-
PDF
- Split View
-
Views
-
Cite
Cite
Andrew R Chapman, Bertil Lindahl, Nicholas L Mills, Christian Mueller, the Study Group on Biomarkers of the ESC for Acute CardioVascular Care, Blood and imaging biomarkers in type 2 myocardial infarction, European Heart Journal. Acute Cardiovascular Care, Volume 11, Issue 3, March 2022, Pages 269–271, https://doi.org/10.1093/ehjacc/zuab130
Close - Share Icon Share
Type 2 myocardial infarction is a heterogeneous condition which can be precipitated by a variety of different illnesses.1 Most commonly, it occurs in the context of tachyarrhythmia, but it can also occur as a consequence of significant haemodynamic instability such as prolonged hypotension, anaemia, or hypoxia. In practice, this diagnosis may be applied where there is evidence of symptoms or signs of myocardial ischaemia, a rise and/or fall in cardiac troponin concentration and evidence of a lack of myocardial oxygen supply, or an unmet increase in oxygen demand, without intracoronary thrombosis. Spontaneous coronary artery dissection, coronary embolism, or vasospasm is additional causes of type 2 myocardial infarction.
In consecutive hospitalized patients with elevated cardiac troponin who are aged over 75 years, type 2 myocardial infarction is the most frequent diagnosis. It is associated with poor short- and long-term outcomes, with as few as one in three patients alive at 5 years after index diagnosis. There is an excess in non-cardiovascular mortality compared to patients with type 1 myocardial infarction, thought to be attributable to increasing age and comorbidity, but the absolute rate of future myocardial infarction or cardiovascular death is broadly comparable.2
Despite a high disease prevalence and poor outcomes, there are few prospective studies to guide diagnosis, investigation, or treatment. Distinguishing type 1 and type 2 myocardial infarction in clinical practice is challenging. Troponin alone cannot distinguish different subtypes of myocardial infarction.3 Combinations of different biomarkers including markers of congestion or atrial stretch including Copeptin, midregional proatrial natriuretic peptide, and others have shown good discrimination, but these have not been widely validated and are not yet available for use in clinical practice in most centres.4
The role of imaging to aid diagnosis and identify underlying coronary or cardiac disease is being explored (Figure 1). The DEMAND-MI study will use computed tomography or invasive coronary angiography with intracoronary imaging, cardiac magnetic resonance imaging, or echocardiography to define the prevalence of coronary disease and left ventricular impairment in patients with type 2 myocardial infarction. The presence of coronary artery disease is known to predict outcomes in type 2 myocardial infarction,5 and given the low rate of investigation in this group of patients, it is likely that this is undiagnosed and suboptimally treated in the majority. Markers of thrombosis on positron emission tomography-computed tomography have been shown to aid in the diagnosis of myocardial infarction. 18F-GP1 binds with high affinity to the glycoprotein IIb/IIIa receptors on activated platelets, highlighting both venous and arterial thrombus. This could be helpful in identifying coronary thrombosis as an alternative to invasive assessment, but this technique requires validation in a larger population.6
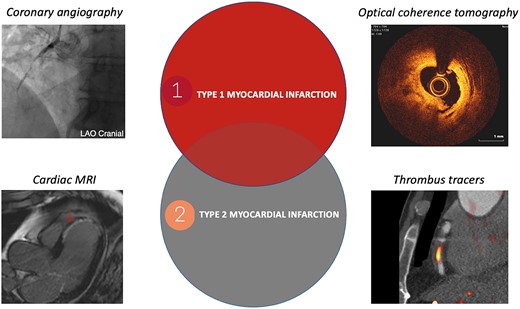
Differentiating type 1 and type 2 myocardial infarction; delineating the ‘grey zone’ using imaging. Coronary angiography demonstrating a thrombotic occlusion in a right coronary artery; cardiac magnetic resonance imaging demonstrating near transmural anteroseptal late gadolinium enhancement; optical coherence tomography demonstrating atherosclerotic plaque rupture and thrombosis; positron emission tomography/computed tomography scan demonstrating thrombus using 18F-GP1 thrombus tracer in a circumflex coronary artery in a patient with type 1 myocardial infarction.
Whilst these novel approaches hold promise, at present, clinicians lack the evidence base to justify routine use of these investigations. New approaches to improve the diagnosis of type 2 myocardial infarction using blood biomarkers would have significant benefits for patients and healthcare providers reducing diagnostic uncertainty, guiding the use of cardiac imaging, and facilitating prompt therapy for type 1 myocardial infarction. It is possible that targeted imaging may also facilitate the use of secondary prevention in patients with type 2 myocardial infarction with the potential to improve outcomes, but this requires evaluation in randomized trials.
Other members of the Study Group on Biomarkers of the ESC Association for Acute Cardiovascular Care include:
Evangelos Giannitsis, MD1, Allan S. Jaffe, MD2, Kurt Huber, MD3, Johannes Mair, MD4, Louise Cullen, MD, PhD5, Ola Hammarsten, MD, PhD6, Martin Möckel, MD7, Konstantin Krychtiuk, MD8, and Kristian Thygesen, MD9
1Department of Cardiology, University Heidelberg, Heidelberg, Germany;
2Mayo Clinic and Medical School, Rochester, MN, USA;
3Department of Medicine, Cardiology and Intensive Care Medicine, Wilhelminenhospital, and Sigmund Freud University, Medical School, Vienna, Austria;
4Department of Internal Medicine III—Cardiology and Angiology, Medical University Innsbruck, Innsbruck, Austria;
5Emergency and Trauma Centre, Royal Brisbane and Women’s Hospital, University of Queensland, St Lucia, QLD, Australia;
6Department of Clinical Chemistry and Transfusion Medicine, University of Gothenburg, Gothenburg, Sweden;
7Division of Emergency Medicine, Charité-Universitätsmedizin Berlin, Berlin, Germany;
8Division of Cardiology, Department of Internal Medicine II, Medical University of Vienna, Vienna, Austria; and
9Department of Cardiology, Aarhus University Hospital, Aarhus, Denmark
Acknowledgements
We thank Dr Evangelos Tzolos, University of Edinburgh, for providing 18F-GP1 thrombus tracer image in Figure 1.
Funding
A.R.C. is supported by a Starter Grant for Clinical Lecturers from the Academy of Medical Sciences (SGL/021/1075). N.L.M. is supported by a Research Excellence Award (RE/18/5/34216) and a Chair Award (CH/F/21/90010) from the British Heart Foundation.
Conflict of interest: A.R.C. has received honoraria from Biosensors. N.L.M. reports research grants awarded to the University of Edinburgh from Abbott Diagnostics and Siemens Healthineers outside the submitted work, and honoraria from Abbott Diagnostics, Siemens Healthineers, Roche Diagnostics and LumiraDx. C.M. has received research support from the Swiss National Science Foundation, the Swiss Heart Foundation, the KTI, the University Hospital Basel, the University of Basel; Abbott, Beckman Coulter, Brahms, Idorsia, Novartis, Ortho Diagnostics, Quidel, Roche, Siemens, Singulex, Sphingotec outside the submitted work, as well as speaker honoraria/consulting honoraria from Amgen, Astra Zeneca, Bayer, Boehringer Ingelheim, BMS, Novartis, Osler, Roche, and Sanofi, all paid to the institution. All other coauthors have no conflicts to report.




Comments