-
PDF
- Split View
-
Views
-
Cite
Cite
Andreas Goette, Gregory Y H Lip, Bulent Gorenek, What acute cardiac care physicians need to know from the latest 2022 ESC Guidelines for ventricular tachycardia and sudden cardiac death, European Heart Journal. Acute Cardiovascular Care, Volume 12, Issue 1, January 2023, Pages 62–68, https://doi.org/10.1093/ehjacc/zuac149
Close - Share Icon Share
Abstract
The present paper summarizes and comments on the latest 2022 ESC guidelines on ventricular tachycardia and sudden cardiac death. Most relevant recommendations for acute cardiovascular care physicians are addressed, particularly, in the fields of coronary artery disease, dilated cardiomyopathy, and inflammatory diseases. New recommendations encompass the implantation of a defibrillator (ICD) in the setting of acute myocarditis. Furthermore, the pathophysiology of the electrical storm including involved molecular pathways as well as the angry Purkinje fibre syndrome is presented and discussed.
In the last years, several news aspects about the therapy of ventricular tachycardia (VT) and sudden cardiac death (SCD) have been published in various ESC guidelines over the last years.1–3 Of note, pathophysiological evidence also suggests that arrhythmia patterns, concomitant disease, and so on influence the occurrence of arrhythmia and prognosis.4–7 The latest ESC guideline on VT and SCD summarizes several important aspects, which are relevant for acute cardiovascular care (ACVC) physicians (Table 1). The present commentary highlights aspects focusing on VT and electrical storms since both conditions are of utmost importance.8–10 Furthermore, new recommendations for VT/SCD management encompassing coronary artery disease and inflammatory diseases are discussed. Both topics have high relevance in the ACVC setting. Importantly, VT has to be differentiated from other mechanisms responsible for wide QRS tachycardia like rapid atrioventricular conduction via antegrade conducting accessory pathway (Figure 1A and B). Thus, specific tachycardia mechanisms may require certain therapies, which are also addressed in the current guidelines. The central figure of the ESC guidelines summarizes clinical factors of different VT/SCD entities, which should also be considered by ACVC physicians (Figure 2).
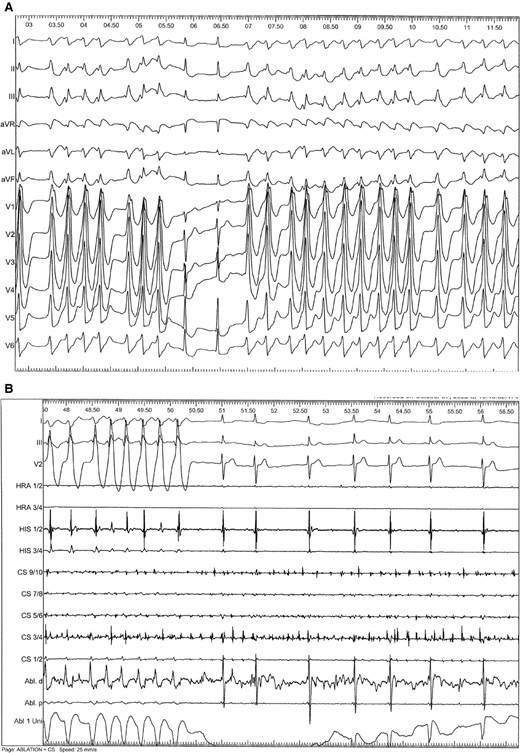
(A) Unusual mechanism of wide QRS tachycardia. Fast atrioventricular conduction of atrial fibrillation via a left-sided posterolateral bypass tract causing a wide QRS tachycardia. Of note, QRS complexes vary in QRS width and the RR intervals are absolute irregular. Intermittently, there is conduction through the AV node, which causes regular QRS complexes during tachycardia. This phenomenon must be differentiated from true fusion beats, which would prove the presence of VT. (B) Catheter ablation a left-sided posterolateral bypass tract (Abl. d = distal ablation catheter) causes narrowing of the QRS complexes. Different intracardiac electrograms are also shown (HRA, HIS, CS). Thereafter, atrial fibrillation is conducted through the AV node only, causing slowing of the ventricular response due to decremental conduction properties of the AV node.
