-
PDF
- Split View
-
Views
-
Cite
Cite
Bernard Cosyns, Mani A Vannan, Global longitudinal strain in severe aortic stenosis, European Heart Journal - Cardiovascular Imaging, Volume 21, Issue 11, November 2020, Pages 1259–1261, https://doi.org/10.1093/ehjci/jeaa260
Close - Share Icon Share
This editorial refers to ‘Incremental value of left ventricular global longitudinal strain in a newly proposed staging classification based on cardiac damage in patients with severe aortic stenosis’ by E.M. Vollema et al., pp. 1248–1258.
More important than the quest for certainty is the quest for clarity
—Francois Gautier
The timing of intervention in asymptomatic severe aortic stenosis (AS) is one of the challenging issues in the management of the disease. Currently, in asymptomatic severe AS, which represent one-half of the patients with severe AS, transcatheter or surgical aortic valve replacement (TAVR or SAVR) is indicated only when left ventricular ejection fraction (LVEF) is <50%, when symptoms are induced during exercise testing or in those who are undergoing cardiac surgery for another reason.1 However, symptoms are notoriously unreliable in AS and exercise testing is not always feasible especially in the elderly. Furthermore, recent data would indicate that there is increased risk of mortality in asymptomatic severe AS even when LVEF is >50%.2 Also, moderate AS based on current echocardiographic data may be associated with reduced survival which in part be related to comorbid clinical factors and co-existing cardiac abnormalities. Hence, the concept of staging AS rather than merely classifying the severity of the valve lesion based on echocardiographic data (Stage 0, no left ventricle damage; Stage 1, left ventricle damage; Stage 2, left atrial enlargement, mitral valve damage; Stage 3, pulmonary hypertension, tricuspid damage; and Stage 4, right ventricular dysfunction) has been proposed to aid the decision to potentially intervene in a more timely fashion.3 One of the main focus of these staging algorithms has been the changes that occur in the myocardium in severe AS, the pattern of LV remodelling and LVEF as the functional index.
The underpinning of our understanding the LV in severe AS comes from elegant work done in the recent past using cardiovascular magnetic resonance (CMR) to delineate the changes in the structure of the myocytes and the interstitial tissue in response to the pressure overload imposed by AS.4 The gross morphological manifestation of these changes is reflected in the pattern of LV remodelling and the functional consequences of these changes are best indicated by alterations in myocardial mechanics. Global longitudinal strain (GLS) has emerged as the most practical and distinctive measure of these abnormal myocardial mechanics in severe AS.5 In this study, Vollema et al.6 have provided data on clinical outcomes when GLS was included in the originally proposed staging system. In 616 patients with severe AS, incorporation of GLS divided by quintiles into the original staging classification demonstrated that the reclassified Stages 2–4 were all independently associated with all-cause mortality. This was unlike the original staging proposal where mortality was mainly driven by Stages 3 and 4. In other words, the addition of GLS to LVEF <50% into the risk stratification model detected more individuals with advanced LV damage, and thus this new Stage 2 was equally predictive of worse clinical outcomes as the original Stages 3 and 4. This single-centre, retrospective, and observational data included patients who all had symptomatic severe AS and underwent AVR. Thus, arguably GLS was not necessary to determine the timing of intervention. There is also no information on the presence of other causes, which may cause reduced GLS like coronary artery disease although the authors argue this may not have changed the findings. More critically, there is no data on myocardial structure (i.e. amyloidosis) or the type of LV remodelling in these patients, except that all of them had a degree of left ventricular hypertrophy (LVH). Notwithstanding these limitations, the report points to the fact that the incorporation of GLS in the staging risk assessment in severe AS may identify those may potentially benefit from timely AVR. The shift in paradigm regarding AVR with the introduction of TAVR and mini-invasive surgery will also have an influence on the risk during the post--AVR period. The choice of the procedure could be oriented depending on this integrated risk calculation including GLS when more than one option is available. Of course, this will also need prospective confirmation in asymptomatic severe AS.
The quest for risk assessment and identification of triggers for intervention in asymptomatic severe AS has included distinguishing clinical features, delineating features of valve biology which may predict rapid progression of the disease, and detection of adverse myocardial remodelling which signify adaptation or maladaptation to the pressure overload. Of these, imaging the adverse myocardial remodelling, denoted by the changes in the myocytes and the interstitium, has been the focus of a considerable amount of studies using CMR. Also, the gross morphological changes in the LV (type of remodelling) that accompanies these structural changes have also been delineated in these CMR studies. Collectively, the data from these studies show that ‘normal’ LV geometry, concentric remodelling (CR), concentric hypertrophy (CH), and eccentric hypertrophy (EH) are accompanied by distinctive changes in the myocytes and interstitium of the myocardium: early myocyte remodelling progressing to expansion of the interstitium (reactive fibrosis) and ultimately to loss of myocytes and scar (replacement fibrosis).7 What is less clear is the functional phenotype corresponding to these changes in the myocardium.
