-
PDF
- Split View
-
Views
-
Cite
Cite
Chiara Robba, Ewoud J van Dijk, Mathieu van der Jagt, Acute ischaemic stroke and its challenges for the intensivist, European Heart Journal. Acute Cardiovascular Care, Volume 11, Issue 3, March 2022, Pages 258–268, https://doi.org/10.1093/ehjacc/zuac004
Close - Share Icon Share
Abstract
Acute ischaemic stroke (AIS) is responsible for almost 90% of all strokes and is one of the leading causes of death and disability. Acute ischaemic stroke is caused by a critical alteration in focal cerebral blood flow (ischaemia) from a variety of causes, resulting in infarction. The primary cerebral injury due to AIS occurs in the first hours, therefore early reperfusion importantly impacts on patient outcome (‘Time is brain’ concept). Secondary cerebral damage progressively evolves over the following hours and days due to cerebral oedema, haemorrhagic transformation, and cerebral inflammation. Systemic complications, such as pneumonia, sepsis, and deep venous thrombosis, could also affect outcome. The risk of a recurrent ischaemic stroke is in particular high in the first days, which necessitate particular attention. The role of intensive care unit physicians is therefore to avoid or reduce the risk of secondary damage, especially in the areas where the brain is functionally impaired and ‘at risk’ of further injury. Therapeutic strategies therefore consist of restoration of blood flow and a bundle of medical, endovascular, and surgical strategies, which—when applied in a timely and consistent manner—can prevent secondary deterioration due to cerebral and systemic complications and recurrent stroke and improve short- and long-term outcomes. A multidisciplinary collaboration between neurosurgeons, interventional radiologists, neurologists, and intensivists is necessary to elaborate the best strategy for the treatment of these patients.
Introduction
Acute ischaemic stroke (AIS) represents an important condition for intensivists, given the risk of clinical deterioration of these patients after admission to hospital resulting in impaired vital functions, often including the respiratory function and consciousness.
This practice review aims to highlight clinical management relevant for physicians dealing with critically ill or vitally impaired AIS patients in critical care or emergency department settings, or those at risk for deterioration in the ward.
Acute ischaemic stroke: organizational models and variability in intensive care unit management
Acute ischaemic stroke is the third leading cause of death and a major cause of permanent disability worldwide.1–3
Acute ischaemic stroke is an acute disease. The primary brain damage evolves from 5 min on to several hours (depending on the collateral circulation). As AIS is a time-dependent pathology4 and early management can importantly improve patients’ outcome, a great effort has been recently made on the definition of organizational models aimed to promptly recognize and treat these patients.3
Although this educational review aims to address management in critically ill AIS patients, it is a priori important to acknowledge that there is no broad consensus on what constitutes a ‘vitally impaired’ or ‘critically ill’ stroke patient. This lack of consensus translates into different organizational models to manage AIS patients: in some hospitals, all AIS patients may be managed at an intensive care unit (ICU) (which may be a dedicated neurocritical care unit or a general ICU also admitting neurocritically ill patients), but on the other hand, hospitals may have chosen to admit all AIS patients in principle to the ward (albeit with varying intensities of monitoring, e.g. electrocardiogram, oxygen saturation), a stroke unit, or an intermediate care unit (with higher level of monitoring of vital functions than the ward, but not constituting an ICU).4,5 This variation likely stems from varying definitions of what constitutes a critically ill AIS patient between hospitals and countries, differences in funding of healthcare and historic differences. For instance, all AIS patients may be considered at high risk of clinical deterioration due to pneumonia, cardiac arrhythmias, haemorrhagic transformation of infarcted brain tissue, or cerebral oedema in case of large middle cerebral artery (MCA) occlusion and it might be argued that intense vital monitoring at an ICU is therefore always warranted. On the other hand, in many hospitals AIS patients are managed by neurologists at the ward or a stroke unit, and consultation by intensivists is only undertaken in case of significant clinical deterioration, most often involving progressive disorders of consciousness (which does not apply to most AIS patients who have intact consciousness) and/or respiratory failure.6 Other variability relates to the 24/7 availability of neurointerventional service, the presence of dedicated neurocritical care units, nurse-patient ratio, and other factors.5,6 There is no firm evidence that favours one organizational model (e.g. neurocritical care vs. a non-dedicated critical care unit) over the other in terms of optimal neurological recovery, except for the fact that evidence favours ‘stroke units’ over non-specialized nursing wards for non-critically ill AIS patients.5
Furthermore, there is large variability in ICU management independently from organization of care. For instance, large variability exists between hospitals in general medical care, including glucose targets, indications for ICU admission, blood pressure (BP) targets, and dysphagia assessment.6
Recent diagnostic and therapeutic advances in acute ischaemic stroke
In order to appreciate the current management of AIS, emergency and critical care physicians should know the current state of the art regarding acute diagnostic and therapeutic interventions in AIS.5 Historically, for AIS there was a lack of effective therapeutic interventions. For decades, antiplatelet therapy and vitamin K antagonists constituted the mainstay of secondary prevention and therapy after AIS, but recent advances have improved this prospect dramatically.7 Significant advances in the treatment of AIS include the implementation of neurological recovery strategies, of ‘stroke units’ and intravenous thrombolysis,8,9 as well as intra-arterial thrombectomy (IAT) which improved the therapeutic management of AIS in eligible patients.7 Importantly, IAT significantly contributed to a better AIS management being this a true medical emergency, given the possibility of acute treatment and reversal of the culprit: an arterial thrombus in the brain. With this most recent major development, the adage ‘time is brain’ got an additional implication, and became more relevant than ever before (Figures 1 and 2).