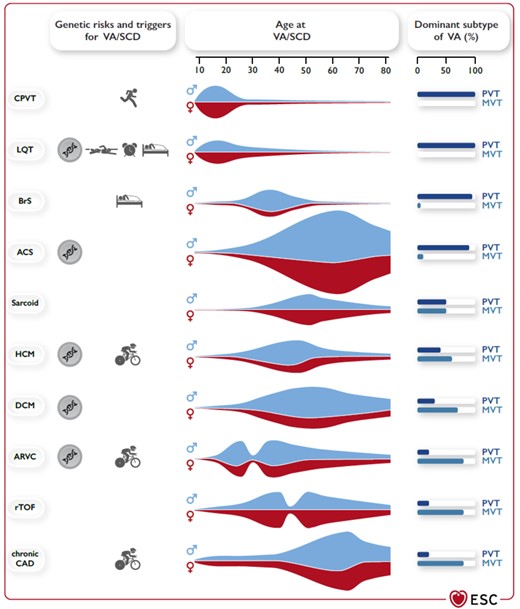
Clinical presentation for VT and SCD in accordance to underlying diseases. Genetic risk for VT/SCD, typical triggers for VT/SCD, age at presentation with VT/SCD, sex predominance, and typical VT. (PVT/VF vs. MVT) in different diseases associated with VA/SCD. ACS, acute coronary syndrome; ARVC, arrhythmogenic right ventricular cardiomyopathy; BrS, Brugada syndrome; CAD, coronary artery disease; CPVT, catecholaminergic polymorphic ventricular tachycardia; DCM, dilated cardiomyopathy; HCM, hypertrophic cardiomyopathy; LQT, long QT syndrome; MVT, monomorphic ventricular tachycardia; PVT, polymorphic ventricular tachycardia; rTOF, repaired tetralogy of Fallot; SCD, sudden cardiac death; VA, ventricular arrhythmia; VF, ventricular fibrillation.(adopted from ref. 2).
| Premature ventricular complex (PVC): Premature occurrence of an abnormal QRS complex (duration typically ≥120 ms, corresponding T-wave typically broad and in the opposite direction of the major QRS deflection, no preceding P-wave). |
| Unifocal or monomorphic PVCs: PVCs with a single QRS morphology. |
| Multifocal, multiform, or polymorphic PVCs: PVCs with different QRS morphologies. |
| Short-coupled PVC: A PVC that interrupts the T-wave of the preceding conducted beat. |
| Ventricular tachycardia (VT): ≥ 3 consecutive beats with a rate. |
| Non-sustained ventricular tachycardia (NSVT): Run of consecutive ventricular beats persisting for 3 beats to 30 s. |
| Monomorphic ventricular tachycardia (MVT): Same QRS morphology from beat to beat. |
| Polymorphic ventricular tachycardia (PVT): Continually changing QRS morphology. |
| Sustained monomorphic/polymorphic ventricular tachycardia (SMVT/SPVT): Continuous VT for at least 30 s, or which requires an intervention for termination. |
| Ventricular fibrillation (VF): A chaotic rhythm with undulations that are irregular in timing and morphology, without discrete QRS complexes on the surface ECG. |
| Electrical storm: VA that occurs 3 or more times within 24 h (separated by at least 5 min), each requiring termination by an intervention. |
| Incessant VT: Continuous sustained VT that recurs promptly despite repeated intervention for termination over several hours. |
| Premature ventricular complex (PVC): Premature occurrence of an abnormal QRS complex (duration typically ≥120 ms, corresponding T-wave typically broad and in the opposite direction of the major QRS deflection, no preceding P-wave). |
| Unifocal or monomorphic PVCs: PVCs with a single QRS morphology. |
| Multifocal, multiform, or polymorphic PVCs: PVCs with different QRS morphologies. |
| Short-coupled PVC: A PVC that interrupts the T-wave of the preceding conducted beat. |
| Ventricular tachycardia (VT): ≥ 3 consecutive beats with a rate. |
| Non-sustained ventricular tachycardia (NSVT): Run of consecutive ventricular beats persisting for 3 beats to 30 s. |
| Monomorphic ventricular tachycardia (MVT): Same QRS morphology from beat to beat. |
| Polymorphic ventricular tachycardia (PVT): Continually changing QRS morphology. |
| Sustained monomorphic/polymorphic ventricular tachycardia (SMVT/SPVT): Continuous VT for at least 30 s, or which requires an intervention for termination. |
| Ventricular fibrillation (VF): A chaotic rhythm with undulations that are irregular in timing and morphology, without discrete QRS complexes on the surface ECG. |
| Electrical storm: VA that occurs 3 or more times within 24 h (separated by at least 5 min), each requiring termination by an intervention. |
| Incessant VT: Continuous sustained VT that recurs promptly despite repeated intervention for termination over several hours. |
| Premature ventricular complex (PVC): Premature occurrence of an abnormal QRS complex (duration typically ≥120 ms, corresponding T-wave typically broad and in the opposite direction of the major QRS deflection, no preceding P-wave). |
| Unifocal or monomorphic PVCs: PVCs with a single QRS morphology. |
| Multifocal, multiform, or polymorphic PVCs: PVCs with different QRS morphologies. |
