-
PDF
- Split View
-
Views
-
Cite
Cite
Jorge Sanz-Sanchez, Hector M Garcia-Garcia, Mattia Branca, Enrico Frigoli, Sergio Leonardi, Andrea Gagnor, Paolo Calabrò, Stefano Garducci, Paolo Rubartelli, Carlo Briguori, Giuseppe Andò, Alessandra Repetto, Ugo Limbruno, Roberto Garbo, Paolo Sganzerla, Filippo Russo, Alessandro Lupi, Bernardo Cortese, Arturo Ausiello, Salvatore Ierna, Giovanni Esposito, Andrea Santarelli, Gennaro Sardella, Fernando Varbella, Simone Tresoldi, Nicoletta de Cesare, Stefano Rigattieri, Antonio Zingarelli, Paolo Tosi, Arnoud van ‘t Hof, Giacomo Boccuzzi, Elmir Omerovic, Manel Sabaté, Dik Heg, Pascal Vranckx, Marco Valgimigli, Coronary calcification in patients presenting with acute coronary syndromes: insights from the MATRIX trial, European Heart Journal. Acute Cardiovascular Care, Volume 12, Issue 11, November 2023, Pages 782–791, https://doi.org/10.1093/ehjacc/zuad122
Close - Share Icon Share
Abstract
The role of coronary calcification on clinical outcomes among different revascularization strategies in patients presenting with acute coronary syndromes (ACSs) has been rarely investigated. The aim of this investigation is to evaluate the role of coronary calcification, detected by coronary angiography, in the whole spectrum of patients presenting with acute ACS.
The present study was a post hoc analysis of the MATRIX programme. The primary endpoint was major adverse cardiovascular events (MACE), defined as the composite of all-cause mortality, myocardial infarction (MI), or stroke up to 365 days. Among the 8404 patients randomized in the MATRIX trial, data about coronary calcification were available in 7446 (88.6%) and therefore were included in this post hoc analysis. Overall, 875 patients (11.7%) presented with severe coronary calcification, while 6571 patients (88.3%) did not present severe coronary calcification on coronary angiography. Fewer patients with severe coronary calcification underwent percutaneous coronary intervention whereas coronary artery bypass grafting or medical therapy-only was more frequent compared with patients without severe calcification. At 1-year follow-up, MACE occurred in 237 (27.1%) patients with severe calcified coronary lesions and 985 (15%) patients without severe coronary calcified lesions [hazard ratio (HR) 1.91; 95% confidence interval (CI) 1.66–2.20, P < 0.001]. All-cause mortality was 8.6% in patients presenting with and 3.7% in those without severe coronary calcification (HR 2.38, 1.84–3.09, P < 0.001). Patients with severe coronary calcification incurred higher rate of MI (20.1% vs. 11.5%, HR 1.81; 95% CI 1.53–2.1, P < 0.001) and similar rate of stroke (0.8% vs. 0.6%, HR 1.35; 95% CI 0.61–3.02, P = 0.46).
Patients with ACS and severe coronary calcification, as compared to those without, are associated with worse clinical outcomes irrespective of the management strategy.
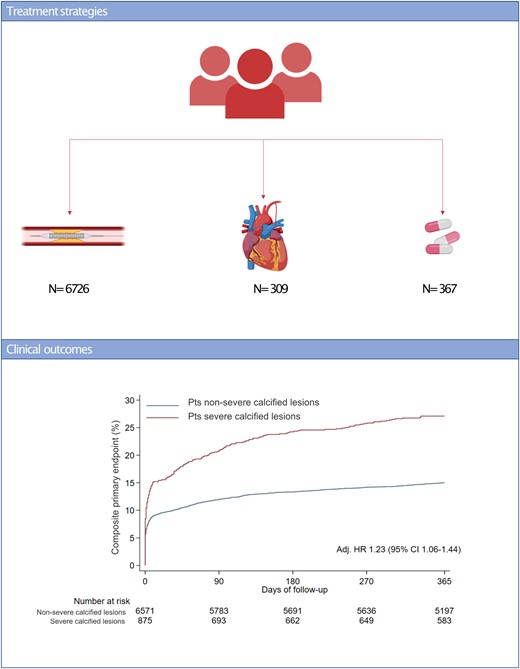
Treatment strategies and clinical outcomes of patients presenting with acute coronary syndromes. (A) Treatment strategies, (B) composite primary endpoint at 365 days based on the presence of severe calcified lesions.
Introduction
Calcified coronary lesions are present in a considerable proportion of patients undergoing coronary revascularization, and its prevalence is expected to further raise in the subsequent years due to ageing of the population and growing incidence of diabetes mellitus and chronic kidney disease.1,2 Despite the development of novel techniques and equipment improvements that have enabled the treatment of increasingly complex lesions, severely calcified coronary lesions yet remain a procedural and clinical challenge.3,4 Severely calcified percutaneous coronary intervention (PCI) requires proper lesion preparation and is associated with higher rates of procedural complications, stent underexpansion, malapposition, polymer disruption, and impaired drug delivery with drug-eluting stents; leading to an increased risk of target lesion failure, restenosis, stent thrombosis, myocardial infarction (MI), and death; as compared to PCI in patients without calcified coronary lesions.5,6 Severe lesion calcification is also associated with increased mortality in patients undergoing coronary artery bypass grafting (CABG).7,8 However, the vast majority of data derives from patients presenting with chronic coronary syndromes, with limited evidence for patients with acute coronary syndrome (ACS).9 In addition, the impact of coronary calcification on clinical outcomes and procedural success among different revascularization strategies in patients with ACS has been rarely investigated.
The Minimizing Adverse Hemorrhagic Events by Trans-radial Access Site and Systemic Implementation of Angiox (MATRIX) was a programme of three nested, randomized, multicentre, open-label superiority trials involving patients with an ACS.10 The aim of the present post hoc subanalysis of the MATRIX programme was to investigate the role of coronary calcification, detected by coronary angiography, in the whole spectrum of patients presenting with ACS undergoing initial invasive management.
Methods
Study design and patient population
The present study was a post hoc analysis of the MATRIX programme. In brief, the MATRIX programme included three randomized, multicentre, open-label superiority trials involving patients with an ACS.10 The first trial (MATRIX-Access) compared radial vs. femoral access in 8404 patients with ACS.11,12 The second study, (MATRIX-Antithrombin) compared bivalirudin with unfractionated heparin, with optional use of glycoprotein IIb/IIIa inhibitors, in 7213 ACS patients in whom PCI was planned.13 The third trial, MATRIX Treatment Duration, compared prolonged bivalirudin administration with a post-PCI infusion vs. short-term bivalirudin administration without a post-PCI infusion in 3610 patients assigned to receive bivalirudin.13
Patients with non-ST-segment elevation acute coronary syndrome (NSTE-ACS) were eligible if they had a history consistent with new or worsening cardiac ischaemia that occurred while they were at rest or with minimal activity within 7 days before randomization and met at least two high-risk criteria among the following: (i) age of 60 years or older, the elevation of cardiac biomarkers, or electrocardiographic changes compatible with ischaemia; and (ii) if they were considered to be candidates for PCI after completion of coronary angiography. Patients with ST-segment elevation myocardial infarction (STEMI) were eligible if they presented within 12 h of the onset of symptoms or between 12 and 24 h after symptom onset if there was evidence of continued ischaemia or previous fibrinolytic treatment.