GLS has emerged as likely candidate among myocardial mechanics indices to be the indicator of the myocardial alterations, especially when the LVEF is normal. The latter is more likely to be seen in the compensated (Stages 0–1) and the subclinical decompensated stages (Stages 1–2), which are exemplified by CR and CH remodelling patterns. Structurally, the myocardium of these stages having varying degrees of myocyte and interstitial remodelling as shown in Figure 1. With regard to LVEF, multiple studies have now shown that LVEF of <60% in severe AS predicts worse outcomes even though the guidelines use EF of <50% as definition of LV dysfunction. In this study, the LVEF was <50% in the vast majority of patients in Stages 3 and 4. In Stage 2, where GLS had the most impact for reclassification, about one-third of the patients had EF <50% and it is unclear how many had EF between 50% and 60% (which would have signalled LV dysfunction). Even in Stage 1, LVEF was <50% in 20% of the patents, and only in Stage 0 was LVEF was >50%. Thus, it remains to be seen if the GLS would have unmasked subclinical LV dysfunction in the original Stage 2 if an EF >60% would have been used. But we do have meta-analysis data of GLS measurements in severe AS and LVEF >60%, which does suggest that it may have the discriminative potential to signal increased mortality at a cut-off of 14.7%.8 The increased mortality is presumed to be due to advanced LV damage. But, about a third of the patients in this meta-analysis had EF between 55% and 60%, and there is no data on LV mass, LV wall thickness, or myocardial structure. So, it remains an open question whether GLS would have been <14.7% during Stages 0–1 or even at Stages 1–2 (early decompensation).
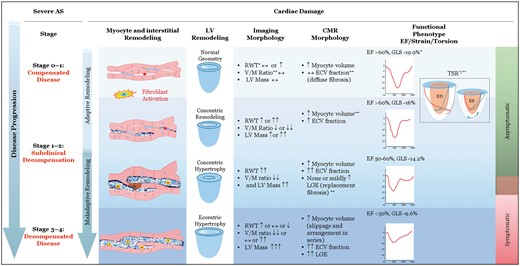
A proposed scheme connecting structural remodelling of the myocardium, morphological pattern of LV remodelling, correlative imaging morphology of the LV, corresponding CMR myocardial structure, and the functional phenotype defined by myocardial mechanics at various stages of severe aortic stenosis. ↔, normal/no change; ↑, ↑↑↑, = mildly or markedly increased; ↓, ↓↓, mildly or markedly decreased; ∗, echocardiography; ∗∗, Cardiovascular magnetic resonance imaging. ECV, extracellular volume; ED, end-diastole; EF, ejection fraction; ES, end-systole; GLS, global longitudinal strain; LGE, late gadolinium enhancement; RWT, relative wall thickness; TSR, torsion-shortening ratio, also see supplement for magnified picture and explanation; V/M, volume to mass.
Thus, GLS <14.7% may be the functional phenotype in the late subclinical decompensation (CH remodelling, diffuse, and replacement myocardial fibrosis). But, despite many arguments for and against the issue of load dependency, myocardial strain is fundamentally a load-dependent measure. GLS corrected for afterload (myocardial work index) and GLS end-systolic strain index are some of the approaches, which may yet improve the reliability of GLS to predict myocardial damage, adverse clinical outcomes, and reverse remodelling of the LV post-AVR.9 Then there is the question of whether a higher GLS cut-off (>14.7% but 20%) will be able to predict predominant myocyte hypertrophy and minimal diffuse fibrosis of Stages 0–1 and early decompensation of Stages 1–2. This is an important question to address. The reversal of adverse myocardial and LV remodelling (regression of LVH and LV mass) are more likely at these compensated (Stages 0–1) and early decompensated stage (Stages 1–2).4 And, regression of LVH and LV mass post-AVR are key predictors of survival post-AVR.3 Furthermore, even normal and CR geometry in hypertrophic remodelling is associated with increased mortality.10 The normal geometry in severe AS is particularly intriguing. It resembles the physiological hypertrophy that occurs with endurance training and pregnancy where a mild dilatation of the LV (10–20%) occurs with a co-ordinated increase in wall thickness so that the RWT is normal.11 So, it is like the EH pattern of LV remodelling but unlike pathological EH, the LV mass in normal. There is possibly little if any expansion of interstitial space at this stage. CR remodelling has increased RWT and minimal expansion of extracellular matrix and is likely a reversible alteration much like Stage A heart failure with preserved EF. If GLS of <14.7% is marker of myocardial fibrosis, perhaps that cut-off my not be able to detect the myocyte contractile dysfunction which may occur with the normal geometry and CR remodelling when there is little no diffuse fibrosis. And GLS >14.7% may be more contaminated by the effect of the afterload of severe AS than the cut-off <14.7%? There is also evidence that GLS may not reliably indicate early myocardial remodelling in the form of expansion of the extracellular matrix determined by CMR.12 Therefore, we still need to investigate whether another cut-off of GLS alone or GLS together with another index of myocardial performance is able to detect contractile dysfunction before the onset of significant myocardial diffuse fibrosis and scar. In this regard, the role of increased LV torsion (or twist or apical rotation) in the presence of a “normal” GLS (albeit rarely >20% in severe AS), and EF >60%, needs to be explored. While, measurements of these indices can be cumbersome compared to GLS, they are rooted in sound physiological basis. Thus, they may have the potential to detect occult myocardial dysfunction at the compensated stages (Stage 0–1) and in the CR phase of subclinical decompensation of severe AS.13,14
In conclusion, GLS measurement by speckle tracking echocardiography has clearly opened up possibilities in our pursuit to define the functional phenotype, which accompany the adverse structural remodelling of the myocardium in severe AS. However, there is work to be done yet to identify other functional correlates of myocardial remodelling which will improve the predictive reliability of GLS in early stages of severe AS. With regard to the value of GLS in severe AS it can be said as the saying goes, ‘Only half the story is true. The rest is necessary’.
Conflict of interest: none declared.
The opinions expressed in this article are not necessarily those of the Editors of EHJCI, the European Heart Rhythm Association or the European Society of Cardiology.