| Short-coupled PVC: A PVC that interrupts the T-wave of the preceding conducted beat. |
| Ventricular tachycardia (VT): ≥ 3 consecutive beats with a rate. |
| Non-sustained ventricular tachycardia (NSVT): Run of consecutive ventricular beats persisting for 3 beats to 30 s. |
| Monomorphic ventricular tachycardia (MVT): Same QRS morphology from beat to beat. |
| Polymorphic ventricular tachycardia (PVT): Continually changing QRS morphology. |
| Sustained monomorphic/polymorphic ventricular tachycardia (SMVT/SPVT): Continuous VT for at least 30 s, or which requires an intervention for termination. |
| Ventricular fibrillation (VF): A chaotic rhythm with undulations that are irregular in timing and morphology, without discrete QRS complexes on the surface ECG. |
| Electrical storm: VA that occurs 3 or more times within 24 h (separated by at least 5 min), each requiring termination by an intervention. |
| Incessant VT: Continuous sustained VT that recurs promptly despite repeated intervention for termination over several hours. |
| Premature ventricular complex (PVC): Premature occurrence of an abnormal QRS complex (duration typically ≥120 ms, corresponding T-wave typically broad and in the opposite direction of the major QRS deflection, no preceding P-wave). |
| Unifocal or monomorphic PVCs: PVCs with a single QRS morphology. |
| Multifocal, multiform, or polymorphic PVCs: PVCs with different QRS morphologies. |
| Short-coupled PVC: A PVC that interrupts the T-wave of the preceding conducted beat. |
| Ventricular tachycardia (VT): ≥ 3 consecutive beats with a rate. |
| Non-sustained ventricular tachycardia (NSVT): Run of consecutive ventricular beats persisting for 3 beats to 30 s. |
| Monomorphic ventricular tachycardia (MVT): Same QRS morphology from beat to beat. |
| Polymorphic ventricular tachycardia (PVT): Continually changing QRS morphology. |
| Sustained monomorphic/polymorphic ventricular tachycardia (SMVT/SPVT): Continuous VT for at least 30 s, or which requires an intervention for termination. |
| Ventricular fibrillation (VF): A chaotic rhythm with undulations that are irregular in timing and morphology, without discrete QRS complexes on the surface ECG. |
| Electrical storm: VA that occurs 3 or more times within 24 h (separated by at least 5 min), each requiring termination by an intervention. |
| Incessant VT: Continuous sustained VT that recurs promptly despite repeated intervention for termination over several hours. |
Acute management of VT/SCD
There is some new information about the acute management of VT/VF in the 2022 VT/SCD guidelines.2,3 The aspect of reversible cause and VT/VF is highlighted in more detail: so-called reversible causes may account for half of SCD cases. Besides underlying cardiac diseases, electrolyte imbalances, such as hypokalaemia, are known to cause VT/SCD. Hypomagnesaemia and/or hypokalaemia may be associated with Torsades de pointes (TdP). Of note, intravenous magnesium is quite effective for TdP even in the absence of hypomagnesaemia. Other reversible factors such as cardiac ischaemia, thrombosis, fever, acute starvation, and so on may trigger VT/SCD. Another cause might be the impact of drug-induced VT/VF. This should be suspected in cases pre-treated with QRS and/or QT-prolonging agents. Relative bradycardia is another factor, which might cause repetitive VT and recurrent TdP in the setting of acquired long QT. In such cases, transvenous pacing at rates between 90 and 110 bpm might help to acutely suppress VT/VF. Overall, even patients who survive SCD caused by reversible conditions have high mortality. Observational data showed that ICD implantation is beneficial and reduces all-cause mortality in SCD patients due to reversible causes. Overall, the implementation of cardiac arrest centres (https://doi.org/10.1007/s10049-021-00920-x) appears as an important concept to improve the treatment of patients with VT and SCD. Furthermore, cardiac arrest centres will help to collect data from patients with SCD in prospective registries.
