is poor and preferentially at least 6 months after intervention. After percutaneous ASD closure, imaging aims to evaluate the proper and stable device position across the atrial septum, the presence, location and extent of residual transseptal flow, and device impingement on neighbouring structures (SVC, IVC, coronary sinus, pulmonary veins, and AV valves). Detailed echocardiographic assessment should be done after surgical repair of the ASD to exclude residual shunts, confirm remodelling of the RV and resolution of RV volume load and rule out pericardial effusion in the early post-operative period.33,45
Primum type—partial atrioventricular septal defect
TTE remains the first-choice modality; TOE or CMR can be performed when image quality is poor. Echocardiographic evaluation should include the evaluation of atrial shunting, biventricular size and function, residual elevated pulmonary artery pressure, AV valve regurgitation and pericardial effusion in the early post-operative period. 3D echocardiography and strain echocardiography should be used for biventricular size and function, and if image quality is adequate for AV valve morphology.33,45,46
Sinus venosus type
TTE is the preferred imaging method. It should demonstrate the unobstructed vena cava flow into the RA, no residual shunt, and unobstructed communication/baffle of the pulmonary veins to LA as well as RV dimensions and pulmonary artery pressures. TOE can be useful if acoustic windows are poor.
A contrast study through a venous cannula can be helpful to exclude residual shunt or misplacement of the atrial septal patch, especially for inferior SVD. Cardiac CT scan or CMR may be useful for more detailed anatomic and functional evaluation.
Sinus coronarius type
Depending on the type of repair,47–49 transthoracic, or if needed, TOE should demonstrate unobstructed coronary sinus flow to the RA, with no residual shunt. In the presence of a left SVC, an agitated saline contrast study through a venous cannula in the left arm is recommended to exclude residual shunt. In the absence of left SVC, imaging of the closed atrial communication is the same as in secundum ASD. If TTE is insufficient, CMR or, if contraindicated, CT may be useful for detailed anatomic and functional evaluation.
Table 1 summarizes the complications after ASD and/or abnormal pulmonary venous drainage repair and preferred imaging modality.
Complications after ASD and/or abnormal pulmonary venous drainage repair and preferred imaging modality
| . | Complication . | Preferred imaging . | . |
|---|---|---|---|
| ASD type I | Septal leakage | TTE TOE CMR | Detection Anatomical details Volume and function Shunt quantification |
| Insufficient MV | TTE TOE Catheterization | Detection Anatomical details Haemodynamic data | |
| PH | TTE Catheterization | Detection PH type determination | |
| ASD type II | Septal leakage | TTE TOE CMR | Detection Anatomical details Volume and function Shunt quantification |
| PH | TTE Catheterization | Detection PH type determination | |
| Device dislocation | TTE TOE CT | Detection Anatomical details Anatomical details | |
| ASD sinus venosus | Septal leakage | TTE TOE/CT CMR | Detection Anatomical details Volume and function Shunt quantification |
| PH | TTE Catheterization | Detection PH type determination | |
| SVC obstruction | TOE/CT Catheterization | Anatomical details Anatomical details | |
| PAPVD | PAPVD obstruction | CT Catheterization | Detection Anatomical details Pulmonary angiogram |
| . | Complication . | Preferred imaging . | . |
|---|---|---|---|
| ASD type I | Septal leakage | TTE TOE CMR | Detection Anatomical details Volume and function Shunt quantification |
| Insufficient MV | TTE TOE Catheterization | Detection Anatomical details Haemodynamic data | |
| PH | TTE Catheterization | Detection PH type determination | |
| ASD type II | Septal leakage | TTE TOE CMR | Detection Anatomical details Volume and function Shunt quantification |
| PH | TTE Catheterization | Detection PH type determination | |
| Device dislocation | TTE TOE CT | Detection Anatomical details Anatomical details | |
| ASD sinus venosus | Septal leakage | TTE TOE/CT CMR | Detection Anatomical details Volume and function Shunt quantification |
| PH | TTE Catheterization | Detection PH type determination | |
| SVC obstruction | TOE/CT Catheterization | Anatomical details Anatomical details | |
| PAPVD | PAPVD obstruction | CT Catheterization | Detection Anatomical details Pulmonary angiogram |
Detection refers to noticing the complication; anatomical details refer to a more detailed description of the lesion of interest; volume and function refer to size and function of the ventricles of interest.
