-
PDF
- Split View
-
Views
-
Cite
Cite
Marco Spagnolo, Giovanni Occhipinti, Claudio Laudani, Antonio Greco, Davide Capodanno, Periprocedural myocardial infarction and injury, European Heart Journal. Acute Cardiovascular Care, Volume 13, Issue 5, May 2024, Pages 433–445, https://doi.org/10.1093/ehjacc/zuae014
Close - Share Icon Share
Abstract
Periprocedural myocardial infarction (PMI) and injury, pertinent to both cardiac and non-cardiac procedures, have gained increasing recognition in clinical practice. Over time, diverse definitions for diagnosing PMI have been developed and validated among patient populations undergoing coronary revascularization. However, this variety in definitions presents considerable challenges in clinical settings and complicates both the design and interpretation of clinical trials. The necessity to accurately diagnose PMI has spurred significant interest in establishing universally accepted and prognostically meaningful thresholds for cardiac biomarkers elevation and supportive ancillary criteria. In fact, elevations in cardiac biomarkers in line with the 4th Universal Definition of Myocardial Infarction, have been extensively confirmed to be associated with increased mortality and cardiovascular events. In the context of non-coronary cardiac procedures, such as Transcatheter Aortic Valve Implantation, there is a growing acknowledgment of both the high incidence rates and the adverse impact of PMI on patient outcomes. Similarly, emerging research underscores the significance of PMI and injury in non-cardiac surgery, highlighting the urgent need for effective prevention and risk management strategies in this domain.
Introduction
Percutaneous coronary intervention (PCI) and coronary artery bypass grafting (CABG) are performed every year in ∼500 000 and 200 000 patients with atherosclerotic coronary artery disease (CAD) requiring myocardial revascularization, respectively.1,2 Despite notable periprocedural refinements and safety advancements over the last decades, both interventions carry a small but sizeable risk of acute complications, including periprocedural death, stroke, and myocardial infarction (MI). While periprocedural death and stroke are relatively infrequent, being reported in less than 3% of patients undergoing revascularization, periprocedural myocardial infarction (PMI) occurs in a larger proportion of subjects, with an incidence that has variably been reported between ∼2% and ∼15% (Figure 1).3–5 PMI is a potential adverse event also in the context of other transcatheter and surgical procedures, with an incidence approaching 14% in patients undergoing non-coronary cardiac interventions (e.g. transcatheter aortic valve implantation [TAVI] or surgical aortic valve replacement) and 15% in high-risk patients undergoing non-cardiac surgery.6,7 Importantly, PMI has been associated with adverse outcomes in all these settings.8–10

Incidences of adverse events and periprocedural myocardial infarction in landmark randomized trials of coronary revascularization. This figure illustrates incidences of death, myocardial infarction and stroke in major randomized trial of coronary revascularization, with a special focus on trial-defined periprocedural myocardial infarction. Numeric data followed by an asterisk (*) are esteemed from Kaplan Meier curves. Abbreviations: MI, myocardial infarction.
There is a growing body of evidence that differentiates PMI from isolated cardiac biomarkers elevation occurring after a procedure in the absence of clinical or diagnostic signs of ischaemia, a condition known as periprocedural myocardial injury. The diagnosis of both PMI and injury has sparked significant interest in the establishment of universally accepted and prognostically meaningful thresholds of cardiac biomarkers elevation. Over time, various consortia and international cardiology societies have published different definitions, each based on criteria that differ in the type and/or cut-off levels of laboratory cardiac biomarkers, as well as in the requirement of supplementary diagnostic criteria.11–13 The absence of robust, comparative data to establish the prognostic significance of these proposed definitions has limited their application in clinical research and practice, fuelling an ongoing controversy.14 This gap in knowledge is particularly evident in the dispute surrounding the interpretation of landmark trials of PCI and/or CABG using PMI as a component of composite endpoints.14
This article offers a state-of-the-art review of PMI and periprocedural myocardial injury in various clinical contexts, such as myocardial revascularization, non-coronary cardiac procedures, and non-cardiac surgery. Special emphasis is placed on examining the characteristics, mitigation strategies, and unmet needs related to these conditions within the realm of PCI, which stands as the pioneering context in PMI and injury research.
Periprocedural myocardial infarction and injury after coronary revascularization
Mechanisms
PMI and injury during PCI and CABG have multiple causes (Figure 2). In the PCI setting, the most common mechanisms involve side-branch occlusion and distal coronary embolization of thrombus or plaque debris, accounting for as much as 60% and 10–15% of cases, respectively.15–20 Additionally, certain steps of PCI, such as lesion preparation, balloon angioplasty, and the use of coronary devices (e.g. intracoronary imaging catheters and devices for plaque modification), can directly or indirectly contribute to myocardial damage. In fact, these procedures increase the risk of iatrogenic events, including air embolization, acute stent thrombosis, or coronary dissection.
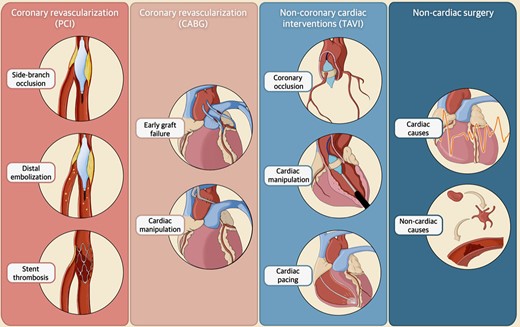
Aetiologies of periprocedural myocardial infarction and injury in cardiac and non-cardiac interventions. The figure illustrates different aetiologies underlying periprocedural myocardial infarction and injury in cardiac (i.e. percutaneous coronary intervention, coronary artery bypass grafting, transcatheter aortic valve implantation) and non-cardiac interventions. Abbreviations: CABG, coronary artery bypass grafting; PCI, percutaneous coronary intervention; TAVI, transcatheter aortic valve implantation.
Conversely, in the case of CABG, early graft failure is the primary cause of periprocedural myocardial injury, responsible for up to 12% of cases, although only up to 3% are clinically evident.21 Various mechanisms have been proposed to explain this event, encompassing both mechanical (e.g. graft kinking or thrombosis, anastomosis stenosis or distal stenoses) and metabolic factors (e.g. global hypoperfusion).22,23 Less frequent causes of myocardial injury in the context of CABG are cardiac manipulation, the use of cardiopulmonary bypass, reperfusion injury, prolonged hypotension, inadequate myocardial protection, cardioplegia, or tachyarrhythmias.24,25
Definitions
Current diagnostic guidelines for PMI and injury have been proposed by leading professional collaborations, including the European Society of Cardiology (ESC), American College of Cardiology (ACC), American Heart Association (AHA), World Heart Federation (WHF), Academic Research Consortium (ARC), and Society for Cardiovascular Angiography and Interventions (SCAI).11–13 While the majority of these guidelines recommend cardiac troponin (cTn) or high-sensitivity cTn (hs-cTn) as biomarkers of choice, SCAI primarily recommends using the isoform MB of creatine kinase (CK-MB), or cTn when CK-MB is unavailable.
The 4th Universal Definition of Myocardial Infarction (UDMI), as established by ESC, ACC, AHA, and WHF, defines periprocedural myocardial injury as any increase in cTn (or hs-cTn) above the 99th percentile upper reference limit (URL) within 48 to 72 h following PCI. By contrast, the ARC-2 set a considerably higher threshold (i.e. an increase greater than 70 times the 99th percentile URL), while SCAI has not provided a precise definition of this condition (Table 1, Figure 3).11–13 In a consensus document of the ESC Working Group on Cellular Biology of the Heart and European Association of Percutaneous Cardiovascular Interventions, major and minor periprocedural myocardial injury have been defined as a rise of at least 5 times and among 1 and 5 times the 99th percentile URL, respectively.23
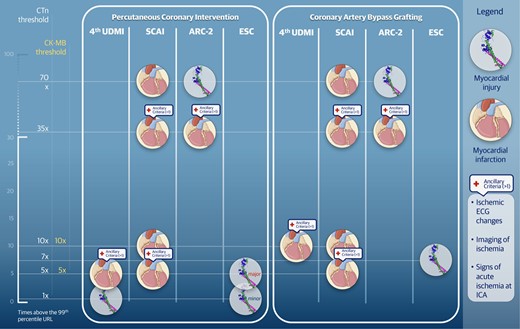
Biomarker concentration cut-offs for the diagnosis of periprocedural myocardial infarction and injury according to main definitions. The figure is informed by publications of consensus documents. However, information classified under the European Society of Cardiology derives from two different consensus documents based on the specific subset (i.e. percutaneous coronary intervention and coronary artery bypass grafting). Abbreviations: ARC, Academic Research Consortium; CK-MB, creatin kinase-myocardial band; CTn, Cardiac Troponin; ESC, European Society of Cardiology. SCAI, Society for Cardiovascular Angiography and Interventions; 4th UDMI, Universal Definition of Myocardial Infarction, fourth iteration; URL, upper reference limit.