Management of electrical storm
An electrical storm is associated with high acute mortality and a worse prognosis during follow-up.2,3,7–10 Pathophysiologically, the impact of the sympathetic nervous system in association with calmodulin-dependent intracellular signalling causing calcium release from the sarcoplasmatic reticulum with subsequent early and delayed after depolarizations have recently been described (Figure 3). It might be realistic to study several molecular drug targets in the context of electrical storms in the future. Inhibition of Ca2+/calmodulin-dependent protein kinase II (CaMKII) appears as one highly interesting target.8 The severity of an electrical storm varies and encompasses asymptomatic VT episodes as well as deleterious electrical instability requiring multiple electrical cardioversions or defibrillation attempts. A recent meta-analysis implies that already two arrhythmia episodes during one day, but also within weeks cause a worsening of the prognosis. Thus, it remains currently unclear if the current definition of electrical storms should be re-defined (Figure 4).
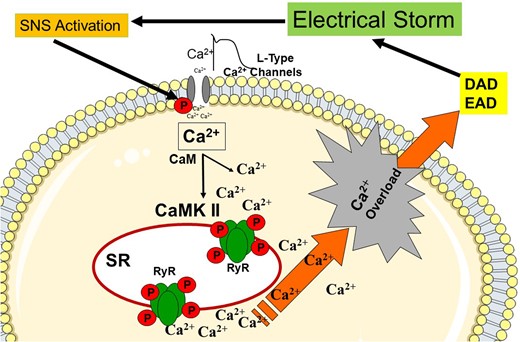
Pathophysiological concept of electrical storm. Activated sympathetic nervous system (SNS) increases the cytosolic Ca2 + concentration by L-type Ca2 + channels and Ca2+/calmodulin-dependent protein kinase II (CaMKII), and ryanodine receptors (RyR). Ca2 + overload triggers after depolarization (early AD, delayed AD) CaM, calmodulin; SR, sarcoplasmic reticulum; DAD, delayed after depolarization; and EAD, early after depolarization.
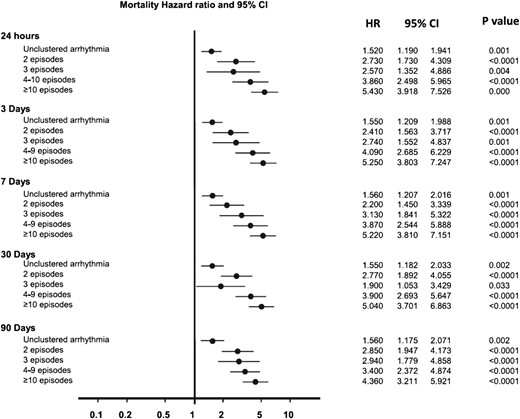
Mortality in association with number of VT episodes in various time intervals. Hazard ratio (HR) for mortality according to ventricular arrhythmia burden. Five separate time varying Cox models were used to correlate mortality HR with the highest cluster burden. (Adopted from ref. 8).