ASD, atrial septal defect; CMR, cardiac magnetic resonance; CT, computed tomography; MV, mitral valve; PAPVD, partial abnormal pulmonary vein drainage; PH, pulmonary hypertension; SVC, superior vena cava; TOE, transoesophageal echocardiogram; TTE, transthoracic echocardiogram; type I, primum type; type II, secundum type.
Complications after ASD and/or abnormal pulmonary venous drainage repair and preferred imaging modality
| . | Complication . | Preferred imaging . | . |
|---|---|---|---|
| ASD type I | Septal leakage | TTE TOE CMR | Detection Anatomical details Volume and function Shunt quantification |
| Insufficient MV | TTE TOE Catheterization | Detection Anatomical details Haemodynamic data | |
| PH | TTE Catheterization | Detection PH type determination | |
| ASD type II | Septal leakage | TTE TOE CMR | Detection Anatomical details Volume and function Shunt quantification |
| PH | TTE Catheterization | Detection PH type determination | |
| Device dislocation | TTE TOE CT | Detection Anatomical details Anatomical details | |
| ASD sinus venosus | Septal leakage | TTE TOE/CT CMR | Detection Anatomical details Volume and function Shunt quantification |
| PH | TTE Catheterization | Detection PH type determination | |
| SVC obstruction | TOE/CT Catheterization | Anatomical details Anatomical details | |
| PAPVD | PAPVD obstruction | CT Catheterization | Detection Anatomical details Pulmonary angiogram |
| . | Complication . | Preferred imaging . | . |
|---|---|---|---|
| ASD type I | Septal leakage | TTE TOE CMR | Detection Anatomical details Volume and function Shunt quantification |
| Insufficient MV | TTE TOE Catheterization | Detection Anatomical details Haemodynamic data | |
| PH | TTE Catheterization | Detection PH type determination | |
| ASD type II | Septal leakage | TTE TOE CMR | Detection Anatomical details Volume and function Shunt quantification |
| PH | TTE Catheterization | Detection PH type determination | |
| Device dislocation | TTE TOE CT | Detection Anatomical details Anatomical details | |
| ASD sinus venosus | Septal leakage | TTE TOE/CT CMR | Detection Anatomical details Volume and function Shunt quantification |
| PH | TTE Catheterization | Detection PH type determination | |
| SVC obstruction | TOE/CT Catheterization | Anatomical details Anatomical details | |
| PAPVD | PAPVD obstruction | CT Catheterization | Detection Anatomical details Pulmonary angiogram |
Detection refers to noticing the complication; anatomical details refer to a more detailed description of the lesion of interest; volume and function refer to size and function of the ventricles of interest.
ASD, atrial septal defect; CMR, cardiac magnetic resonance; CT, computed tomography; MV, mitral valve; PAPVD, partial abnormal pulmonary vein drainage; PH, pulmonary hypertension; SVC, superior vena cava; TOE, transoesophageal echocardiogram; TTE, transthoracic echocardiogram; type I, primum type; type II, secundum type.