| . | 4th UDMI . | ESC/EAPCI . | SCAI . | ARC-2 . |
|---|---|---|---|---|
| Patients with normal baseline cardiac biomarkers | ||||
| cTn threshold | ||||
| >1× above the 99th percentile URL | • | (•) | ||
| >5× above the 99th percentile URL | [•] | |||
| >7× above the 99th percentile URL (in CABG) | • | |||
| >70× above the 99th percentile URL | • | |||
| Patients with elevated baseline cardiac biomarkers | ||||
| High-sensitivity cTn threshold | ||||
| >20% above the 99th percentile URLa | • | |||
| >70× above the 99th percentile URL | • | |||
| . | 4th UDMI . | ESC/EAPCI . | SCAI . | ARC-2 . |
|---|---|---|---|---|
| Patients with normal baseline cardiac biomarkers | ||||
| cTn threshold | ||||
| >1× above the 99th percentile URL | • | (•) | ||
| >5× above the 99th percentile URL | [•] | |||
| >7× above the 99th percentile URL (in CABG) | • | |||
| >70× above the 99th percentile URL | • | |||
| Patients with elevated baseline cardiac biomarkers | ||||
| High-sensitivity cTn threshold | ||||
| >20% above the 99th percentile URLa | • | |||
| >70× above the 99th percentile URL | • | |||
(•) Minor periprocedural myocardial injury; [•] Major periprocedural myocardial injury.
aConsidering stable or falling baseline cardiac biomarkers.
Abbreviations: ARC, Academic Research Consortium 2; cTn, cardiac troponin; CABG, Coronary Artery Bypass Grafting; EAPCI, European Association of Percutaneous Cardiovascular Interventions; ESC, European Society of Cardiology; SCAI, Society for Cardiovascular Angiography and Interventions; UDMI, Universal Definition of Myocardial Infarction; URL, upper reference limit.
| . | 4th UDMI . | ESC/EAPCI . | SCAI . | ARC-2 . |
|---|---|---|---|---|
| Patients with normal baseline cardiac biomarkers | ||||
| cTn threshold | ||||
| >1× above the 99th percentile URL | • | (•) | ||
| >5× above the 99th percentile URL | [•] | |||
| >7× above the 99th percentile URL (in CABG) | • | |||
| >70× above the 99th percentile URL | • | |||
| Patients with elevated baseline cardiac biomarkers | ||||
| High-sensitivity cTn threshold | ||||
| >20% above the 99th percentile URLa | • | |||
| >70× above the 99th percentile URL | • | |||
| . | 4th UDMI . | ESC/EAPCI . | SCAI . | ARC-2 . |
|---|---|---|---|---|
| Patients with normal baseline cardiac biomarkers | ||||
| cTn threshold | ||||
| >1× above the 99th percentile URL | • | (•) | ||
| >5× above the 99th percentile URL | [•] | |||
| >7× above the 99th percentile URL (in CABG) | • | |||
| >70× above the 99th percentile URL | • | |||
| Patients with elevated baseline cardiac biomarkers | ||||
| High-sensitivity cTn threshold | ||||
| >20% above the 99th percentile URLa | • | |||
| >70× above the 99th percentile URL | • | |||
(•) Minor periprocedural myocardial injury; [•] Major periprocedural myocardial injury.
aConsidering stable or falling baseline cardiac biomarkers.
Abbreviations: ARC, Academic Research Consortium 2; cTn, cardiac troponin; CABG, Coronary Artery Bypass Grafting; EAPCI, European Association of Percutaneous Cardiovascular Interventions; ESC, European Society of Cardiology; SCAI, Society for Cardiovascular Angiography and Interventions; UDMI, Universal Definition of Myocardial Infarction; URL, upper reference limit.
For the diagnosis of PMI in patients who have normal cardiac biomarkers levels at baseline, three definitions apply (i.e. the 4th UDMI, ARC-2, and SCAI), all incorporating specific biomarker thresholds along with additional diagnostic criteria, such as electrocardiographic, imaging, and angiographic signs of ischaemia.11–13 Notably, the SCAI definition differs from the 4th UDMI and ARC-2 as it requires additional diagnostic criteria only for lower cTn increases (i.e. > 5 and >35 times the 99th percentile URL, respectively); conversely, ancillary criteria are not mandatory for higher biomarker levels (i.e. >10 and >70 times the 99th percentile URL, respectively).11–13 In contrast, the 4th UDMI and ARC-2 definitions consistently require the presence of ancillary criteria (e.g. new ischaemic electrocardiographic changes, imaging evidence of ischaemia, angiographic findings of acute ischaemia). A detailed description of these definitions, including their respective biomarker cut-offs and the requirements for ancillary criteria, is presented in Table 2 and Figure 3.
Definitions of periprocedural myocardial infarction in patients undergoing percutaneous coronary intervention
| . | 4th UDMI . | SCAI . | ARC-2 . |
|---|---|---|---|
| cTn threshold | |||
| >5× above the 99th percentile URL | • | ||
| >35× above the 99th percentile URL | • | • | |
| >70× above the 99th percentile URL | (•) | ||
| CK-MB threshold | |||
| >5× above the 99th percentile URL | • | ||
| >10× above the 99th percentile URL | (•) | ||
| Ancillary criteria | Required (>1) | Additional | Required |
| New ischaemic electrocardiographic changes | • | ||
| New Q waves | • | • | • |
| New LBBB | • | ||
| Imaging evidence of ischaemia | • | • | |
| Angiographic findings of acute ischaemia | • | • | |
| . | 4th UDMI . | SCAI . | ARC-2 . |
|---|---|---|---|
| cTn threshold | |||
| >5× above the 99th percentile URL | • | ||
| >35× above the 99th percentile URL | • | • | |
| >70× above the 99th percentile URL | (•) | ||
| CK-MB threshold | |||
| >5× above the 99th percentile URL | • | ||
| >10× above the 99th percentile URL | (•) | ||
| Ancillary criteria | Required (>1) | Additional | Required |
| New ischaemic electrocardiographic changes | • | ||
| New Q waves | • | • | • |
| New LBBB | • | ||
| Imaging evidence of ischaemia | • | • | |
| Angiographic findings of acute ischaemia | • | • | |
(•) No ancillary criteria are required to meet the diagnosis.
Abbreviations: ARC, Academic Research Consortium, second iteration; CK-MB, creatin kinase-myocardial band; cTn, cardiac troponin; LBBB, left bundle branch block; SCAI, Society for Cardiovascular Angiography and Interventions; 4th UDMI, Universal Definition of Myocardial Infarction, fourth iteration; URL, upper reference limit.
Definitions of periprocedural myocardial infarction in patients undergoing percutaneous coronary intervention
| . | 4th UDMI . | SCAI . | ARC-2 . |
|---|---|---|---|
| cTn threshold | |||
| >5× above the 99th percentile URL | • | ||
| >35× above the 99th percentile URL | • | • | |
| >70× above the 99th percentile URL | (•) | ||
| CK-MB threshold | |||
| >5× above the 99th percentile URL | • | ||
| >10× above the 99th percentile URL | (•) | ||
| Ancillary criteria | Required (>1) | Additional | Required |
| New ischaemic electrocardiographic changes | • | ||
| New Q waves | • | • | • |
| New LBBB | • | ||
| Imaging evidence of ischaemia | • | • | |
| Angiographic findings of acute ischaemia | • | • | |
| . | 4th UDMI . | SCAI . | ARC-2 . |
|---|---|---|---|
| cTn threshold | |||
| >5× above the 99th percentile URL | • | ||
| >35× above the 99th percentile URL | • | • | |
| >70× above the 99th percentile URL | (•) | ||
| CK-MB threshold | |||
| >5× above the 99th percentile URL | • | ||
| >10× above the 99th percentile URL | (•) | ||
| Ancillary criteria | Required (>1) | Additional | Required |
| New ischaemic electrocardiographic changes | • | ||
| New Q waves | • | • | • |
| New LBBB | • | ||
| Imaging evidence of ischaemia | • | • | |
| Angiographic findings of acute ischaemia | • | • | |
(•) No ancillary criteria are required to meet the diagnosis.