All patients enrolled in MATRIX programme were eligible for this post hoc analysis, except those without angiographic data regarding the severity of coronary calcification. The trial was approved by the institutional review board at each participating site, and all patients gave written informed consent. Follow-up was performed under local law and the regulations of each participating institution and complied with the Declaration of Helsinki.
Angiographic analysis
The presence of severe coronary calcification was evaluated, on the basis of coronary angiography, during the index procedure by each investigator at the participating centres. As defined in the SYNTAX score, severe coronary calcification was defined as multiple persisting opacifications of the coronary wall visible in more than one projection surrounding the complete lumen of the coronary artery at the site of the lesion.14 A patient was classified as presenting with severe coronary calcification when at least one obstructive coronary lesion was defined as severely calcified. A total number of severe calcified lesions per patient were reported.
Outcomes
The primary objective of this post hoc analysis was to investigate the impact of coronary calcification in the whole spectrum of ACS initially undergoing invasive management.
The primary outcome of this post hoc analysis was major adverse cardiovascular events (MACE), defined as the composite of all-cause mortality, MI, or stroke up to 365 days. Secondary endpoints included the composite of cardiovascular death, MI, stroke, target vessel revascularization or stent thrombosis, all single components of the composite outcomes, and target lesion revascularization (TLR).
An independent clinical events committee masked to treatment allocation adjudicated all suspected events, with the exception of TLR that was investigator-reported. Detailed outcome definitions and clinical events committee procedures have been published elsewhere.11
Statistical analysis
Analyses were done according to the intention-to-treat principle. Categorical data were compared by the χ2 test or Fisher exact test if the expected number of observations in any cell was <5. Continuous data are presented as mean ± SD or median with interquartile range and were compared using the analysis of variance or Kruskal–Wallis test as appropriate. Adjustment was done for baseline characteristics with complete data and P-value < 0.2. Primary and secondary outcomes were analysed as time to first event using Cox regression, accompanied by log-rank tests to calculate corresponding two-sided P-values. Survival curves using Kaplan–Meier estimates were constructed. Landmark analysis was also created using 30-day as a timepoint. Subgroup analyses were performed according to age, sex, presenting syndrome, diabetes mellitus, management strategy (PCI, CABG, medical therapy), number of calcified lesions, access site, anticoagulant therapy, and accompanied by formal tests for subgroup by treatment interaction or tests for trend across ordered groups. All analyses were done using Stata release 17.0 (StataCorp LLC, College Station, TX, USA).
Results
Among the 8404 patients randomized in the MATRIX trial to radial or femoral access, data about coronary calcification were available in 7446 (88.6%) patients, who were selected for the present analysis. A total of 875 patients (11.7%) involving 2672 lesions presented with severe coronary calcification, while 6571 patients (88.3%) involving 13 912 lesions did not present severe coronary calcification on coronary angiography for index ACS.
Patient characteristics
As shown in Table 1, patients with severe coronary calcification, as compared to those without, were older, more frequently women, and had higher rates of hypertension, anaemia, diabetes mellitus, chronic kidney disease, previous cerebrovascular disease, peripheral vascular disease, and previous CABG. Patients with severe coronary calcification presented more frequently with NSTE-ACS, lower left ventricular ejection fraction, and higher rates of Killip class III or IV at clinical presentation.
| . | Overall (n = 7446) . | Patients with severe calcification (n = 875) . | Patients without severe calcification (n = 6571) . | P-value . |
|---|---|---|---|---|
| Clinical characteristics | ||||
| Age ≥ 75, years | 1914 (26) | 387 (44) | 1527 (23) | <0.001 |
| Male | 5704 (77) | 633 (72) | 5071 (77) | 0.002 |
| BMI, kg/m2 | 27.09 ± 4.07 | 26.89 ± 4.15 | 27.12 ± 4.06 | 0.125 |
| Hypertension | 4695 (63) | 657 (75) | 4038 (61) | <0.001 |
| Anaemia | 1478 (20) | 271 (31) | 1207 (18) | <0.001 |
| Diabetes mellitus | 1755 (24) | 294 (34) | 1461 (22) | <0.001 |
| Renal failure | 93 (1) | 31 (4) | 62 (1) | <0.001 |
| Dialysis | 7 (0) | 3 (0) | 4 (0) | 0.039 |
| Peripheral vascular disease | 642 (9) | 165 (19) | 477 (7) | <0.001 |
| Previous CVA | 385 (5) | 80 (9) | 305 (5) | <0.001 |
| Previous PCI | 1099 (15) | 145 (17) | 954 (15) | 0.116 |
| Previous CABG | 246 (3) | 68 (8) | 178 (3) | <0.001 |
| Killip class III or IV | 221 (3) | 55 (6) | 166 (3) | <0.001 |
| STEMI | 3776 (51) | 353 (40) | 3423 (52) | <0.001 |
| NSTE-ACS | 3299 (44) | 458 (52) | 2841 (43) | <0.001 |
| LVEF < 35% | 643 (9) | 136 (16) | 507 (8) | <0.001 |
| Lesion characteristics | ||||
| Total number of lesions | 2.23 ± 1.36 | 3.05 ± 1.46 | 2.12 ± 1.31 | <0.001 |
| Number of calcified lesions | ||||
| None | 6571 (88) | 0 | 0 | <0.001 |
| One | 577 (8) | 577 (66) | 0 | <0.001 |
| Two | 198 (3) | 198 (23) | 0 | <0.001 |
| Three or more | 100 (1) | 100 (11) | 0 | <0.001 |
| LM or LAD | 5453 (73) | 769 (88) | 4684 (71) | <0.001 |