Ventricular septal defect
Perimembranous type
TTE is the first-line imaging modality1 in the follow-up after VSD closure and should focus on detecting residual shunts or lesions (e.g. outflow tract obstruction, aortic, and tricuspid valve regurgitation) and persistent pulmonary hypertension. LV dysfunction soon after repair is quite common and is mainly attributed to reduction of preload after shunt closure. Two-dimensional speckle tracking-derived global longitudinal strain may discern pre-operative changes and allow follow-up.1,50 Full recovery is generally expected within the following 9 months. Abnormal parameters of RV function may last longer, particularly in the presence of post-operative right bundle branch block.51
Muscular type
LV dysfunction may be more persistent in patients after left ventriculotomy for either surgical repair or percutaneous access. Residual/remaining VSDs are common in patients with pre-existing multiple muscular defects. In case of device closure, an adequate deployment of the device must be assured. Persistent flow through the device may be present for the first weeks until complete endothelization. TTE is first line for assessment but ventriculotomy sites, apical residual/remaining defects, and apical devices may be more difficult to assess and cardiac CT (anatomical and functional assessment) or CMR (anatomical, functional, and flow assessment) may be considered.
Doubly committed type
In a doubly committed VSD, the defect is in most cases closed by a surgical patch or more recently by device.52 Because the patch has a close connection with the ventriculo-arterial valves, their function has to be followed over time.53 TTE is the preferred routine imaging method. In case of unclear relationship between valve dysfunction and patch repair, TOE is the second choice because of a better image resolution. Also, when residual shunting is suspected, TOE might offer more detailed anatomical information. Similar to most other post-interventional or post-operative statuses, additional non-invasive haemodynamic data can be obtained by CMR. Invasive measurements by catheterization are useful when PAH has to be excluded or a re-intervention at any level is considered.
Inlet type
The preferred imaging method after repair of an inlet VSD is TTE. In most cases, the patch closure can be visualized in detail and eventually a residual shunt detected. Because an inlet VSD can be considered as a part of an AVSD, (bilateral) AV surgery is often needed.54 For valve evaluation, TTE is also the first-choice imaging modality. TOE can be of added value when more anatomical and functional details of the valves are requested. Sequels of preceding volume loading of the systemic ventricle and signs of persistent pulmonary hypertension can easy be detected by TTE. CMR might provide more haemodynamic information in case of persistent pressure or volume overload. Invasive measurements are useful when PAH has to be excluded or a re-intervention at any level is considered.
Table 2 summarizes the complications after VSD repair.
| . | Complication . | Preferred imaging . | . |
|---|---|---|---|
| Perimembranous VSD | Residual leakage | TTE TOE CMR | Detection Anatomical details Volume and function Shunt quantification |
| Aortic valve dysfunction | TTE TOE Catheterization | Detection Anatomical details Haemodynamic data | |
| PH | TTE Catheterization | Detection PH type determination | |
| Device dislocation | TTE TOE CT | Detection Anatomical details Anatomical details | |
| Muscular VSD | Residual leakage | TTE TOE CMR | Detection Anatomical details Volume and function Shunt quantification |
| PH | TTE Catheterization | Detection PH type determination | |
| Device dislocation | TTE TOE CT | Detection Anatomical details Anatomical details | |
| Doubly committed VSD | Residual leakage | TTE TOE CMR | Detection Anatomical details Volume and function Shunt quantification |
| Aortic or pulmonary valve dysfunction | TTE TOE Catheterization | Detection Anatomical details Haemodynamic data | |
| PH | TTE Catheterization | Detection PH type determination | |
| Inlet VSD | Residual leakage | TTE TOE CMR | Detection Anatomical details Volume and function Shunt quantification |
| AV-valve dysfunction | TTE TOE Catheterization | Detection Anatomical details Haemodynamic data | |
| PH | TTE Catheterization | Detection PH type determination |
| . | Complication . | Preferred imaging . | . |
|---|---|---|---|
| Perimembranous VSD | Residual leakage | TTE TOE CMR | Detection Anatomical details Volume and function Shunt quantification |
| Aortic valve dysfunction | TTE TOE Catheterization | Detection Anatomical details Haemodynamic data | |
| PH | TTE Catheterization | Detection PH type determination | |
| Device dislocation | TTE TOE CT | Detection Anatomical details Anatomical details | |
| Muscular VSD | Residual leakage | TTE TOE CMR | Detection Anatomical details Volume and function Shunt quantification |
| PH | TTE Catheterization | Detection PH type determination | |
| Device dislocation | TTE TOE CT | Detection Anatomical details Anatomical details | |
| Doubly committed VSD | Residual leakage | TTE TOE CMR | Detection Anatomical details Volume and function Shunt quantification |
| Aortic or pulmonary valve dysfunction | TTE TOE Catheterization | Detection Anatomical details Haemodynamic data | |
| PH | TTE Catheterization | Detection PH type determination | |
| Inlet VSD | Residual leakage | TTE TOE CMR | Detection Anatomical details Volume and function Shunt quantification |
| AV-valve dysfunction | TTE TOE Catheterization | Detection Anatomical details Haemodynamic data | |
| PH | TTE Catheterization | Detection PH type determination |
Detection refers to noticing the complication; anatomical details refer to a more detailed description of the lesion of interest; volume and function refer to size and function of the ventricles of interest.