Abbreviations: ARC, Academic Research Consortium, second iteration; CK-MB, creatin kinase-myocardial band; cTn, cardiac troponin; LBBB, left bundle branch block; SCAI, Society for Cardiovascular Angiography and Interventions; 4th UDMI, Universal Definition of Myocardial Infarction, fourth iteration; URL, upper reference limit.
In patients with pre-existing elevated cardiac biomarkers or conditions affecting biomarker kinetics [e.g. acute coronary syndrome (ACS), end-stage renal disease, musculoskeletal diseases], diagnosing PMI is more challenging. The ARC-2 has refrained from defining PMI in this context, while both the 4th UDMI and SCAI task forces suggest using relative biomarker increases rather than baseline levels.11–13
In the context of CABG, while the ARC-2 and SCAI definitions recommend the same cut-offs used for PCI, the 4th UDMI advocates for the use of higher cTn thresholds due to the extensive cardiac manipulation associated with surgery.11–13 In 2017, a consensus document by the ESC Working Group on Cellular Biology of the Heart and European Association of Percutaneous Cardiovascular Interventions, suggested specific cTn thresholds (i.e. >7 times the 99th percentile URL) for CABG-related periprocedural myocardial injury, while using the same cut-off as the 4th UDMI for PMI (Table 3).23
Definitions of periprocedural myocardial infarction in patients undergoing coronary artery bypass grafting
| . | 4th UDMI . | SCAI . | ARC-2 . |
|---|---|---|---|
| cTn threshold | |||
| >10x above the 99th percentile URL | • | ||
| >35x above the 99th percentile URL | • | • | |
| >70x above the 99th percentile URL | (•) | ||
| CK-MB threshold | |||
| >5x above the 99th percentile URL | • | ||
| >10x above the 99th percentile URL | (•) | ||
| Ancillary criteria | |||
| New Q waves | • | • | • |
| New LBBB | • | ||
| Imaging evidence of ischaemia | • | • | |
| Angiographic findings of acute ischaemia | • | • | |
| . | 4th UDMI . | SCAI . | ARC-2 . |
|---|---|---|---|
| cTn threshold | |||
| >10x above the 99th percentile URL | • | ||
| >35x above the 99th percentile URL | • | • | |
| >70x above the 99th percentile URL | (•) | ||
| CK-MB threshold | |||
| >5x above the 99th percentile URL | • | ||
| >10x above the 99th percentile URL | (•) | ||
| Ancillary criteria | |||
| New Q waves | • | • | • |
| New LBBB | • | ||
| Imaging evidence of ischaemia | • | • | |
| Angiographic findings of acute ischaemia | • | • | |
(•) No ancillary criteria are required to meet the diagnosis.
Abbreviations: ARC-2, Academic Research Consortium, second iteration; CK-MB, creatin kinase-myocardial band; cTn, cardiac troponin; LBBB, left bundle branch block; SCAI, Society for Cardiovascular Angiography and Interventions; 4th UDMI, Universal Definition of Myocardial Infarction, fourth iteration; URL, upper reference limit.
Definitions of periprocedural myocardial infarction in patients undergoing coronary artery bypass grafting
| . | 4th UDMI . | SCAI . | ARC-2 . |
|---|---|---|---|
| cTn threshold | |||
| >10x above the 99th percentile URL | • | ||
| >35x above the 99th percentile URL | • | • | |
| >70x above the 99th percentile URL | (•) | ||
| CK-MB threshold | |||
| >5x above the 99th percentile URL | • | ||
| >10x above the 99th percentile URL | (•) | ||
| Ancillary criteria | |||
| New Q waves | • | • | • |
| New LBBB | • | ||
| Imaging evidence of ischaemia | • | • | |
| Angiographic findings of acute ischaemia | • | • | |
| . | 4th UDMI . | SCAI . | ARC-2 . |
|---|---|---|---|
| cTn threshold | |||
| >10x above the 99th percentile URL | • | ||
| >35x above the 99th percentile URL | • | • | |
| >70x above the 99th percentile URL | (•) | ||
| CK-MB threshold | |||
| >5x above the 99th percentile URL | • | ||
| >10x above the 99th percentile URL | (•) | ||
| Ancillary criteria | |||
| New Q waves | • | • | • |
| New LBBB | • | ||
| Imaging evidence of ischaemia | • | • | |
| Angiographic findings of acute ischaemia | • | • | |
(•) No ancillary criteria are required to meet the diagnosis.
Abbreviations: ARC-2, Academic Research Consortium, second iteration; CK-MB, creatin kinase-myocardial band; cTn, cardiac troponin; LBBB, left bundle branch block; SCAI, Society for Cardiovascular Angiography and Interventions; 4th UDMI, Universal Definition of Myocardial Infarction, fourth iteration; URL, upper reference limit.
Incidence
Unsurprisingly, the incidence rates of PMI and myocardial injury vary depending on their definitions.5,14 This variation is particularly evident for myocardial injury, as this condition relies solely on biomarkers with no additional criteria. Consequently, the reported incidence of myocardial injury ranges from 4.3% to 79.8% in the context of PCI, with more sensitive markers like hs-cTn resulting in higher rates.26–28 Similarly, the incidence of PMI differs across definitions due to variations in biomarker elevation thresholds. The 4th UDMI is more sensitive, with PMI rates as high as ∼15%, while the more specific ARC-2 and SCAI definitions are associated with lower rates of PMI (i.e. ∼2%) in patients with chronic coronary syndrome.5
In the context of CABG, the choice of biomarker class used to estimate the incidence of PMI and injury has a notable effect. For example, the incidence rate of CABG-related myocardial injury has been reported at ∼95% with cTnI, and at ∼30% with CK-MB.13 Similar to PCI, different thresholds for biomarker elevation have a significant impact on the incidence rates of CABG-related PMI.23 In the VISION study, recommended troponin thresholds of >10, ≥ 35, and ≥70 times the URL (roughly corresponding to the thresholds introduced by the 4th UDMI, ARC-2, and SCAI) were exceeded in 97.5%, 89.4%, and 74.7% of patients, respectively, within the first day after surgery.29 In the EXCEL trial, the SCAI definition demonstrated greater sensitivity in detecting CABG-related PMI than the 4th UDMI (6.1% vs. 2.2%).6,14 This difference was confirmed in a cohort study of 2829 patients undergoing CABG, reporting incidences of 49.5%, 2.9%, and 2.6% using the SCAI, 4th UDMI, and ARC-2 criteria, respectively.30 In the same study, 51.5% of patients were diagnosed with an ARC-defined myocardial injury, while 9.9% had also electrocardiogram changes.