In cases of inappropriate ICD shocks, disabling ICD therapies is recommended. If hemodynamic instability is present institution of advanced life support is recommended.2 Reversible concomitant factors must be treated. Specific treatment of an electrical storm often requires ICD reprogramming, AAD therapy, sedation, catheter ablation, autonomic modulation, and mechanical circulatory support. Importantly, deep sedation and mechanical ventilation are often indicated to reduce psychological distress and reduce pro-arrhythmogenic sympathetic tone.2,3,7–10 Initial beta-blocker therapy, e.g. non-selective beta-blockers such as propranolol, was found to be superior to metoprolol. Most commonly, beta-blockers are combined with amiodarone. In patients with recurrent haemodynamically not-tolerated VTs resistant to amiodarone, landiolol (ultra-short-acting selective blocker) was found to be effective for arrhythmia suppression.2 If beta-blockers are insufficient or not tolerated, autonomic modulation, i.e. percutaneous ganglionic stellate blockade, thoracic epidural anaesthesia, or left cardiac sympathetic denervation might be used. In the presence of structural heart diseases, catheter ablation should be considered in particular in patients with incessant slow monomorphic VT.2 Nevertheless, catheter ablation should be considered in recurrent symptomatic episodes of polymorphic VT or VF episodes triggered by premature ventricular complexes. Therefore, the recording of a 12-lead ECG at the initiation of VT or during VT is of high importance in all cases of an electrical storm or recurrent VT. Institution of mechanical circulatory support is debatable. In the latest guideline, it is clearly shown that the underlying aetiology determines further management. Recently, different approaches to defibrillate refractory VF were introduced. These highly interesting results are not included in the current VT/SCD Guidelines.2 Nevertheless, the use of two separate defibrillators or an anterior–posterior position was the defibrillation pads appearing to be helpful to terminate VF in such critical situations.11
Current ESC guideline recommendations to treat an electrical storm are as follows:
Mild to moderate sedation is recommended in patients with an electrical storm to alleviate psychological distress and reduce sympathetic tone (Level I C).
Antiarrhythmic therapy with beta-blockers (non-selective preferred) in combination with intravenous amiodarone is recommended in patients with SHD and electrical storm unless contraindicated (Level I B).
Intravenous magnesium with supplementation of potassium is recommended in patients with polymorphic VT (Level I C).
Isoproterenol or transvenous pacing to increase heart rate is recommended in patients with acquired LQT syndrome and recurrent TdP despite correction of precipitating conditions and magnesium. Level I C catheter ablation is recommended in patients presenting with incessant VT or electrical storm due to SMVT refractory to AADs (Level I B).
Deep sedation/intubation should be considered in patients with an intractable electrical storm refractory to drug treatment (Level IIa C).
Catheter ablation should be considered in patients with recurrent episodes of polymorphic VT/VF triggered by a similar ectopic premature ventricular complex (PVC), non-responsive to medical treatment or coronary revascularization (Level IIa C).
Angry Purkinje fibre syndrome
ST-elevation myocardial infarction (STEMI) patients have an incidence 4–12% of VT/VF within the first 48 h after the onset of clinical presentation.1–3 Pre-reperfusion VT/VF are more common than reperfusion-induced or post-reperfusion arrhythmias. Electrical storms and/or recurrent VF in the early post-MI period are life-threatening.1–3 Therefore ischaemia needs to be excluded as the triggering factor. Nevertheless, Friedman et al.12 described the pathophysiology of the so-called ‘angry Purkinje fibre syndrome’ in 1973. Bipolar electrograms recorded from subendocardial regions of infarcted myocardium demonstrated the persistence of Purkinje fibre activity. Using transmembrane action potential recordings, they could record subendocardial Purkinje fibre activity within infarcted regions.12 Of note, action potentials of ventricular myocytes were absent. Thus, despite the presence of myocardial infarction subendocardial Purkinje fibres survived in the infarct area.12 In addition, these fibres had reduced maximum diastolic potentials, action potential amplitudes, and maximum depolarization velocities compared with normal subendocardial Thus, surviving subendocardial Purkinje fibres in infarcted regions can develop abnormal action potentials, and thereby, participate in the genesis of VT/VF.12–15 In clinical practice, focally triggered VF storm in post-MI patients catheter ablation targeting the culprit triggers might be lifesaving. Reports showed improved short- and long-term freedom from recurrent VF storms.2,12–15 Of note, the triggering ectopic activities most commonly originate from the surviving Purkinje tissue over the border zone of an ischaemic scar.12 Furthermore, early intervention after the occurrence of a VF storm reduces the risk of cardiovascular mortality.2 Taken together, this angry Purkinje fibre syndrome requires aggressive antiarrhythmic therapy including ICD implantation or/or catheter ablation.