AV, atrioventricular; CMR, cardiac magnetic resonance; CT, computed tomography; PH, pulmonary hypertension; TOE, transoesophageal echocardiogram; TTE, transthoracic echocardiogram; VSD, ventricular septal defect.
| . | Complication . | Preferred imaging . | . |
|---|---|---|---|
| Perimembranous VSD | Residual leakage | TTE TOE CMR | Detection Anatomical details Volume and function Shunt quantification |
| Aortic valve dysfunction | TTE TOE Catheterization | Detection Anatomical details Haemodynamic data | |
| PH | TTE Catheterization | Detection PH type determination | |
| Device dislocation | TTE TOE CT | Detection Anatomical details Anatomical details | |
| Muscular VSD | Residual leakage | TTE TOE CMR | Detection Anatomical details Volume and function Shunt quantification |
| PH | TTE Catheterization | Detection PH type determination | |
| Device dislocation | TTE TOE CT | Detection Anatomical details Anatomical details | |
| Doubly committed VSD | Residual leakage | TTE TOE CMR | Detection Anatomical details Volume and function Shunt quantification |
| Aortic or pulmonary valve dysfunction | TTE TOE Catheterization | Detection Anatomical details Haemodynamic data | |
| PH | TTE Catheterization | Detection PH type determination | |
| Inlet VSD | Residual leakage | TTE TOE CMR | Detection Anatomical details Volume and function Shunt quantification |
| AV-valve dysfunction | TTE TOE Catheterization | Detection Anatomical details Haemodynamic data | |
| PH | TTE Catheterization | Detection PH type determination |
| . | Complication . | Preferred imaging . | . |
|---|---|---|---|
| Perimembranous VSD | Residual leakage | TTE TOE CMR | Detection Anatomical details Volume and function Shunt quantification |
| Aortic valve dysfunction | TTE TOE Catheterization | Detection Anatomical details Haemodynamic data | |
| PH | TTE Catheterization | Detection PH type determination | |
| Device dislocation | TTE TOE CT | Detection Anatomical details Anatomical details | |
| Muscular VSD | Residual leakage | TTE TOE CMR | Detection Anatomical details Volume and function Shunt quantification |
| PH | TTE Catheterization | Detection PH type determination | |
| Device dislocation | TTE TOE CT | Detection Anatomical details Anatomical details | |
| Doubly committed VSD | Residual leakage | TTE TOE CMR | Detection Anatomical details Volume and function Shunt quantification |
| Aortic or pulmonary valve dysfunction | TTE TOE Catheterization | Detection Anatomical details Haemodynamic data | |
| PH | TTE Catheterization | Detection PH type determination | |
| Inlet VSD | Residual leakage | TTE TOE CMR | Detection Anatomical details Volume and function Shunt quantification |
| AV-valve dysfunction | TTE TOE Catheterization | Detection Anatomical details Haemodynamic data | |
| PH | TTE Catheterization | Detection PH type determination |
Detection refers to noticing the complication; anatomical details refer to a more detailed description of the lesion of interest; volume and function refer to size and function of the ventricles of interest.