Prognosis
Studies investigating the short-term prognostic implications of periprocedural myocardial injury after PCI yielded mixed results (Table 4), with recent and larger studies contradicting earlier studies reporting a significant association with 1-year major adverse cardiac events (MACE) and death.26,27 Part of the reason for these mixed findings may lie in the different biomarkers used in these studies.28 In a pooled analysis of 9 081 patients undergoing elective PCI from 10 studies, only major periprocedural myocardial injury (defined as a post-PCI troponin increase ≥5 times the 99th percentile URL) showed a significant association with death at 1 year, regardless of the assay adopted [e.g. conventional or high-sensitivity cTn (hs-cTn)].27 More compelling associations of PMI have been reported with 30-day MACE, 1-year MACE, all-cause death, and cardiovascular death.27
Studies evaluating the prognostic impact of periprocedural myocardial injury in percutaneous coronary interventions
| Study . | Study design . | Year . | Population . | Definition of minor injury . | Definition of major injury . | Troponin assay . | Incidence of minor injury . | Incidence of major injury . | Results . |
|---|---|---|---|---|---|---|---|---|---|
| Liou et al. | Retrospective, single-centre | 2015 | 459 mixed CCS and ACS patients undergoing PCI | >1× above the 99th percentile URL or >20% rise from baseline | NR | hs-cTnT | 4.3% | NR | Periprocedural myocardial injury independently predicted 1-year MACE |
| Christensen et al. | Historical prospective, single-centre. | 2016 | 2760 CCS patients undergoing elective PCI. | >1× above the 99th percentile URL 1–5× above the 99th percentile URL | >5× 99th URL | cTnT and CK-MB | 37.7% (cTnT) 23.4% (CK-MB) | 15.2% (cTnT) 4.1% (CK-MB) | No significant predictive role for all-cause death Dose-dependent relationship with the combined end-point of all-cause death and heart failure |
| Di Serafino et al. | Retrospective, propensity matched, single-centre. | 2016 | 1110 single-vessel CTO patients undergoing elective PCI | >5× above the 99th percentile URL | NR | cTnI | 4.7% | NR | Periprocedural myocardial injury predicted 1-year cardiac death and MACE, but did not predict long-term outcomes |
| Ferreira et al. | Retrospective, single-centre. | 2017 | 383 CCS patients undergoing elective PCI | >5× above the 99th percentile URL | NR | cTnI | 41.3% | NR | Dose-response relationship between any cTnI elevation and 1-year mortality Threshold of >5× 99th URL strongly predicted 1-year mortality |
| Cottens et al. | Retrospective, two-centre | 2018 | 409 CTO patients undergoing elective PCI | >1× above the 99th percentile | >5× 99th URL | hs-cTnT | 85% | 42% | No predictive role of major and minor periprocedural myocardial injury |
| Zeitouni et al. | Prospective, single-centre | 2018 | 1390 CCS patients undergoing elective PCI | >1× above the 99th percentile | NA | hs-cTnT | 21.6 | NA | Periprocedural myocardial injury predicted 30-day, but not 1-year, events |
| Silvain et al. | Pooled analysis | 2020 | 9081 CCS patients undergoing elective PCI | >1× above the 99th percentile | >5× 99th URL | Mixed cTn and hs-cTn | 52.8% (ranging from 23.8% to 79.8% depending on the assay used) | 18.2% (ranging from 10.1% to 25.8% depending on the assay used) | Periprocedural myocardial injury did not predict 1-year mortality Major periprocedural myocardial injury was a strong independent predictor of 1-year mortality, independently of the biomarker assay used |
| Zhou et al. | Retrospective, single-centre | 2021 | 3249 CCS patients undergoing elective PCI | > 99% URL | >5× 99th URL | hs-cTnT | 78.3% | 23.4% | Neither minor nor major periprocedural myocardial injury were predictor of long-term MACE. Higher thresholds hold prognostic significance |
| Wang et al. | Prospective, single-centre | 2021 | 4013 patients without myocardial necrosis undergoing LMCAD PCI | >1× 99th URL | NA | CK-MB and cTnI | 16.3% (CK-MB)67.6% (cTnI) | NA | Only CK-MB-defined periprocedural myocardial injury was predictive of 3-year cardiovascular death and all-cause death |
| Study . | Study design . | Year . | Population . | Definition of minor injury . | Definition of major injury . | Troponin assay . | Incidence of minor injury . | Incidence of major injury . | Results . |
|---|---|---|---|---|---|---|---|---|---|
| Liou et al. | Retrospective, single-centre | 2015 | 459 mixed CCS and ACS patients undergoing PCI | >1× above the 99th percentile URL or >20% rise from baseline | NR | hs-cTnT | 4.3% | NR | Periprocedural myocardial injury independently predicted 1-year MACE |
| Christensen et al. | Historical prospective, single-centre. | 2016 | 2760 CCS patients undergoing elective PCI. | >1× above the 99th percentile URL 1–5× above the 99th percentile URL | >5× 99th URL | cTnT and CK-MB | 37.7% (cTnT) 23.4% (CK-MB) | 15.2% (cTnT) 4.1% (CK-MB) | No significant predictive role for all-cause death Dose-dependent relationship with the combined end-point of all-cause death and heart failure |
| Di Serafino et al. | Retrospective, propensity matched, single-centre. | 2016 | 1110 single-vessel CTO patients undergoing elective PCI | >5× above the 99th percentile URL | NR | cTnI | 4.7% | NR | Periprocedural myocardial injury predicted 1-year cardiac death and MACE, but did not predict long-term outcomes |
| Ferreira et al. | Retrospective, single-centre. | 2017 | 383 CCS patients undergoing elective PCI | >5× above the 99th percentile URL | NR | cTnI | 41.3% | NR | Dose-response relationship between any cTnI elevation and 1-year mortality Threshold of >5× 99th URL strongly predicted 1-year mortality |
| Cottens et al. | Retrospective, two-centre | 2018 | 409 CTO patients undergoing elective PCI | >1× above the 99th percentile | >5× 99th URL | hs-cTnT | 85% | 42% | No predictive role of major and minor periprocedural myocardial injury |
| Zeitouni et al. | Prospective, single-centre | 2018 | 1390 CCS patients undergoing elective PCI | >1× above the 99th percentile | NA | hs-cTnT | 21.6 | NA | Periprocedural myocardial injury predicted 30-day, but not 1-year, events |
| Silvain et al. | Pooled analysis | 2020 | 9081 CCS patients undergoing elective PCI | >1× above the 99th percentile | >5× 99th URL | Mixed cTn and hs-cTn | 52.8% (ranging from 23.8% to 79.8% depending on the assay used) | 18.2% (ranging from 10.1% to 25.8% depending on the assay used) | Periprocedural myocardial injury did not predict 1-year mortality Major periprocedural myocardial injury was a strong independent predictor of 1-year mortality, independently of the biomarker assay used |
| Zhou et al. | Retrospective, single-centre | 2021 | 3249 CCS patients undergoing elective PCI | > 99% URL | >5× 99th URL | hs-cTnT | 78.3% | 23.4% | Neither minor nor major periprocedural myocardial injury were predictor of long-term MACE. Higher thresholds hold prognostic significance |
| Wang et al. | Prospective, single-centre | 2021 | 4013 patients without myocardial necrosis undergoing LMCAD PCI | >1× 99th URL | NA | CK-MB and cTnI | 16.3% (CK-MB)67.6% (cTnI) | NA | Only CK-MB-defined periprocedural myocardial injury was predictive of 3-year cardiovascular death and all-cause death |
Abbreviations: ACS, acute coronary syndromes; CCS, chronic coronary syndromes; CK-MB, creatine kinase-myocardial band; cTn, cardiac troponin; cTnI, cardiac troponin I; CTO, coronary total occlusion; cTnT, cardiac troponin T; hs-cTn, high-sensitive cardiac troponin; hs-cTnT, high-sensitive cardiac troponin T; LMCAD, left main coronary artery disease; MACE, major adverse cardiovascular events; NA, not available; PCI, percutaneous coronary intervention; URL, upper reference limit.