| LCX | 3705 (50) | 585 (67) | 3120 (48) | <0.001 |
| RCA | 3967 (53) | 628 (72) | 3339 (51) | <0.001 |
| Bypass graft | 56 (1) | 11 (1) | 45 (1) | 0.091 |
| TIMI flow pre-procedure | ||||
| 0 or 1 | 4817 (29) | 742 (28) | 4075 (29) | 0.114 |
| 2 | 1355 (8) | 212 (8) | 1143 (8) | 0.644 |
| 3 | 10 412 (63) | 1718 (64) | 8694 (62) | 0.080 |
| Management strategies | ||||
| PCI | 6726 (90) | 725 (83) | 6001 (91) | <0.001 |
| CABG | 309 (4) | 80 (9) | 229 (3) | <0.001 |
| Medical therapy | 367 (5) | 68 (8) | 299 (5) | <0.001 |
| Index PCI characteristics | ||||
| More than one vessel treated | 891 (13) | 170 (23) | 721 (12) | <0.001 |
| Lesions treated per patient | ||||
| One | 5296 (79) | 498 (69) | 4798 (80) | <0.001 |
| Two | 1165 (17) | 173 (24) | 992 (17) | <0.001 |
| Three or more | 259 (4) | 53 (7) | 206 (3) | <0.001 |
| Complex PCI | 3090 (46) | 465 (64) | 2625 (44) | <0.001 |
| Rotational atherectomy | 78 (1) | 55 (6) | 23 (0) | <0.001 |
| IVUS | 225 (3) | 45 (5) | 180 (3) | <0.001 |
| Type of stent | ||||
| DES | 7178 (75) | 883 (74) | 6295 (75) | 0.298 |
| BMS | 2350 (25) | 308 (26) | 2041 (24) | 0.315 |
| Direct stenting | 1742 (23) | 131 (15) | 1611 (25) | <0.001 |
| Post-dilation | 4483 (47) | 611 (51) | 3872 (46) | 0.002 |
| Number of stents per patient | 1.44 ± 0.87 | 1.65 ± 1.13 | 1.42 ± 0.84 | <0.001 |
| Procedural success | 6238 (93) | 616 (85) | 5622 (94) | <0.001 |
| Duration of procedure, min | 52.50 ± 28.28 | 58.05 ± 34.31 | 51.75 ± 27.28 | <0.001 |
| Total contrast volume, mL | 179.54 ± 85.47 | 199.58 ± 98.06 | 176.86 ± 83.28 | <0.001 |
| Average stent diameter per lesion, mm | 3.07 ± 0.48 | 3.04 ± 0.48 | 3.07 ± 0.48 | 0.043 |
| Average stent length per lesion, mm | 26.39 ± 14.88 | 30.88 ± 18.64 | 25.75 ± 14.15 | <0.001 |
| TIMI flow post-procedure | ||||
| 0 or 1 | 1560 (9) | 420 (16) | 1140 (8) | <0.001 |
| 2 | 371 (2) | 90 (3) | 281 (2) | <0.001 |
| 3 | 14 653 (88) | 2162 (81) | 12 491 (90) | <0.001 |
| Residual stenosis < 30% | 6420 (95) | 634 (87) | 5786 (96) | <0.001 |
| . | Overall (n = 7446) . | Patients with severe calcification (n = 875) . | Patients without severe calcification (n = 6571) . | P-value . |
|---|---|---|---|---|
| Clinical characteristics | ||||
| Age ≥ 75, years | 1914 (26) | 387 (44) | 1527 (23) | <0.001 |
| Male | 5704 (77) | 633 (72) | 5071 (77) | 0.002 |
| BMI, kg/m2 | 27.09 ± 4.07 | 26.89 ± 4.15 | 27.12 ± 4.06 | 0.125 |
| Hypertension | 4695 (63) | 657 (75) | 4038 (61) | <0.001 |
| Anaemia | 1478 (20) | 271 (31) | 1207 (18) | <0.001 |
| Diabetes mellitus | 1755 (24) | 294 (34) | 1461 (22) | <0.001 |
| Renal failure | 93 (1) | 31 (4) | 62 (1) | <0.001 |
| Dialysis | 7 (0) | 3 (0) | 4 (0) | 0.039 |
| Peripheral vascular disease | 642 (9) | 165 (19) | 477 (7) | <0.001 |
| Previous CVA | 385 (5) | 80 (9) | 305 (5) | <0.001 |
| Previous PCI | 1099 (15) | 145 (17) | 954 (15) | 0.116 |
| Previous CABG | 246 (3) | 68 (8) | 178 (3) | <0.001 |
| Killip class III or IV | 221 (3) | 55 (6) | 166 (3) | <0.001 |
| STEMI | 3776 (51) | 353 (40) | 3423 (52) | <0.001 |
| NSTE-ACS | 3299 (44) | 458 (52) | 2841 (43) | <0.001 |
| LVEF < 35% | 643 (9) | 136 (16) | 507 (8) | <0.001 |
| Lesion characteristics | ||||
| Total number of lesions | 2.23 ± 1.36 | 3.05 ± 1.46 | 2.12 ± 1.31 | <0.001 |
| Number of calcified lesions | ||||
| None | 6571 (88) | 0 | 0 | <0.001 |
| One | 577 (8) | 577 (66) | 0 | <0.001 |
| Two | 198 (3) | 198 (23) | 0 | <0.001 |
| Three or more | 100 (1) | 100 (11) | 0 | <0.001 |
| LM or LAD | 5453 (73) | 769 (88) | 4684 (71) | <0.001 |
| LCX | 3705 (50) | 585 (67) | 3120 (48) | <0.001 |
| RCA | 3967 (53) | 628 (72) | 3339 (51) | <0.001 |
| Bypass graft | 56 (1) | 11 (1) | 45 (1) | 0.091 |
| TIMI flow pre-procedure | ||||
| 0 or 1 | 4817 (29) | 742 (28) | 4075 (29) | 0.114 |
| 2 | 1355 (8) | 212 (8) | 1143 (8) | 0.644 |
| 3 | 10 412 (63) | 1718 (64) | 8694 (62) | 0.080 |
| Management strategies | ||||
| PCI | 6726 (90) | 725 (83) | 6001 (91) | <0.001 |
| CABG | 309 (4) | 80 (9) | 229 (3) | <0.001 |
| Medical therapy | 367 (5) | 68 (8) | 299 (5) | <0.001 |
| Index PCI characteristics | ||||
| More than one vessel treated | 891 (13) | 170 (23) | 721 (12) | <0.001 |
| Lesions treated per patient | ||||
| One | 5296 (79) | 498 (69) | 4798 (80) | <0.001 |
| Two | 1165 (17) | 173 (24) | 992 (17) | <0.001 |
| Three or more | 259 (4) | 53 (7) | 206 (3) | <0.001 |
| Complex PCI | 3090 (46) | 465 (64) | 2625 (44) | <0.001 |
| Rotational atherectomy | 78 (1) | 55 (6) | 23 (0) | <0.001 |
| IVUS | 225 (3) | 45 (5) | 180 (3) | <0.001 |
| Type of stent | ||||
| DES | 7178 (75) | 883 (74) | 6295 (75) | 0.298 |
| BMS | 2350 (25) | 308 (26) | 2041 (24) | 0.315 |
| Direct stenting | 1742 (23) | 131 (15) | 1611 (25) | <0.001 |
| Post-dilation | 4483 (47) | 611 (51) | 3872 (46) | 0.002 |
| Number of stents per patient | 1.44 ± 0.87 | 1.65 ± 1.13 | 1.42 ± 0.84 | <0.001 |
| Procedural success | 6238 (93) | 616 (85) | 5622 (94) | <0.001 |
| Duration of procedure, min | 52.50 ± 28.28 | 58.05 ± 34.31 | 51.75 ± 27.28 | <0.001 |
| Total contrast volume, mL | 179.54 ± 85.47 | 199.58 ± 98.06 | 176.86 ± 83.28 | <0.001 |
| Average stent diameter per lesion, mm | 3.07 ± 0.48 | 3.04 ± 0.48 | 3.07 ± 0.48 | 0.043 |
| Average stent length per lesion, mm | 26.39 ± 14.88 | 30.88 ± 18.64 | 25.75 ± 14.15 | <0.001 |
| TIMI flow post-procedure | ||||
| 0 or 1 | 1560 (9) | 420 (16) | 1140 (8) | <0.001 |
| 2 | 371 (2) | 90 (3) | 281 (2) | <0.001 |
| 3 | 14 653 (88) | 2162 (81) | 12 491 (90) | <0.001 |
| Residual stenosis < 30% | 6420 (95) | 634 (87) | 5786 (96) | <0.001 |
Values are mean ± SD or n (%).