AV, atrioventricular; CMR, cardiac magnetic resonance; CT, computed tomography; PH, pulmonary hypertension; TOE, transoesophageal echocardiogram; TTE, transthoracic echocardiogram; VSD, ventricular septal defect.
Persistent arterial duct
TTE is the examination of choice for long-term follow-up after PDA closure. A reduction in LV diastolic volume along with a transient decrease in LV ejection fraction is observed after repair the latter being more prominent in surgically than percutaneously treated patients.55 Residual flow due to incomplete closure can be visualized from suprasternal and high short-axis views and is more common when coils are used for repair.
Flow disturbances caused by protruding, not well aligned, or to large disks (left pulmonary artery and descending aorta) are anecdotal in adults but should be ruled out in low-bodyweight infants, particularly when large devices are used.56 Moreover, Doppler gradient estimates in the obstructed pulmonary branch might be underestimated because of a decreased flow in the obstructed branch (low flow gradient), whereas the flow in the unobstructed branch is increased. In these cases, a careful assessment of the branch anatomy and flow measurements with CMR is advised.
Table 3 summarizes the complications after PDA repair.
Complications after persistent arterial duct repair and preferred imaging modality
| . | Complication . | Preferred imaging . | . |
|---|---|---|---|
| Persistent duct | Residual leakage | TTE | Detection |
| CT | Anatomical details | ||
| Catheterization | Aortogram anatomical details Haemodynamic data | ||
| Peripheral pulmonary stenosis | TTE | Detection | |
| CT | Anatomical details | ||
| Catheterization | Pulmonary angiogram Anatomical details Haemodynamic data |
| . | Complication . | Preferred imaging . | . |
|---|---|---|---|
| Persistent duct | Residual leakage | TTE | Detection |
| CT | Anatomical details | ||
| Catheterization | Aortogram anatomical details Haemodynamic data | ||
| Peripheral pulmonary stenosis | TTE | Detection | |
| CT | Anatomical details | ||
| Catheterization | Pulmonary angiogram Anatomical details Haemodynamic data |
Detection refers to noticing the complication; anatomical details refer to a more detailed description of the lesion of interest.
CT, computed tomography; TTE, transthoracic echocardiogram.
Complications after persistent arterial duct repair and preferred imaging modality
| . | Complication . | Preferred imaging . | . |
|---|---|---|---|
| Persistent duct | Residual leakage | TTE | Detection |
| CT | Anatomical details | ||
| Catheterization | Aortogram anatomical details Haemodynamic data | ||
| Peripheral pulmonary stenosis | TTE | Detection | |
| CT | Anatomical details | ||
| Catheterization | Pulmonary angiogram Anatomical details Haemodynamic data |
| . | Complication . | Preferred imaging . | . |
|---|---|---|---|
| Persistent duct | Residual leakage | TTE | Detection |
| CT | Anatomical details | ||
| Catheterization | Aortogram anatomical details Haemodynamic data | ||
| Peripheral pulmonary stenosis | TTE | Detection | |
| CT | Anatomical details | ||
| Catheterization | Pulmonary angiogram Anatomical details Haemodynamic data |
Detection refers to noticing the complication; anatomical details refer to a more detailed description of the lesion of interest.
CT, computed tomography; TTE, transthoracic echocardiogram.
Conclusions
This document intends to give an overview of the diagnostic process in patients with simple shunt lesions. TTE plays an utmost important role for the detection of a shunt lesion, but advanced imaging techniques might be required to obtain more anatomical or haemodynamic data. Intraprocedural imaging is in most cases TOE guided. Knowledge of post-procedural complications is important to apply the most appropriate imaging technique. The proposed algorithms are based on expert opinion and might guide healthcare providers with no expertise in ACHD to choose for the most accurate imaging approach.
Conflict of interest: none declared.