Studies evaluating the prognostic impact of periprocedural myocardial injury in percutaneous coronary interventions
| Study . | Study design . | Year . | Population . | Definition of minor injury . | Definition of major injury . | Troponin assay . | Incidence of minor injury . | Incidence of major injury . | Results . |
|---|---|---|---|---|---|---|---|---|---|
| Liou et al. | Retrospective, single-centre | 2015 | 459 mixed CCS and ACS patients undergoing PCI | >1× above the 99th percentile URL or >20% rise from baseline | NR | hs-cTnT | 4.3% | NR | Periprocedural myocardial injury independently predicted 1-year MACE |
| Christensen et al. | Historical prospective, single-centre. | 2016 | 2760 CCS patients undergoing elective PCI. | >1× above the 99th percentile URL 1–5× above the 99th percentile URL | >5× 99th URL | cTnT and CK-MB | 37.7% (cTnT) 23.4% (CK-MB) | 15.2% (cTnT) 4.1% (CK-MB) | No significant predictive role for all-cause death Dose-dependent relationship with the combined end-point of all-cause death and heart failure |
| Di Serafino et al. | Retrospective, propensity matched, single-centre. | 2016 | 1110 single-vessel CTO patients undergoing elective PCI | >5× above the 99th percentile URL | NR | cTnI | 4.7% | NR | Periprocedural myocardial injury predicted 1-year cardiac death and MACE, but did not predict long-term outcomes |
| Ferreira et al. | Retrospective, single-centre. | 2017 | 383 CCS patients undergoing elective PCI | >5× above the 99th percentile URL | NR | cTnI | 41.3% | NR | Dose-response relationship between any cTnI elevation and 1-year mortality Threshold of >5× 99th URL strongly predicted 1-year mortality |
| Cottens et al. | Retrospective, two-centre | 2018 | 409 CTO patients undergoing elective PCI | >1× above the 99th percentile | >5× 99th URL | hs-cTnT | 85% | 42% | No predictive role of major and minor periprocedural myocardial injury |
| Zeitouni et al. | Prospective, single-centre | 2018 | 1390 CCS patients undergoing elective PCI | >1× above the 99th percentile | NA | hs-cTnT | 21.6 | NA | Periprocedural myocardial injury predicted 30-day, but not 1-year, events |
| Silvain et al. | Pooled analysis | 2020 | 9081 CCS patients undergoing elective PCI | >1× above the 99th percentile | >5× 99th URL | Mixed cTn and hs-cTn | 52.8% (ranging from 23.8% to 79.8% depending on the assay used) | 18.2% (ranging from 10.1% to 25.8% depending on the assay used) | Periprocedural myocardial injury did not predict 1-year mortality Major periprocedural myocardial injury was a strong independent predictor of 1-year mortality, independently of the biomarker assay used |
| Zhou et al. | Retrospective, single-centre | 2021 | 3249 CCS patients undergoing elective PCI | > 99% URL | >5× 99th URL | hs-cTnT | 78.3% | 23.4% | Neither minor nor major periprocedural myocardial injury were predictor of long-term MACE. Higher thresholds hold prognostic significance |
| Wang et al. | Prospective, single-centre | 2021 | 4013 patients without myocardial necrosis undergoing LMCAD PCI | >1× 99th URL | NA | CK-MB and cTnI | 16.3% (CK-MB)67.6% (cTnI) | NA | Only CK-MB-defined periprocedural myocardial injury was predictive of 3-year cardiovascular death and all-cause death |
| Study . | Study design . | Year . | Population . | Definition of minor injury . | Definition of major injury . | Troponin assay . | Incidence of minor injury . | Incidence of major injury . | Results . |
|---|---|---|---|---|---|---|---|---|---|
| Liou et al. | Retrospective, single-centre | 2015 | 459 mixed CCS and ACS patients undergoing PCI | >1× above the 99th percentile URL or >20% rise from baseline | NR | hs-cTnT | 4.3% | NR | Periprocedural myocardial injury independently predicted 1-year MACE |
| Christensen et al. | Historical prospective, single-centre. | 2016 | 2760 CCS patients undergoing elective PCI. | >1× above the 99th percentile URL 1–5× above the 99th percentile URL | >5× 99th URL | cTnT and CK-MB | 37.7% (cTnT) 23.4% (CK-MB) | 15.2% (cTnT) 4.1% (CK-MB) | No significant predictive role for all-cause death Dose-dependent relationship with the combined end-point of all-cause death and heart failure |
| Di Serafino et al. | Retrospective, propensity matched, single-centre. | 2016 | 1110 single-vessel CTO patients undergoing elective PCI | >5× above the 99th percentile URL | NR | cTnI | 4.7% | NR | Periprocedural myocardial injury predicted 1-year cardiac death and MACE, but did not predict long-term outcomes |
| Ferreira et al. | Retrospective, single-centre. | 2017 | 383 CCS patients undergoing elective PCI | >5× above the 99th percentile URL | NR | cTnI | 41.3% | NR | Dose-response relationship between any cTnI elevation and 1-year mortality Threshold of >5× 99th URL strongly predicted 1-year mortality |
| Cottens et al. | Retrospective, two-centre | 2018 | 409 CTO patients undergoing elective PCI | >1× above the 99th percentile | >5× 99th URL | hs-cTnT | 85% | 42% | No predictive role of major and minor periprocedural myocardial injury |
| Zeitouni et al. | Prospective, single-centre | 2018 | 1390 CCS patients undergoing elective PCI | >1× above the 99th percentile | NA | hs-cTnT | 21.6 | NA | Periprocedural myocardial injury predicted 30-day, but not 1-year, events |
| Silvain et al. | Pooled analysis | 2020 | 9081 CCS patients undergoing elective PCI | >1× above the 99th percentile | >5× 99th URL | Mixed cTn and hs-cTn | 52.8% (ranging from 23.8% to 79.8% depending on the assay used) | 18.2% (ranging from 10.1% to 25.8% depending on the assay used) | Periprocedural myocardial injury did not predict 1-year mortality Major periprocedural myocardial injury was a strong independent predictor of 1-year mortality, independently of the biomarker assay used |
| Zhou et al. | Retrospective, single-centre | 2021 | 3249 CCS patients undergoing elective PCI | > 99% URL | >5× 99th URL | hs-cTnT | 78.3% | 23.4% | Neither minor nor major periprocedural myocardial injury were predictor of long-term MACE. Higher thresholds hold prognostic significance |
| Wang et al. | Prospective, single-centre | 2021 | 4013 patients without myocardial necrosis undergoing LMCAD PCI | >1× 99th URL | NA | CK-MB and cTnI | 16.3% (CK-MB)67.6% (cTnI) | NA | Only CK-MB-defined periprocedural myocardial injury was predictive of 3-year cardiovascular death and all-cause death |
Abbreviations: ACS, acute coronary syndromes; CCS, chronic coronary syndromes; CK-MB, creatine kinase-myocardial band; cTn, cardiac troponin; cTnI, cardiac troponin I; CTO, coronary total occlusion; cTnT, cardiac troponin T; hs-cTn, high-sensitive cardiac troponin; hs-cTnT, high-sensitive cardiac troponin T; LMCAD, left main coronary artery disease; MACE, major adverse cardiovascular events; NA, not available; PCI, percutaneous coronary intervention; URL, upper reference limit.
Different definitions of PMI may possess diverse prognostic implications. A pivotal study by Ueki et al. has elucidated this variation in prognostic potential among patients with chronic coronary syndrome.5 Notably, the study found that PMI defined by the ARC-2 and SCAI criteria though observed less frequently (2.0% of patients), was more prognostically significant for cardiac death at 1 year compared to the 3rd (18.0%) and 4th (14.9%) UDMI.5 These findings underscore the critical importance of careful consideration in the selection of criteria for PMI diagnosis, as they can significantly influence prognostic outcomes (Table 4).
In the context of CABG, the optimal thresholds for defining a clinically significant periprocedural myocardial injury that carries prognostic significance remain a topic of debate. Current data suggest that higher increments of cTnI and CK-MB play a prognostic role.31 This role has also been confirmed for hs-cTn in the VISION study, where the recommended troponin thresholds (i.e. >10, ≥ 35, and ≥70 times the URL) were exceeded in 97.5%, 89.4%, and 74.7% of patients, respectively, within the first day after surgery.29 However, the levels of hs-cTn after cardiac surgery that were associated with an increased risk of death within 30 days were substantially higher than the levels currently recommended to define clinically significant periprocedural myocardial injury.29 This suggests that current injury definitions may result in some degree of overdiagnosis, missing the identification of those elevations that have prognostic relevance.29 A sub-analysis of the EXCEL trial concluded that CABG-related PMI, based on the 3rd UDMI, was a better predictor of 5-year all-cause death than the SCAI definition.14 In 2017 an ESC consensus, defined prognostically significant myocardial injury as a rise >20 times the 99th URL of cTnI.23 This was somewhat confirmed in a pooled analysis of seven studies involving 19 908 patients undergoing CABG, both the 4th UDMI and the ARC-2 definition, compared to the SCAI definition, were associated with 5-year MACE, 30-day, and 1-year all-cause death.30
Risk mitigation strategies
Pre-procedural strategies
Pre-procedural strategies to mitigate the risk of PMI or injury primarily rely on the identification of factors related to patients (e.g. age), their comorbidities (e.g. renal failure, congestive heart failure), specific characteristics of coronary lesions (e.g. multi-vessel, left main disease or bifurcation lesions), or cTn elevation at baseline.8 Also, factors associated with an increased risk of MACE should be considered, including diabetes, peripheral artery disease, smoking status, and a history of cardiovascular disease.8,32–34
Interestingly, among influential baseline characteristics, nutritional status has been linked to the incidence of PMI. An observational study involving 22 267 patients undergoing elective PCI revealed a linear relationship between nutritional assessment tool scores and the increase in cTnI, as well as PMI rates, suggesting that pre-procedural nutritional status may have a significant association with PMI in patients undergoing elective PCI.35 The risk of PMI is significantly affected by the nature of coronary lesions.