BMI, body max index; BMS, bare-metal stent; CABG, coronary artery bypass grafting; CVA, cerebrovascular accident; DES, drug-eluting stent; IVUS, intravascular ultrasound; LAD, left anterior descending artery; LCX, left circumflex; LM, left main; LVEF, left ventricle ejection fraction; NSTE-ACS, non-ST-segment elevation acute coronary syndrome; PCI, percutaneous coronary intervention; RCA, right coronary artery; STEMI, ST-segment elevation myocardial infarction; TIMI, Thrombolysis in Myocardial Infarction.
| . | Overall (n = 7446) . | Patients with severe calcification (n = 875) . | Patients without severe calcification (n = 6571) . | P-value . |
|---|---|---|---|---|
| Clinical characteristics | ||||
| Age ≥ 75, years | 1914 (26) | 387 (44) | 1527 (23) | <0.001 |
| Male | 5704 (77) | 633 (72) | 5071 (77) | 0.002 |
| BMI, kg/m2 | 27.09 ± 4.07 | 26.89 ± 4.15 | 27.12 ± 4.06 | 0.125 |
| Hypertension | 4695 (63) | 657 (75) | 4038 (61) | <0.001 |
| Anaemia | 1478 (20) | 271 (31) | 1207 (18) | <0.001 |
| Diabetes mellitus | 1755 (24) | 294 (34) | 1461 (22) | <0.001 |
| Renal failure | 93 (1) | 31 (4) | 62 (1) | <0.001 |
| Dialysis | 7 (0) | 3 (0) | 4 (0) | 0.039 |
| Peripheral vascular disease | 642 (9) | 165 (19) | 477 (7) | <0.001 |
| Previous CVA | 385 (5) | 80 (9) | 305 (5) | <0.001 |
| Previous PCI | 1099 (15) | 145 (17) | 954 (15) | 0.116 |
| Previous CABG | 246 (3) | 68 (8) | 178 (3) | <0.001 |
| Killip class III or IV | 221 (3) | 55 (6) | 166 (3) | <0.001 |
| STEMI | 3776 (51) | 353 (40) | 3423 (52) | <0.001 |
| NSTE-ACS | 3299 (44) | 458 (52) | 2841 (43) | <0.001 |
| LVEF < 35% | 643 (9) | 136 (16) | 507 (8) | <0.001 |
| Lesion characteristics | ||||
| Total number of lesions | 2.23 ± 1.36 | 3.05 ± 1.46 | 2.12 ± 1.31 | <0.001 |
| Number of calcified lesions | ||||
| None | 6571 (88) | 0 | 0 | <0.001 |
| One | 577 (8) | 577 (66) | 0 | <0.001 |
| Two | 198 (3) | 198 (23) | 0 | <0.001 |
| Three or more | 100 (1) | 100 (11) | 0 | <0.001 |
| LM or LAD | 5453 (73) | 769 (88) | 4684 (71) | <0.001 |
| LCX | 3705 (50) | 585 (67) | 3120 (48) | <0.001 |
| RCA | 3967 (53) | 628 (72) | 3339 (51) | <0.001 |
| Bypass graft | 56 (1) | 11 (1) | 45 (1) | 0.091 |
| TIMI flow pre-procedure | ||||
| 0 or 1 | 4817 (29) | 742 (28) | 4075 (29) | 0.114 |
| 2 | 1355 (8) | 212 (8) | 1143 (8) | 0.644 |
| 3 | 10 412 (63) | 1718 (64) | 8694 (62) | 0.080 |
| Management strategies | ||||
| PCI | 6726 (90) | 725 (83) | 6001 (91) | <0.001 |
| CABG | 309 (4) | 80 (9) | 229 (3) | <0.001 |
| Medical therapy | 367 (5) | 68 (8) | 299 (5) | <0.001 |
| Index PCI characteristics | ||||
| More than one vessel treated | 891 (13) | 170 (23) | 721 (12) | <0.001 |
| Lesions treated per patient | ||||
| One | 5296 (79) | 498 (69) | 4798 (80) | <0.001 |
| Two | 1165 (17) | 173 (24) | 992 (17) | <0.001 |
| Three or more | 259 (4) | 53 (7) | 206 (3) | <0.001 |
| Complex PCI | 3090 (46) | 465 (64) | 2625 (44) | <0.001 |
| Rotational atherectomy | 78 (1) | 55 (6) | 23 (0) | <0.001 |
| IVUS | 225 (3) | 45 (5) | 180 (3) | <0.001 |
| Type of stent | ||||
| DES | 7178 (75) | 883 (74) | 6295 (75) | 0.298 |
| BMS | 2350 (25) | 308 (26) | 2041 (24) | 0.315 |
| Direct stenting | 1742 (23) | 131 (15) | 1611 (25) | <0.001 |
| Post-dilation | 4483 (47) | 611 (51) | 3872 (46) | 0.002 |
| Number of stents per patient | 1.44 ± 0.87 | 1.65 ± 1.13 | 1.42 ± 0.84 | <0.001 |
| Procedural success | 6238 (93) | 616 (85) | 5622 (94) | <0.001 |
| Duration of procedure, min | 52.50 ± 28.28 | 58.05 ± 34.31 | 51.75 ± 27.28 | <0.001 |
| Total contrast volume, mL | 179.54 ± 85.47 | 199.58 ± 98.06 | 176.86 ± 83.28 | <0.001 |
| Average stent diameter per lesion, mm | 3.07 ± 0.48 | 3.04 ± 0.48 | 3.07 ± 0.48 | 0.043 |
| Average stent length per lesion, mm | 26.39 ± 14.88 | 30.88 ± 18.64 | 25.75 ± 14.15 | <0.001 |
| TIMI flow post-procedure | ||||
| 0 or 1 | 1560 (9) | 420 (16) | 1140 (8) | <0.001 |
| 2 | 371 (2) | 90 (3) | 281 (2) | <0.001 |
| 3 | 14 653 (88) | 2162 (81) | 12 491 (90) | <0.001 |
| Residual stenosis < 30% | 6420 (95) | 634 (87) | 5786 (96) | <0.001 |
| . | Overall (n = 7446) . | Patients with severe calcification (n = 875) . | Patients without severe calcification (n = 6571) . | P-value . |
|---|---|---|---|---|
| Clinical characteristics | ||||
| Age ≥ 75, years | 1914 (26) | 387 (44) | 1527 (23) | <0.001 |
| Male | 5704 (77) | 633 (72) | 5071 (77) | 0.002 |
| BMI, kg/m2 | 27.09 ± 4.07 | 26.89 ± 4.15 | 27.12 ± 4.06 | 0.125 |
| Hypertension | 4695 (63) | 657 (75) | 4038 (61) | <0.001 |
| Anaemia | 1478 (20) | 271 (31) | 1207 (18) | <0.001 |
| Diabetes mellitus | 1755 (24) | 294 (34) | 1461 (22) | <0.001 |
| Renal failure | 93 (1) | 31 (4) | 62 (1) | <0.001 |
| Dialysis | 7 (0) | 3 (0) | 4 (0) | 0.039 |
| Peripheral vascular disease | 642 (9) | 165 (19) | 477 (7) | <0.001 |
| Previous CVA | 385 (5) | 80 (9) | 305 (5) | <0.001 |
| Previous PCI | 1099 (15) | 145 (17) | 954 (15) | 0.116 |
| Previous CABG | 246 (3) | 68 (8) | 178 (3) | <0.001 |
| Killip class III or IV | 221 (3) | 55 (6) | 166 (3) | <0.001 |
| STEMI | 3776 (51) | 353 (40) | 3423 (52) | <0.001 |
| NSTE-ACS | 3299 (44) | 458 (52) | 2841 (43) | <0.001 |
| LVEF < 35% | 643 (9) | 136 (16) | 507 (8) | <0.001 |
| Lesion characteristics | ||||
| Total number of lesions | 2.23 ± 1.36 | 3.05 ± 1.46 | 2.12 ± 1.31 | <0.001 |