The current ESC Guideline recommendation is as follows:
ICD implantation is recommended in patients without ongoing ischaemia with documented VF or haemodynamically not-tolerated VT occurring later than 48 h after myocardial infraction (Level I A)
Survivors of sudden cardiac arrest
An algorithm for the evaluation of sudden cardiac arrest survivors has been clearly presented in the present ESC Guideline.2 Coronary angiography is recommended in STEMI patients. Of note, randomized controlled trials have found no benefit for early coronary angiography in SCD patients without ST elevation. In case of electrical instability, or suspicious of ongoing ischaemia, a coronary angiography might be performed. Brain and chest CT scans may identify non-cardiac causes of SCD, as well as toxicological analyses. Assertion of suitable blood samples is also important to allow further diagnostic work up, including DNA analysis. A 12-lead ECG at baseline and during recovery is of high importance in all patients with VT and SCD. Furthermore, an echocardiogram and coronary artery imaging are important to find a definite diagnosis. MRI has been demonstrated to add significantly to make to correct diagnosis, in particular in inflammatory diseases and cardiomyopathies. In patients with primary electrical diseases provocative manoeuvers such as sodium channel blocker challenge, adenosine challenge, epinephrine challenge, ergonovine/acetylcholine, and so on might be useful. Retaining tissue or biosamples for DNA analyses appears to be of importance for post-mortem evaluation. In addition, the implementation of cardiac arrest centres (https://doi.org/10.1007/s10049-021-00920-x) appears as an important concept to improve the treatment of patients with VT and SCD. Enrolment of patients into prospective SCD registries will also help to improve patient care in the future.
The recommendation in CAD patients with aborted SCD:
In electrically unstable patients after aborted SCD, with suspicion of ongoing myocardial ischaemia, a coronary angiogram is indicated (Level I C)
New 2020 ESC guideline recommendations
Several new recommendations, as well as changes to previous guidelines, are presented in the field of coronary artery disease (CAD) in the present version of the ESC Guidelines.2 Most relevant in the field of ACVC are as follows:
In patients with CAD and recurrent, symptomatic stable monomorphic VT (SMVT), or ICD shocks for SMVT despite chronic amiodarone therapy, catheter ablation is recommended in preference to escalating antiarrhythmic drug (AAD) therapy (Level I). Thus, amiodarone is a drug of choice and the first step of therapy in SMVT patients, but thereafter, catheter ablation should be performed in case of SMVT recurrence.
In SCD survivors with coronary artery spasms, implantation of an ICD should be considered (Level IIa). Thus, coronary artery spasms must be taken seriously if detected on detected coronary angiography. Patients should be closely followed to assess the effect of medical therapy.
Catheter ablation should be considered in patients with CAD and recurrent, symptomatic SMVT, or ICD shocks for SMVT despite beta-blocker or sotalol treatment (Level IIa). Treatment with sotalol is controversial in many countries. Thus, beta-blockers are commonly used in CAD patients in particular after myocardial infarction to reduce the risk of VT and SCD. In the case of SMVT, amiodarone might be used in addition to beta-blockers as first treatment approach.
In addition to new ESC recommendations, some recommendations have been changed in patients with coronary artery disease (2015 ESC guideline vs. 2022 guideline):
In patients with syncope and previous STEMI, invasive electrophysiological study (programmed electrical stimulation) is indicated when syncope remains unexplained after non-invasive evaluation from Level IIa to Level I.
Intravenous amiodarone treatment should be considered for patients with recurrent polymorphic VT/VF during the acute phase of an acute coronary syndrome (ACS) from Level I to Level IIa.