Disease burden (e.g. multi-vessel disease, bifurcation disease, left main disease) and plaque characteristics (e.g. plaque length and distribution, ulcerated plaques, presence of intracoronary thrombus) are particularly important in this context. Non-invasive pre-procedural tests, such as cardiac computed tomography angiography (CCTA) and cardiac magnetic resonance (CMR), offer the potential to gain insights into plaque characteristics. High-risk plaque features identified by CCTA were independently associated with the occurrence of PMI and MACE.36 Also, high-intensity plaques identified by CMR and intravascular near-infrared spectroscopy ultrasound (NIRS-IVUS) were associated with adverse events.37 An early identification of such features may help to identify high-risk patients to prompt risk mitigation strategies.8
Various drug-based pre-procedural strategies aim to prevent PMI, including antithrombotic therapy. Dual antiplatelet therapy, which combines aspirin and a P2Y12 inhibitor, has demonstrated effectiveness in reducing complications in PCI patients and improving graft patency in CABG cases.38,39 In patients with chronic coronary syndrome undergoing PCI, a 600 mg loading dose of clopidogrel was found to be more effective in reducing PMI compared to a 300 mg dose.40 It's worth noting that it is recommended to delay PCI for at least 2 h after administering the loading dose for optimal absorption.40,41 In specific high-risk situations of elective stenting, such as suboptimal stent deployment or other procedural characteristics associated with a high risk of stent thrombosis, complex left main stem, or multi-vessel stenting, guidelines endorse the use of prasugrel or ticagrelor due to their faster onset of action, at least as initial therapy. This recommendation is at a class IIb and level of evidence C, with consideration for de-escalation to clopidogrel thereafter.8,39
High-intensity statins have shown promise in reducing PMI and injury, likely due to their pleiotropic effects on inflammation and endothelial cell production; however, current evidence remains contentious.42–46 Current guidelines support the use of high-dose statin therapy as early as possible in the context of planned PCI in high-risk patients (class II, level of evidence B) and as soon as possible after an ACS event (class I, level of evidence A), both for prognostic benefit and to increase patient adherence after discharge.
Remote ischaemic conditioning has also been considered as a potential strategy, with most studies confirming its ability to reduce acute myocardial ischaemia-reperfusion injury.47–49 The use of parenteral antioxidants demonstrated that vitamin C administration could reduce the overall incidence of myocardial injury and improve microcirculatory reperfusion.50
Based on the key role of inflammation, acute pre-procedural colchicine has been tested to prevent myocardial injury in the COLCHICINE-PCI and the smaller COPE-PCI randomized trials, with controversial results.51,52 An ongoing trial is further investigating the role of pre-procedural colchicine administered 6–24 h before elective PCI in reducing periprocedural myocardial injury and MACE (NCT05745818).
Intra-procedural strategies
Procedure-related factors influencing the risk of PMI or injury include the complexity of the PCI procedure and associated complications. PCI complexity is determined by various factors, such as the number of stents used, the duration of balloon inflation, procedures involving left main bifurcation, the use of plaque-modifying devices (e.g. atherectomy, lithotripsy), and interventions on saphenous vein grafts or chronic total occlusions. These factors can increase the risk of PMI and injury.8,53,54 Additionally, complications like side-branch occlusion, dissection, spasm, distal embolization, slow or no reflow, and disrupted collaterals can further exacerbate myocardial damage.53 Therefore, optimizing procedural strategies and effectively managing complications are crucial steps in the prevention of periprocedural myocardial injury.27,29
Intra-procedural strategies place significant emphasis on the beneficial role of antiplatelet therapy in reducing myocardial damage. Pooled data from the CHAMPION programme, including the CHAMPION-PCI, CHAMPION PLATFORM, and CHAMPION PHOENIX trials, demonstrated that early use of cangrelor during PCI reduced the incidence of PMI at 48 h, regardless of the PMI definition adopted.55 Platelet glycoprotein IIb/IIIa receptor inhibitors (GPI) have also been hypothesized as potential preventive agents, but failed in reducing PMI in patients undergoing elective PCI.56–58 Consequently, their use is recommended (class IIa level C recommendation) for bailout situations, such as high thrombus burden, slow or no reflow.59 In such bailout situations, intracoronary vasodilators like calcium channel blockers, nitroglycerine, nitroprusside, or adenosine may also be helpful, although there is currently no data to recommend one over the others.8
Other strategies have also been investigated. The intracoronary use of enalaprilat (i.e. active metabolite of the oral prodrug enalapril maleate) has been demonstrated to reduce microvascular resistance and the periprocedural increase of high-sensitivity cardiac troponin T (hs-cTnT).60 Proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors have already been shown to reduce the risk of acute MI.61,62 Currently, the use of the PCSK9 inhibitor alirocumab on top of standard therapy is under investigation to prevent PMI or injury in patients undergoing elective PCI.63
In addition to pharmacological interventions, advances in procedural techniques and devices have shown promise in mitigating PMI and injury. These advancements include the use of latest-generation stents and techniques, intravascular imaging, and distal coronary filters in venous-graft lesions.64–66 Intravascular imaging, such as optical coherence tomography (OCT), IVUS, and NIRS can help the prediction of PMI, and inform periprocedural decisions.59 OCT-based fibrous cap thickness emerged as a key predictor of PMI, while NIRS lipid core and IVUS plaque burden were better at determining the likelihood of a periprocedural event.67,68 Finally, despite confirming the predictive value of NIRS, the CANARY trial showed that using a distal protection device during PCI was not associated with improved outcomes in patients undergoing PCI.16,69
Conversely, when it comes to mitigation strategies during CABG procedures, there is limited information available, and mechanical preventive measures play a more dominant role. The ‘no-touch technique’ and internal mammary artery skeletonization have demonstrated particular effectiveness.70,71 Additionally, the use of cardioplegic solutions and ischaemic preconditioning emerge as promising tools in preventing PMI and injury during CABG procedures.72 An algorithm has been proposed for managing patients with possible PMI or injury after CABG, including serial electrocardiograms and measurements of cardiac biomarkers, with coronary angiography in patients presenting issues when wearing off bypass.23
Periprocedural myocardial infarction in non-coronary cardiac interventions
In the realm of percutaneous or surgical cardiac interventions, the risk of PMI and injury is closely tied to the invasiveness of the procedure. Over the last decade, there has been growing attention given to PMI and injury in aortic valve procedures, especially TAVI, due to its increasing global prevalence.73,74 While myocardial injury post-catheter ablation for atrial fibrillation (e.g. regardless the adopted technique) has been linked to inflammation, its impact on long-term prognosis, procedural efficacy, or recurrence of AF remains less clear, warranting additional investigation.75,76 Therefore, our discussion will centre on TAVI, given the clearer understanding of its features and associated outcomes. Figure 4 encompasses definitions, incidence, prognosis, and risk mitigation strategies of PMI in cardiac and non-cardiac interventions.
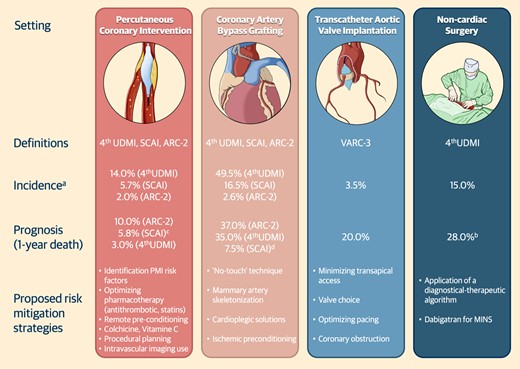
Definitions, incidence, prognosis, and risk mitigation strategies of periprocedural myocardial infarction after cardiac and non-cardiac procedures. (A) Reports the maximum value registered within 1-year; (B) Refers to type 1 myocardial infarction; (C) Refers to cardiac death at 1-year. (D) Esteemed from Kaplan Meier curves. Abbreviations: ARC, academic research consortium; MINS, myocardial infarction after non-cardiac surgery; SCAI, society for cardiovascular angiography and interventions; UDMI, universal definition of myocardial infarction.