| Number of calcified lesions | ||||
| None | 6571 (88) | 0 | 0 | <0.001 |
| One | 577 (8) | 577 (66) | 0 | <0.001 |
| Two | 198 (3) | 198 (23) | 0 | <0.001 |
| Three or more | 100 (1) | 100 (11) | 0 | <0.001 |
| LM or LAD | 5453 (73) | 769 (88) | 4684 (71) | <0.001 |
| LCX | 3705 (50) | 585 (67) | 3120 (48) | <0.001 |
| RCA | 3967 (53) | 628 (72) | 3339 (51) | <0.001 |
| Bypass graft | 56 (1) | 11 (1) | 45 (1) | 0.091 |
| TIMI flow pre-procedure | ||||
| 0 or 1 | 4817 (29) | 742 (28) | 4075 (29) | 0.114 |
| 2 | 1355 (8) | 212 (8) | 1143 (8) | 0.644 |
| 3 | 10 412 (63) | 1718 (64) | 8694 (62) | 0.080 |
| Management strategies | ||||
| PCI | 6726 (90) | 725 (83) | 6001 (91) | <0.001 |
| CABG | 309 (4) | 80 (9) | 229 (3) | <0.001 |
| Medical therapy | 367 (5) | 68 (8) | 299 (5) | <0.001 |
| Index PCI characteristics | ||||
| More than one vessel treated | 891 (13) | 170 (23) | 721 (12) | <0.001 |
| Lesions treated per patient | ||||
| One | 5296 (79) | 498 (69) | 4798 (80) | <0.001 |
| Two | 1165 (17) | 173 (24) | 992 (17) | <0.001 |
| Three or more | 259 (4) | 53 (7) | 206 (3) | <0.001 |
| Complex PCI | 3090 (46) | 465 (64) | 2625 (44) | <0.001 |
| Rotational atherectomy | 78 (1) | 55 (6) | 23 (0) | <0.001 |
| IVUS | 225 (3) | 45 (5) | 180 (3) | <0.001 |
| Type of stent | ||||
| DES | 7178 (75) | 883 (74) | 6295 (75) | 0.298 |
| BMS | 2350 (25) | 308 (26) | 2041 (24) | 0.315 |
| Direct stenting | 1742 (23) | 131 (15) | 1611 (25) | <0.001 |
| Post-dilation | 4483 (47) | 611 (51) | 3872 (46) | 0.002 |
| Number of stents per patient | 1.44 ± 0.87 | 1.65 ± 1.13 | 1.42 ± 0.84 | <0.001 |
| Procedural success | 6238 (93) | 616 (85) | 5622 (94) | <0.001 |
| Duration of procedure, min | 52.50 ± 28.28 | 58.05 ± 34.31 | 51.75 ± 27.28 | <0.001 |
| Total contrast volume, mL | 179.54 ± 85.47 | 199.58 ± 98.06 | 176.86 ± 83.28 | <0.001 |
| Average stent diameter per lesion, mm | 3.07 ± 0.48 | 3.04 ± 0.48 | 3.07 ± 0.48 | 0.043 |
| Average stent length per lesion, mm | 26.39 ± 14.88 | 30.88 ± 18.64 | 25.75 ± 14.15 | <0.001 |
| TIMI flow post-procedure | ||||
| 0 or 1 | 1560 (9) | 420 (16) | 1140 (8) | <0.001 |
| 2 | 371 (2) | 90 (3) | 281 (2) | <0.001 |
| 3 | 14 653 (88) | 2162 (81) | 12 491 (90) | <0.001 |
| Residual stenosis < 30% | 6420 (95) | 634 (87) | 5786 (96) | <0.001 |
Values are mean ± SD or n (%).
BMI, body max index; BMS, bare-metal stent; CABG, coronary artery bypass grafting; CVA, cerebrovascular accident; DES, drug-eluting stent; IVUS, intravascular ultrasound; LAD, left anterior descending artery; LCX, left circumflex; LM, left main; LVEF, left ventricle ejection fraction; NSTE-ACS, non-ST-segment elevation acute coronary syndrome; PCI, percutaneous coronary intervention; RCA, right coronary artery; STEMI, ST-segment elevation myocardial infarction; TIMI, Thrombolysis in Myocardial Infarction.
Angiographic findings and procedural characteristics
Patients with severe coronary calcification presented more extensive coronary artery disease as shown by a higher number of lesions. As compared to those without severe calcification, fewer patients with severe coronary calcification underwent PCI, whereas CABG or medical therapy-only were more frequent in this population (Table 1).
Among patients who underwent PCI, more than one vessel, were more frequently treated during index procedure in patients with severe coronary artery calcification as compared to those without (Table 1). While intracoronary imaging use was low in both groups, patients with severe coronary calcification underwent more frequent use of intravascular ultrasound. Direct stenting was less frequent in patients with severe coronary calcification, whereas post-dilation occurred more frequently. The proportion of DES use was similar in the two study groups (Table 1), although the average stent length of treated the segment was longer in severe calcified patients.
Patients with severe coronary calcification, as compared to those without, were less likely to achieve full procedural success with lower rates of final TIMI 3 and higher residual stenosis.
Clinical outcomes
At 1-year follow-up, the primary endpoint of MACE occurred in 237 (27.1%) patients with severe calcified coronary lesions and 985 (15%) patients without severe coronary calcified lesions [unadjusted hazard ratio (HR) 1.91; 95% confidence interval (CI) 1.66–2.20, P < 0.001; adjusted HR 1.23; 95% CI 1.06–1.44, P = 0.008] (Graphical abstract). The composite of cardiovascular death, MI, stroke, target vessel revascularization, or stent thrombosis occurred in 230 (26.4%) patients with and 928 (14.2%) patients without severe coronary calcified lesions (HR 1.97; 95% CI 1.70–2.27, P < 0.001). All-cause mortality rates were 8.6% in patients presenting with and 3.7% in patients without severe coronary calcified lesions (HR 2.38, 1.84–3.09, P < 0.001) (Table 2, Figures 1 and 2). Patients with severe coronary calcification had also higher rates of cardiovascular death, MI, target vessel revascularization, and definite stent thrombosis (Table 2, Supplementary material online, Appendix Supplementary material online, Figures S1–S5). After adjusting for baseline characteristics, results remained consistent (Table 2).
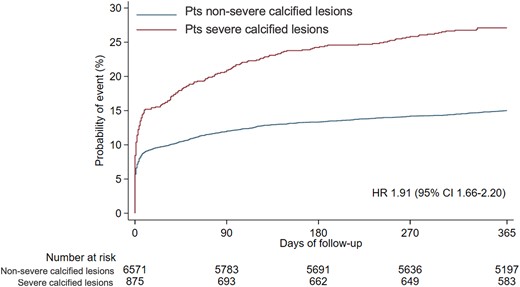
Composite primary endpoint at 365 days. CI, confidence interval; HR, hazard ratio.