In addition to the field of CAD, new recommendations have also been implemented for patients with inflammatory diseases.2,3 Of note, the presence of late gadolinium enhancement (LGE) on MRI has become one new aspect to stratify patients with inflammatory cardiac diseases with regard to the development of VT and SCD. Nevertheless, the diagnosis of myocarditis is still difficult to make.2,15–18 Occurrence of heart failure, AV block, bundle branch block, VT, or aborted SCD may be signs of acute myocarditis. Myocarditis can be clearly diagnosed by positive endomyocardial biopsies showing histological and immunohistochemical myocarditis. Polymerase chain reaction (PCR) is useful for the detection of viral genomes. Thus, an endomyocardial biopsy must be still considered as the gold standard for myocarditis. Nevertheless, the current ESC guidelines rely on clinical presentation, elevated troponin level, ECG changes, evidence of LV dysfunction, absence of significant CAD or valvular heart disease, and suggestive findings on CMR. The presence of LGE at CMR has also been associated with the late occurrence of ventricular arrhythmias in cases of endomyocardial biopsy-proven myocarditis.2,15,16 Consequently, the development of dilatative cardiomyopathy may occur in up to 21%.2,17 In patients with SMVT of unclear aetiology, myocarditis should be suspected especially when CMR reveals subepicardial and/or intramural abnormal fibrotic myocardial tissue.2 Recently, new data were published about the prognosis of VT/SCD in patients with acute myocarditis. A retrospective study showed major adverse event (MAE) rates at one and three years were 19% and 45% in cases of the acute myocarditis.17 Of note, adverse events occurred in 43% and 64% of patients who had sequelae of previous myocarditis. The first MAE occurred after one year for 73% in patients with acute myocarditis compared with 29% of patients with myocarditis sequelae.17 Thus, the occurrence of cardiac arrhythmia in acute myocarditis is common. About 30% of all arrhythmia in this situation are VT or ventricular fibrillation (VF). In conclusion, patients who experienced VT/SCD during acute myocarditis have had a high risk of VT/SCD recurrence.15–18 Therefore, the current ESC Guidelines took these findings into account:
In patients with haemodynamically not-tolerated sustained VT or VF during the acute phase of myocarditis, ICD implantation before hospital discharge should be considered (Level IIa). This aggressive concept of immediate ICD implantation needs to be balanced against the possibility to use wearable ICDs for the first period of acute myocarditis. The presence of LGE on MRI (sequelae of myocarditis) is an interesting concept to evaluate patients after myocarditis and in DCM to assess the risk for recurrent VT and SCD besides the left ventricular ejection fraction.
In post-myocarditis patients with recurrent, symptomatic VT, AAD treatment should be considered (Level IIa).
In patients with hemodynamically tolerated SMVT occurring in the chronic phase of myocarditis, ICD implantation should be considered (Level IIa).
In addition to new ESC recommendations, some recommendations have been changed in patients with inflammatory diseases (2015 ESC Guideline vs. 2022 Guideline):
In patients with haemodynamically not-tolerated SMVT occurring in the chronic phase of myocarditis, ICD implantation is recommended change from Level IIa to Level I. In addition to the new recommendation to implant an ICD even in the acute phase of myocarditis, the benefit of an ICD is also strengthened for patients with chronic inflammatory disease in the latest 2022 ESC Guideline.
ICD implantation is recommended in patients with cardiac sarcoidosis who have an LVEF ≤35% was changed from Level IIb to Level I.
Conclusion
The 2022 ESC Guideline for VT and SCD is of great importance for ACVC physicians. All details about specific issues regarding VT/SCD prevention, primary and secondary prophylaxis and treatment regiments can be appreciated in the full version of the ESC guidelines. The original version of the latest ESC Guideline encompasses several diagnostic and treatment algorithms, which are of great practical relevance for adequate VT/SCD therapy. Risk stratification and criteria for primary preventive ICD implantation, however, are still controversial topics. Several knowledge gaps remain unfortunately.19
Funding
None declared.
Data availability
No new data were generated in support of the article.
References
Author notes
The views and opinions expressed in this article are those of the authors; they do not necessarily reflect the views of the Editors.
Conflict of interest: A.G. received EU Grant Horizon 2020 MAESTRIA Consortium; grant number 965 286. Speaker fees from Abbott, Astra Zeneca, Bayer Health Care, Berlin Chemie, Biotronik, Boehringer Ingelheim, BMS/Pfizer, Boston Scientific, Daiichi-Sankyo, Medtronic, Omeicos, and Sanofi-Aventis. G.Y.H.L. is a consultant and speaker for BMS/Pfizer, Boehringer Ingelheim, Daiichi-Sankyo, Anthem. No fees are received personally. B.G declared no conflict of interest.
- tachycardia
- cardiomyopathy, dilated
- coronary arteriosclerosis
- tachycardia, ventricular
- sudden cardiac death
- myocarditis, acute
- purkinje fibers
- anger
- cardiac care facilities
- cardiovascular system
- guidelines
- inflammatory disorders
- defibrillators
- ablation
- european society of cardiology
- cardiac electrical storm




Comments