Mechanisms
The aetiology of PMI and injury following aortic valve replacement is primarily attributed to cardiac manipulation.77 On the other hand, in the context of TAVI, mechanical trauma from valve positioning, trans-apical access, coronary obstruction, hypotensive episodes, rapid cardiac pacing, or calcific microembolization during the procedure might also contribute to these complications.78–80
Definition
The Valve Academic Research Consortium (VARC), which aims to establish appropriate endpoints and standardize definitions for clinical trials of valvular heart diseases, has also considered the issue of PMI. Despite being conceptually close to the 4th UDMI about MI, VARC-3 endorsed the definitions provided by the SCAI and ARC-2 as it relates to PMI.73
In VARC-3, CK-MB is proposed as the biomarker of choice, with an increase of ≥10 times the 99th percentile URL or ≥5 times the 99th percentile URL along with ancillary criteria (i.e. new acute electrocardiographic signs, evidence of loss of viable myocardium or regional wall motion abnormalities at echocardiogram, angiographic evidence suggestive of ischaemia) to define PMI after TAVI.73 In cases where CK-MB measurements are not available, cTn cut-off thresholds are considered suggestive of TAVI-related PMI when there is an increase is ≥70 times the 99th percentile URL or ≥35 times the 99th percentile URL, along with ancillary criteria.73
VARC-3 also recognizes the existence of ‘myocardial injury not meeting PMI criteria’ referring to any biomarker elevation that does not meet the specific thresholds or ancillary criteria.73 Importantly, biomarker elevations in the context of valve-related complications, such as acute or delayed coronary occlusion or failure to appropriately engage the coronary ostium, should also be classified as cardiac structural complications and deserve further attention in future studies.73
Incidence
Similar to PMI following coronary revascularization, the incidence of PMI after TAVI can vary based on the adopted definition, ranging from 0.4% to 3.5% in 1 year.81,82 Periprocedural release of cardiac biomarkers is an extremely frequent event after TAVI, and varies accordingly to the adopted definition.78,83 The incidence of VARC-3 defined myocardial injury has been reported in up to 14% of patients.78,83 It's worth noting that periprocedural myocardial injury is more frequent after trans-apical access or the implantation of self-expandable valves. Independent predictors of periprocedural myocardial injury included also female sex and peripheral artery disease.10,84
Prognosis
Regarding prognostic implications, it’s important to note that greater elevations in cardiac biomarkers are associated with poorer short- and long-term outcomes, particularly when reaching a threefold or greater increase.78 An increase of more than 70 times in troponin levels was associated with a higher risk of 30-day and 1-year mortality.78,83,85 By contrast, periprocedural myocardial injury as defined by the older VARC-2 criteria did not appear to impact on survival.10
Risk mitigation strategies
Risk mitigation strategies in the context of TAVI have not received the same level of attention as interventions for preventing PMI in coronary revascularization procedures. Efforts to reduce practices associated with an increased incidence of periprocedural myocardial injury and PMI in TAVI should be considered, including minimizing trans-apical access or the implantation of valves causing more mechanical trauma, and optimizing pacing. Pre-procedural planning plays a key role to decrease the risk of coronary obstruction after TAVI.86 It’s worth noting that the topic of coronary revascularization in TAVI candidates is closely linked to the incidence of PMI in this population and deserves extensive investigation.87–89
Periprocedural myocardial infarction in non-cardiac surgery
Emerging evidence highlights the importance of PMI and injury following non-cardiac surgery. Of note, these complications have a detrimental impact on patient prognosis.90–92
Mechanisms
PMI and injury in the setting of non-cardiac surgery may be attributable to cardiac or extra-cardiac causes. Among cardiac causes, underlying mechanisms for PMI mainly include type 1 (i.e. atherothrombotic) or type 2 (i.e. supply demand imbalance) MI, tachyarrhythmias, and acute heart failure. Extra-cardiac causes of PMI encompass severe systemic disorders, such as pulmonary embolism, stroke—which can also result from a prothrombotic condition or embolization—or sepsis.93–95 The acknowledgment of this diverse range of potential causes is crucial to customize preventive measures and therapeutic interventions.
Definition
In the context of non-cardiac surgery, the definition of PMI and injury is based on the 4th UDMI.13 This definition relies on the measurement of an absolute increase in cTn or hs-cTn levels above the 99th percentile URL from baseline to postoperative readings.13
Incidence
In the prospective BASEL-PMI study, periprocedural myocardial injury was detected in up to 15% of patients undergoing non-cardiac surgery who were at high cardiovascular risk.96 PMI after non-cardiac surgery has often been overlooked, primarily due to inconsistent protocols for cardiac biomarkers assessment across hospitals.90,92 In addition, the administration of potent analgesics (e.g. morphine, oxycodone, codeine) during the perioperative period represents another challenge in the diagnosis PMI as they can mask traditional ischaemic symptoms.97
Prognosis
PMI aetiology is significantly associated with adverse cardiovascular outcomes, with a different magnitude depending on the specific cause; in particular, 30-day mortality due to tachyarrhythmia, acute heart failure, or extra-cardiac PMI were higher than those of type 1 or type 2 MI.95 At 1 year, there were also higher rates of all-cause death and MACE, therefore highlighting the need for appropriate management strategies.97 Importantly, no difference in mortality was noted between symptomatic and asymptomatic PMI patients.90–92
Risk mitigation
Prognostic implications of PMI are responsible for the great emphasis on integrating preoperative and postoperative cTn measurements (e.g. at 24 and 48 h after surgery) into standard practice, as endorsed by ESC guidelines on non-cardiac surgery with a class I recommendation and a level of evidence B.6,13 The recommended workup for identifying the aetiology of PMI includes a 12-lead electrocardiogram, the assessment of haemoglobin levels, symptoms evaluation, and a transthoracic echocardiography.
Aetiological diagnosis is crucial to inform downstream management strategies. Interventions for type 1 MI include invasive coronary angiography, administration of aspirin or dual antiplatelet therapy in case of coronary stenting, statins, and vigilant monitoring. In contrast, the management of type 2 myocardial infarctions focuses on identifying and treating the underlying causes of supply demand imbalance, such as administering blood transfusions for anaemia.6
Myocardial injury after non-cardiac surgery
Myocardial injury after non-cardiac surgery (MINS) encompasses a selected subset of patients in whom cardiac injury was deemed most likely due to CAD with myocardial ischaemia (i.e. secondary to supply demand mismatch or thrombosis), in the absence of an atypical surgical physiological stress, and no evidence of a cardiac non-coronary aetiology, and occurs within a 30-day postoperative period.6 MINS does not include perioperative myocardial injury resulting from non-ischaemic causes such as sepsis, rapid atrial fibrillation, pulmonary embolism, or chronically elevated troponin levels.6 Importantly, no ischaemic symptoms are required for the diagnosis of MINS, so more than 80% of MINS remain undetected in the absence of routine perioperative troponin testing.90–92
In a recent study of 22 552 patients undergoing major general surgery, MINS occurred in ∼16% of cases and was independently associated with a nearly five-fold increase in 30-day mortality. Notably, the majority of patients with MINS were asymptomatic, reinforcing that routine postoperative troponin measurement is crucial to detect this condition.98
In the MANAGE randomized trial, the activated factor II inhibitor dabigatran was compared to placebo in patients diagnosed with MINS after non-cardiac surgery; dabigatran, initiated after a median of 6 days from the event, reduced major vascular complications, including vascular mortality, MI, non-hemorrhagic stroke, peripheral arterial thrombosis, amputation, or symptomatic venous thromboembolism, without significantly increasing the risk of major bleeding.99 Hence, initiating dabigatran ∼1 week after non-cardiac surgery is a potential therapeutic strategy for MINS patients with a low bleeding risk.6
Unmet needs and future challenges
Beyond the areas of uncertainty that have been already discussed, additional challenges merit separate consideration. First, a precise definition of PMI is necessary when conceiving clinical trials as it can significantly impact on the results of most studies in the field. Second, despite the effectiveness of current assays, future biotechnological innovations could further refine the assessment of troponin levels, aiming for best sensitivity and specificity. Finally, the occurrence of PMI can identify patients at high thrombotic risk, potentially benefiting from more intense antithrombotic treatments.
Implications of different periprocedural myocardial infarction definitions in clinical trials
The use of different PMI definitions, each corresponding to diverse incidences and prognostic implications, becomes an issue when PMI is selected as an endpoint in clinical trials, especially in the context of myocardial revascularization. A compelling example of this challenge is provided by the EXCEL trial, which compared PCI to CABG for the treatment of left main disease with regards to a composite endpoint of death, MI, or stroke.100 When a protocol definition of PMI (i.e. similar to the SCAI definition) was used, the primary endpoint occurred in 3.6% of PCI group and in 6.1% of the CABG group. However, when the results were analyzed using the 3rd UDMI, there was a lower incidence of PMI with CABG than PCI (e.g. 4.0% vs. 2.2%, respectively) compromising the interpretation of study results.14,100 Notably, these definitions also influenced the ability of PMI to predict long-term outcomes after revascularization.14
Conversely, definitions can also be confirmative as occurred in studies of cangrelor in urgent PCI. While the CHAMPION-PCI showed higher event rates with cangrelor (7.1% vs. 6.6%), cangrelor was beneficial in the CHAMPION-PHOENIX (3.8% vs. 4.7%).101,102 In addition, this latter finding was confirmed also when applying different definitions of PMI to the CHAMPION-PHOENIX trial.103
Moreover, applying different PMI definitions to the SYNTAX trial impacted on study results: when transitioning from the SCAI to the 4th UDMI definition, PMI rates increased from 3% to 5.7% in the PCI group and even more (i.e. from 2.1% to 16.5%) in the CABG group.104 These changes profoundly affected the rates of the primary endpoint, which rose from 17.8% to 19.5 with PCI and from 11.6% from 24.1% with CABG.