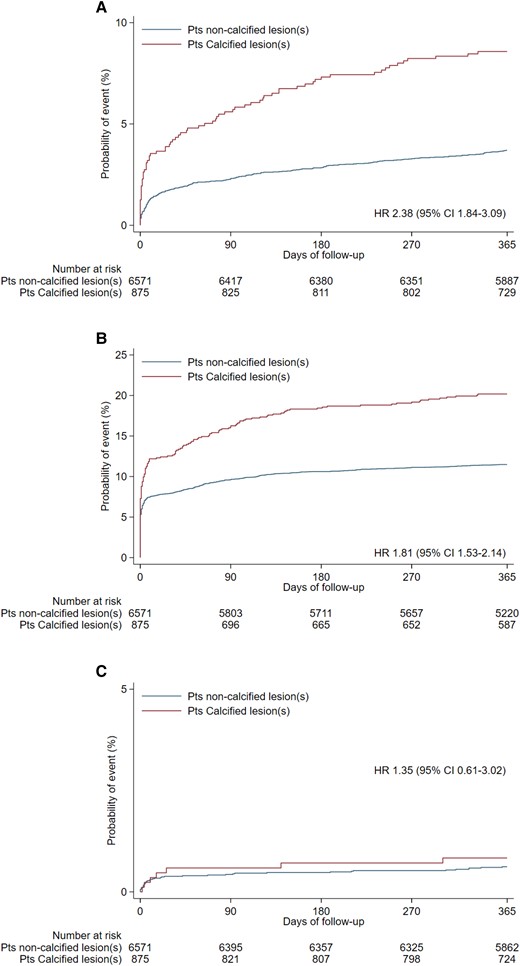
Individual components of composite primary endpoint at 365 days. (A) All-cause mortality, (B) myocardial infarction, (C) stroke. CI, confidence interval; HR, hazard ratio.
| Outcome . | Severe calcification (n = 875) . | Non-severe calcification (n = 6571) . | Hazard ratio (95% CI) . | P-value . | Adjusted hazard ratio (95%) . | P-value . |
|---|---|---|---|---|---|---|
| Primary composite endpoint of all-cause mortality, MI, or stroke | 237 (27.1) | 985 (15.0) | 1.91 (1.66–2.20) | <0.001 | 1.23 (1.06–1.44) | 0.008 |
| Composite endpoint of CV death, MI, stroke TVR, or ST | 230 (26.4) | 928 (14.2) | 1.97 (1.70–2.27) | <0.001 | 1.31 (1.11–1.53) | 0.001 |
| All-cause mortality | 75 (8.6) | 243 (3.7) | 2.38 (1.84–3.09) | <0.001 | 1.34 (1.01–1.77) | 0.039 |
| Cardiovascular death | 52 (6.0) | 154 (2.4) | 2.59 (1.89–3.55) | <0.001 | 1.48 (1.06–2.09) | 0.023 |
| MI | 171 (20.1) | 746 (11.5) | 1.81 (1.53–2.14) | <0.001 | 1.23 (1.02–1.47) | 0.029 |
| Stroke | 7 (0.8) | 40 (0.6) | 1.35 (0.61–3.02) | 0.463 | 0.94 (0.39–2.26) | 0.895 |
| TVR | 50 (6.0) | 160 (2.5) | 2.46 (1.79–3.38) | <0.001 | 1.82 (1.28–2.58) | 0.001 |
| TLR | 57 (6.8) | 225 (3.5) | 1.99 (1.48–2.65) | <0.001 | 1.47 (1.07–2.03) | 0.018 |
| Definite ST | 13 (1.5) | 47 (0.7) | 2.11 (1.14–3.90) | 0.017 | 2.32 (1.17–4.60) | 0.016 |
| Outcome . | Severe calcification (n = 875) . | Non-severe calcification (n = 6571) . | Hazard ratio (95% CI) . | P-value . | Adjusted hazard ratio (95%) . | P-value . |
|---|---|---|---|---|---|---|
| Primary composite endpoint of all-cause mortality, MI, or stroke | 237 (27.1) | 985 (15.0) | 1.91 (1.66–2.20) | <0.001 | 1.23 (1.06–1.44) | 0.008 |
| Composite endpoint of CV death, MI, stroke TVR, or ST | 230 (26.4) | 928 (14.2) | 1.97 (1.70–2.27) | <0.001 | 1.31 (1.11–1.53) | 0.001 |
| All-cause mortality | 75 (8.6) | 243 (3.7) | 2.38 (1.84–3.09) | <0.001 | 1.34 (1.01–1.77) | 0.039 |
| Cardiovascular death | 52 (6.0) | 154 (2.4) | 2.59 (1.89–3.55) | <0.001 | 1.48 (1.06–2.09) | 0.023 |
| MI | 171 (20.1) | 746 (11.5) | 1.81 (1.53–2.14) | <0.001 | 1.23 (1.02–1.47) | 0.029 |
| Stroke | 7 (0.8) | 40 (0.6) | 1.35 (0.61–3.02) | 0.463 | 0.94 (0.39–2.26) | 0.895 |
| TVR | 50 (6.0) | 160 (2.5) | 2.46 (1.79–3.38) | <0.001 | 1.82 (1.28–2.58) | 0.001 |
| TLR | 57 (6.8) | 225 (3.5) | 1.99 (1.48–2.65) | <0.001 | 1.47 (1.07–2.03) | 0.018 |
| Definite ST | 13 (1.5) | 47 (0.7) | 2.11 (1.14–3.90) | 0.017 | 2.32 (1.17–4.60) | 0.016 |
CV, cardiovascular; CI, confidence interval; MI, myocardial infarction; ST, stent thrombosis; TLR, target lesion revascularization; TVR, target vessel revascularization.