In a sub-analysis of the ISCHEMIA trial, comparing PCI and medical therapy in patients with chronic coronary syndrome, results were also sensitive to different definitions of PMI.105 Specifically, while invasive and conservative approaches were associated with similar rates of the primary endpoint (∼5% in each group), applying the 4th UDMI definition (e.g. proved to be a predictor of adverse prognosis) favoured the conservative approach (11% vs. 5%).105
All these examples highlight the challenges posed by the diversity in PMI definitions and their varying prognostic significance when used as primary endpoints in randomized trials, often leading to misinterpretations.106 As a mitigation strategy, excluding PMI from primary composite endpoints could prevent skewed interpretations. However, the prognostic value of certain widely recognized definitions, such as the 4th UDMI, cannot be overlooked. This is likely why these definitions continue to be included in major clinical trials’ composite endpoints and considered in patient-centric evaluations, despite applicability concerns, such as in trials comparing revascularization methods like PCI vs. CABG, which differ in biomarker release profiles. This ongoing debate calls for a cautious approach. Until more conclusive evidence becomes available, it may be prudent to assess the inclusion of PMI in composite endpoints on a case-by-case basis, ensuring alignment with each study’s specific design. The need for further research to develop a more universally accepted approach to PMI in clinical trial outcomes remains a key concern.
Biomarkers essay sensitivity
Adding to ancillary criteria detection, serial measurements of CTn are key in diagnosing PMI.107,108 The use of hs-cTn has enabled the detection of lower concentrations of these cardiac biomarkers, improving diagnosis in acute cardiac care.108
While concerns have been raised regarding the potential for cross-reactivity of cTnT in cases of musculoskeletal disease, current evidence suggests that this is a rare occurrence, and the question of cross-reactivity and false positives remains a topic of ongoing investigation and debate. Nonetheless, cTnT maintains a significant role in clinical practice. By contrast, hs-cTnI demonstrated high specificity for myocardial injury, even amidst conditions like skeletal muscle diseases, chronic kidney diseases, or intense physical activity.109
Areas of uncertainty
The performance of available biomarkers assays is hindered by some weaknesses. There a wide variety of assays is currently available, ranging from older less-sensitive versions to modern high-sensitivity ones, posing a challenge for standardization across different platforms.110 Furthermore, hs-cTn concentrations can be influenced by various factors, including race, physical activity, and comorbidities, such as known CAD, cardiac arrhythmias, hypertension, chronic pulmonary disease, and cancer.108 Therefore, different cut-offs can be applied for age, sex, and estimated glomerular filtration rate, but it may not fully account for all the potential confounders.
Future perspectives
To overcome the limitations associated with assay sensitivity, especially when conducting evaluations at different timeframes, a combined approach using different biomarkers essay as part of a comprehensive diagnostic strategy has been suggested. For instance, the AROMI study evaluated an accelerated dual-marker rule-out strategy for patients with suspected ACS. This approach combined prehospital copeptin, a novel biomarker in this field, with in-hospital hs-cTnT. Although copeptin alone might have limited utility in the perioperative setting due to its nature as a stress marker, this combination strategy in the AROMI study reduced the average hospital stay by 0.9 h and proved non-inferior to the standard rule-out strategy in terms of 30-day MACE.111 Furthermore, the role of combined troponin and copeptin assay is currently under investigation in the CopSCA study, which aims to demonstrate their potential in excluding ACS in patients with chest pain occurring within the last 6 h (NCT05902117).
Personalized diagnostic approaches, especially machine learning techniques, are being explored to address the limitations of assay sensitivity in patient care.112 These methods show potential for creating individualized diagnostic thresholds, potentially improving sensitivity. However, current data on their effectiveness in predicting post-PCI complications, like PMI and injury, is limited.113 This area is still under research, and further studies are needed to determine the true applicability and benefits of these strategies in PCI risk assessment.
Recent studies, including secondary analyses from the ALPHEUS trial, emphasize the need to refine the ancillary criteria in defining Type 4a MI.114 While troponin levels are well-validated, non-troponin criteria such as electrocardiographic or clinical manifestations remain underexamined and lack standardization. These criteria have shown variable correlation with biomarkers elevation.115,116 Notably, angiographic criteria, including stent length, atheroma volume, angiographic complications, may be crucial for predicting prognostically significant PMI.116 This highlights the need for systematic evaluation of these criteria and their predictive capabilities alone and coupled with cardiac biomarkers in apposite studies.
Finally, in light of the numerous validation studies published since the 2018 release of the 4th UDMI, there is an opportunity for the committee to reconvene and consider updating their document. Over the years, the definition has been globally validated in various cohorts, used both as a component of composite endpoints and as a standalone element, and its prognostic prediction power has been compared to other definitions. Updating the UDMI would allow it to reflect the latest clinical evidence, ensuring alignment with current understanding and practices.
Antithrombotic regimens
Antithrombotic therapy is crucial for patients undergoing myocardial revascularization and represents a risk mitigation strategy also in the context of PMI and injury. In particular, more intense antithrombotic regimens can be administered in different modes to patients undergoing complex PCI or at high thrombotic risk.117,118 Antiplatelet therapy escalation, which involves increasing the intensity of platelet inhibition, is a strategy aimed at reducing thrombotic or ischaemic complications during the period when the risk of such events is considered to be greater than the risk of bleeding.119–122 This approach is based on the wide variability in platelet activation among individuals of different race or with genetic polymorphisms influencing the response to antiplatelet drugs such as clopidogrel.123
A study conducted as part of the ALPHEUS trial aimed to assess the effect of P2Y12 loading time on PMI in patients undergoing elective PCI. The findings indicated that the administration of an oral P2Y12 inhibitor at the time of PCI could potentially be associated with a higher frequency of PMI than administering it earlier.124 However, the study did not show a consistent, stepwise decrease in periprocedural myocardial necrosis based on the time interval between loading and PCI.124 As a result, the optimal timing for loading with P2Y12 inhibitors may vary according to patient profiles, and further investigation is needed to better understand the clinical impact of different loading strategies and their long-term consequences.125–128 This ongoing research will help refine the timing of P2Y12 inhibitor administration to improve patient outcomes during and after PCI.
While the concept of modifying antithrombotic regimens to improve secondary prevention of ischaemic events in PMI seems intuitively beneficial, the effectiveness of these strategies in terms of clinical outcomes still needs to be proven.
Conclusions
PMI and injury, whether associated with cardiac or non-cardiac procedures, have emerged as a topic of growing interest due to its significant impact on patient outcomes.
Over the years, various definitions for diagnosing PMI after coronary revascularization have been developed and validated across diverse patient populations. However, this heterogeneity in definitions poses substantial challenges when dealing with PMI and injury in clinical practice. This issue extends to clinical trials, with inconsistent definitions complicating study design and the interpretation of study findings. In the realm of non-coronary cardiac procedures, such as TAVI, the adverse impact of PMI on patient outcomes is increasingly recognized. In addition, the evolving field of PMI related to non-cardiac surgery is expanding the scale of this issue, therefore reinforcing the need for preventive and risk mitigation strategies.
Finally, further research on endpoint definition is warranted to harmonize data and provide reliable information on prognostic significance of PMI and injury, ultimately contributing to a more accurate interpretations of clinical trials when PMI is adopted as a clinical endpoint. This approach will allow a deeper understanding of PMI features to improve patient outcomes after cardiac and non-cardiac procedures.
Funding
None.
Data availability
The data utilized in this review article can be retrieved through PubMed by searching for the corresponding publication using the provided citation details.
References
Author notes
Marco Spagnolo and Giovanni Occhipinti contributed equally to the study.
Conflict of interest: The authors declare no conflict of interest related to topic of the review.




Comments