| Outcome . | Severe calcification (n = 875) . | Non-severe calcification (n = 6571) . | Hazard ratio (95% CI) . | P-value . | Adjusted hazard ratio (95%) . | P-value . |
|---|---|---|---|---|---|---|
| Primary composite endpoint of all-cause mortality, MI, or stroke | 237 (27.1) | 985 (15.0) | 1.91 (1.66–2.20) | <0.001 | 1.23 (1.06–1.44) | 0.008 |
| Composite endpoint of CV death, MI, stroke TVR, or ST | 230 (26.4) | 928 (14.2) | 1.97 (1.70–2.27) | <0.001 | 1.31 (1.11–1.53) | 0.001 |
| All-cause mortality | 75 (8.6) | 243 (3.7) | 2.38 (1.84–3.09) | <0.001 | 1.34 (1.01–1.77) | 0.039 |
| Cardiovascular death | 52 (6.0) | 154 (2.4) | 2.59 (1.89–3.55) | <0.001 | 1.48 (1.06–2.09) | 0.023 |
| MI | 171 (20.1) | 746 (11.5) | 1.81 (1.53–2.14) | <0.001 | 1.23 (1.02–1.47) | 0.029 |
| Stroke | 7 (0.8) | 40 (0.6) | 1.35 (0.61–3.02) | 0.463 | 0.94 (0.39–2.26) | 0.895 |
| TVR | 50 (6.0) | 160 (2.5) | 2.46 (1.79–3.38) | <0.001 | 1.82 (1.28–2.58) | 0.001 |
| TLR | 57 (6.8) | 225 (3.5) | 1.99 (1.48–2.65) | <0.001 | 1.47 (1.07–2.03) | 0.018 |
| Definite ST | 13 (1.5) | 47 (0.7) | 2.11 (1.14–3.90) | 0.017 | 2.32 (1.17–4.60) | 0.016 |
| Outcome . | Severe calcification (n = 875) . | Non-severe calcification (n = 6571) . | Hazard ratio (95% CI) . | P-value . | Adjusted hazard ratio (95%) . | P-value . |
|---|---|---|---|---|---|---|
| Primary composite endpoint of all-cause mortality, MI, or stroke | 237 (27.1) | 985 (15.0) | 1.91 (1.66–2.20) | <0.001 | 1.23 (1.06–1.44) | 0.008 |
| Composite endpoint of CV death, MI, stroke TVR, or ST | 230 (26.4) | 928 (14.2) | 1.97 (1.70–2.27) | <0.001 | 1.31 (1.11–1.53) | 0.001 |
| All-cause mortality | 75 (8.6) | 243 (3.7) | 2.38 (1.84–3.09) | <0.001 | 1.34 (1.01–1.77) | 0.039 |
| Cardiovascular death | 52 (6.0) | 154 (2.4) | 2.59 (1.89–3.55) | <0.001 | 1.48 (1.06–2.09) | 0.023 |
| MI | 171 (20.1) | 746 (11.5) | 1.81 (1.53–2.14) | <0.001 | 1.23 (1.02–1.47) | 0.029 |
| Stroke | 7 (0.8) | 40 (0.6) | 1.35 (0.61–3.02) | 0.463 | 0.94 (0.39–2.26) | 0.895 |
| TVR | 50 (6.0) | 160 (2.5) | 2.46 (1.79–3.38) | <0.001 | 1.82 (1.28–2.58) | 0.001 |
| TLR | 57 (6.8) | 225 (3.5) | 1.99 (1.48–2.65) | <0.001 | 1.47 (1.07–2.03) | 0.018 |
| Definite ST | 13 (1.5) | 47 (0.7) | 2.11 (1.14–3.90) | 0.017 | 2.32 (1.17–4.60) | 0.016 |
CV, cardiovascular; CI, confidence interval; MI, myocardial infarction; ST, stent thrombosis; TLR, target lesion revascularization; TVR, target vessel revascularization.
Among patients with severe coronary calcification who underwent PCI, higher rates of the primary endpoint and secondary composite endpoint, as well as all-cause mortality, cardiovascular death, MI, TLR, target vessel revascularization, and definite stent thrombosis compared to those patients without severe calcification (Table 1 Supplementary material online, Appendix). After adjusting for baseline characteristics, results remained largely consistent (Table 1 Supplementary material online, Appendix).
Subgroup and landmark analysis
In Figure 3, the stratified analysis across the clinical and angiographic covariates showed consistency with the main analysis, except for a more pronounced difference in patients under 75 years, those presenting with STEMI, and in patients with a higher number of severely calcified lesions. After stratifying according to the revascularization strategy (PCI vs. CABG), non-statistically significant differences were found for the primary endpoint and its individual components between groups (see Supplementary material online, Appendix Supplementary material online, Figure S6).
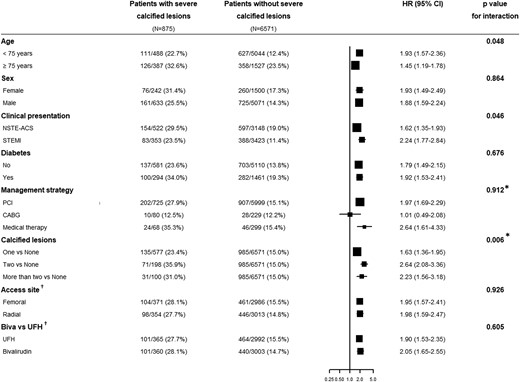
Subgroup analysis of the primary endpoint. Biva, bivalirudin; CABG, coronary artery bypass grafting; CI, confidence interval; HR, hazard ratio; NSTE-ACS, non-ST-segment elevation acute coronary syndrome; PCI, percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction; UFH, unfractionated heparin. *P-value for trend across ordered groups. †For patients undergoing PCI.
Landmark analysis showed an increased risk of the primary composite endpoint as well as all-cause mortality and MI in patients with severe coronary calcification at 30-day post-procedure and also for the period between 30-day and 1-year follow-up (see Supplementary material online, Appendix Supplementary material online, Figures S7–S10).
Discussion
This post hoc analysis of the MATRIX programme including 7446 patients and 16 584 lesions represents the largest study that investigated the role of coronary calcification in the whole spectrum of patients presenting with ACS undergoing initial invasive management. The main findings of this study can be summarized as follows:
Severe coronary calcification lesions were frequent in patients presenting with ACS.
Fewer patients with severe coronary calcification, as compared to those without, underwent PCI whereas CABG or medical therapy-only was more frequent in patients with severe coronary calcification.
Patients with severe coronary calcification who underwent PCI required more and longer stents and were less likely to achieve full procedural success with lower rates of final TIMI 3 and higher residual stenosis, as compared to patients without severe coronary calcification.
Patients with severe coronary calcification undergoing initial invasive management had worse clinical outcomes in terms of MACE, all-cause mortality, cardiovascular death, MI, TLR, target vessel revascularization, and define stent thrombosis, as compared to patients without severe coronary calcification.
Previous reports have shown that patients with calcified lesions undergoing PCI have worse clinical outcomes in terms of MACE, all-cause mortality, MI, and TLR, compared to those without coronary calcification; identifying calcification of the coronary tree as an independent predictor of worse prognosis.6,8,9,15–17 However, most of the data derives from studies of the bare-metal stent era, from studies mainly including patients with chronic coronary syndromes or focusing on procedural outcomes in ACS patients.9,15,16,18–20 The pooled analysis from the Harmonizing Outcomes With Revascularization and Stents in Acute Myocardial Infarction (HORIZONS-AMI) and Acute Catheterization and Urgent Intervention Triage Strategy (ACUITY) trials that included 6855 patients presenting with ACS in whom PCI was performed, was the largest available study evaluating the impact of coronary calcification among patients undergoing PCI for ACS. In such study, moderate/severe coronary calcification was associated with an increased unadjusted 1-year rate of death, cardiac death, definite stent thrombosis, and TLR. The presence of moderate/severe target lesion calcification was an independent predictor for 1-year definite stent thrombosis and TLR.9 However, no prior study had examined the role of severe coronary calcification in the whole spectrum of patients presenting with ACS. The present investigation provides the first and largest evidence about the impact of coronary calcification in patients presenting with ACS undergoing PCI, CABG, or medical therapy management-only. In our study, after initial coronary angiography, a higher proportion of patients with severe coronary calcification did not undergo PCI but more underwent CABG or medical therapy-only more frequently. Patients with severe coronary calcification were associated with an increased adjusted 1-year rate of MACE, all-cause mortality, cardiovascular death, MI, TLR, target vessel revascularization, and define stent thrombosis, as compared to patients without severe coronary calcification, irrespective of the management strategy. In patients undergoing PCI, severe coronary calcification remained associated with lower rates of procedural success, final TIMI 3, and higher residual stenosis; leading to increased rate adverse cardiovascular events. Several mechanisms such as stent underexpansion, malapposition, polymer disruption, impaired drug delivery with drug-eluting stents, and procedural complications (slow flow, no reflow, dissection or coronary perforation) have been postulated to explain the worse clinical outcomes in patients undergoing PCI of severe calcified coronary lesions.21–24 In our study, rotational atherectomy was very infrequently used in patients with severe coronary calcification. Of note, when the study was performed, more contemporary tools for the management of severe calcified lesions such as lithotripsy were not available. Despite that rotational atherectomy has been the predominant available technology for ages, its use in the setting of ACS remains low; probably due to the concerns for further platelet activation and higher risk of slow or no reflow as well as for the technical difficulties linked to its use.25 Whether more operator-friendly tools such as coronary lithotripsy, cutting/scoring balloons, or super high-pressure balloons might improve the outcomes for these patients, at least in terms of procedural success, yet remains unknown and deserves further investigation.
Severe coronary calcification has also shown to be associated with worse clinical outcomes in patients undergoing CABG. In the ACUITY trial, 755 patients presenting with ACS underwent CABG. Severe coronary calcification was identified as an independent predictor of 1-year mortality and MACE or MI. Recently, a substudy of the Synergy Between PCI With Taxus and Cardiac Surgery Extended Survival (SYNTAXES) among 1800 patients with three-vessel disease and/or left main disease randomized to PCI or CABG has shown that patients with heavily calcified lesions had higher 10-year all-cause mortality compared with those without calcified lesions who showed similar mortality rates among PCI and CABG in patients with heavily calcified lesions. Our findings support the increased risk of cardiovascular events in patients presenting with ACS and severely coronary calcified lesions irrespective of the management strategy, as shown by the lack of significant interaction for MACE between treatment (PCI, CABG, or medical therapy) and the presence or absence of severe calcified lesions (P = 0.912, trend test). At subgroup analysis according to the revascularization strategy (PCI vs. CABG), no differences for the primary endpoint and its individual components were found between groups, though a numerically higher rate of MI was observed among patients undergoing PCI. Interestingly, the outcomes of medically treated patients with severe coronary calcification were worse in patients with, compared with those without severe coronary calcification, whereas it was similar with CABG in the two study groups. Notwithstanding, these results should be interpreted with caution given the limited number of patients undergoing CABG (n = 309) or medical therapy-only (n = 367).
Coronary calcification in patients presenting with ACS does not only impact at the level of the culprit lesion. In our study, calcification of the coronary tree was associated with advanced age, higher rates of hypertension, anaemia, diabetes mellitus, chronic kidney disease, previous cerebrovascular disease, and peripheral vascular disease; translating a more extensive coronary artery disease. This has been shown in the landmark analysis of the present investigation, where severe coronary calcification was associated with worse prognosis not only at short-term (i.e. within 30-day post-procedure) but also at long-term follow-up. Coronary calcification is correlated with the overall extent of plaque burden, as such it is associated with an increase of the overall risk for non-stent-related adverse cardiovascular events, as well as complications.26–28 The present large-scale study confirms and extends these findings into the clinical setting of patients presenting with ACS.
Coronary artery calcium assessment by computed tomography has shown to be a strong predictive value for incident cardiovascular disease events. In the Multi-Ethnic Study of Atherosclerosis (MESA) study, coronary artery calcification volume was positively and independently associated with the risk of cardiovascular events. However, at any level of coronary artery calcium volume, density was inversely and significantly associated with the risk of cardiovascular events.27 In a SYNTAX III substudy, Andreini et al.29 assessed whether severe coronary calcification significantly limited coronary computed tomography angiography (CCTA) evaluation and the impact it had on heart team’s treatment decision. A total of 222 patients with available CCTA and coronary angiography were included. The presence of heavy coronary calcification moderately influenced the agreement between CCTA and ICA on the anatomical SYNTAX score. However, agreement on the treatment decision and planning was high and irrespective of the presence of calcified lesions.29
The results of our investigation support the concept that angiography-detected coronary calcification is a marker of extensive atherosclerosis disease associated with worse clinical outcomes irrespective of the management strategy in patients presenting with ACS; providing additional information to current prognostic models aimed to stratify the risk of patients with coronary artery disease.30,31 Therefore, given the difficulties of coronary calcium assessment by computed tomography in patients presenting with ACS, the development of angiography-based methods would be of paramount clinical relevance in order to improve cardiovascular risk prediction in this population.
Study limitations
The present study should be interpreted considering some limitations. First, coronary calcification was based on coronary angiography and not in intravascular imaging, which has been shown to have higher sensitivity/specificity in detecting the presence of coronary calcium.32,33 However, angiography has a high specificity and has been shown to provide useful prognostic information. Second, coronary calcification was classified in a binary fashion as follows: (i) patients with severe coronary calcification, and (ii) patients without severe coronary calcification. Despite that this might be considered a limitation of the present investigation, we based our decision on the low intraobserver and interobserver variability for the discrimination between none/mild and moderate/severe calcification.34 Such strategy has been previously adopted and validated in several clinical studies.15 Third, coronary calcification was not adjudicated by an angiographic core laboratory, increasing the risk of operator variability and subsequent bias. Fourth, despite an independent clinical events committee masked to treatment allocation adjudicated all suspected events, such adjudication was not lesion-based. Therefore, TLR was investigator-reported, leading to reporting bias.
Conclusion
Patients with severe coronary calcification presenting with ACS are associated with worse clinical outcomes irrespective of the management strategy. Coronary calcification assessment by coronary angiography is a marker of extensive atherosclerosis disease and should be taken into account in future prognostic risk models.
Supplementary material
Supplementary material is available at European Heart Journal: Acute Cardiovascular Care online.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
References
Author notes
The first two authors contributed equally to the study.
In line with the Journal’s conflict of interest policy, this paper was handled by Elke Platz.
Conflict of interest: J.S.S. has received minor speaking honoraria from Terumo, Cordis, Biotronik, and Medtronic. H.M.G.-G. reports the following institutional grant support: Biotronik, Boston Scientific, Medtronic, Abbott, Neovasc, Shockwave, Phillips, and Corflow. G.A. reports minor speaking honoraria from Chiesi, Daiichi Sankyo, Boeringer Ingelheim, Bayer, Pfizer, and Biosensors. D.H. has participated on data safety monitoring board or advisory board of switch. S.L. has received consulting fees from AstraZeneca, Bayer, BMS/Pfizer, Chiesi, Daiichi-Sankyo, Icon, and Novonordisk. M.S. has received consulting fees from Abbott Vascular and iVascular. A.v.H. reports unrestricted grants from Medtronic, Abbott Vascular, and Boehringer Ingelheim and consulting fees from Celecor Therapeutics. P.V. reports consulting fees from Daiichi Sankyo, CSL Behring, Pfizer/Bristol Meyers Squibb alliance, Bayer AG, and Novartis; minor speaking honoraria from Daicchi Sankyo and Pfizer/Bristol Meyers Squibb alliance. M.V. reports consulting fees from Abbott, Alvimedica, Bayer Healthcare, Biotronik, Boston Scientific Corporation, Chiesi Farmaceutici, CoreFlow, Daiichi Sankyo, Idorsia, Medtronic, Novartis Pharma, PHASEBIO, Terumo, University of Basel, Vesalio, and Vifor Pharma. The rest of authors have no disclosures to declare.




Comments