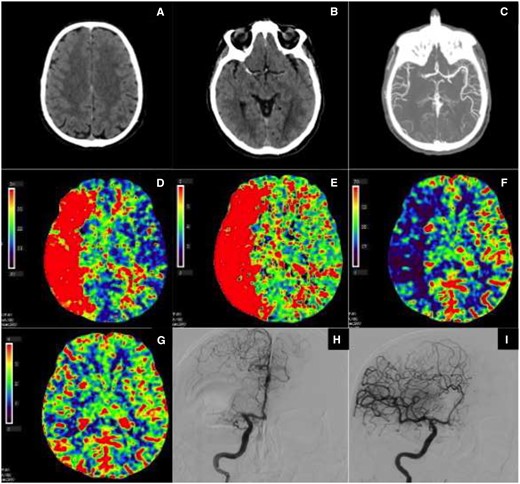
(A–I) Right middle cerebral artery occlusion with no infarct core and large penumbra, due to good collateral circulation. Automated computed tomography perfusion software packages with different algorithms that calculate infarct core and penumbra volumes are used. The input variables for these algorithms are the following computed tomography perfusion variables: CBV (cerebral blood volume, mL/100 g), CBF (cerebral blood flow, mL/100 g/min), MTT (mean transit time, s), and TTP (time to peak, s). (A) Non-contrast computed tomography scan: no early signs of ischaemia. (B) Non-contrast computed tomography scan: dense vessel sign (right middle cerebral artery). (C) Computed tomography angiography: proximal right middle cerebral artery occlusion with collateral circulation in distal middle cerebral artery branches. (D) Computed tomography perfusion, time to peak: delayed contrast peak in the right middle cerebral artery area, compatible with area at risk for infarction (penumbra and potential infarct core together). (E) Computed tomography perfusion, mean transit time: slow contrast transit in right middle cerebral artery area, compatible with area at risk for infarction (penumbra and potential infarct core together). (F) Computed tomography perfusion, cerebral blood flow: diminished cerebral blood flow in the right middle cerebral artery area, compatible with area at risk for infarction (penumbra and potential infarct core together). (G) Computed tomography perfusion, cerebral blood volume: no marked defect in relative cerebral blood volume in the right middle cerebral artery area, compatible with no important infarct core. (H) Digital subtraction angiography, start of intra-arterial thrombectomy procedure: proximal right middle cerebral artery occlusion with collateral flow through the ACA-middle cerebral artery anastomosis. (I) Digital subtraction angiography, end of intra-arterial thrombectomy procedure: recanalization of right middle cerebral artery occlusion with complete perfusion of middle cerebral artery without delay (TICI 3).
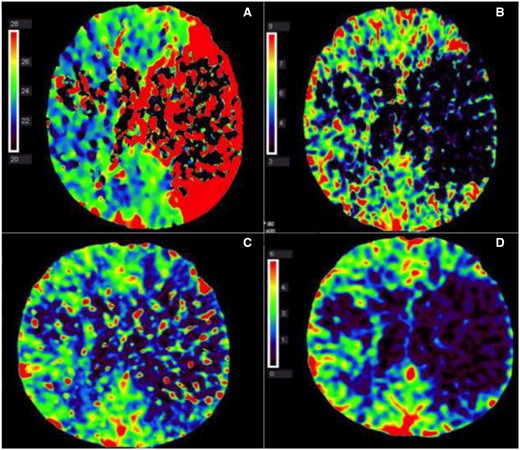
(A–D) Left middle cerebral artery occlusion with mainly infarct core and almost no penumbra. (A) Computed tomography perfusion, time to peak: delayed contrast peak in left middle cerebral artery area, compatible with area at risk for infarction (penumbra and potential infarct core together). (B) Computed tomography perfusion, mean transit time: no contrast transit in the left middle cerebral artery area (mean transit time drop out), compatible with infarction core. (C) Computed tomography perfusion, cerebral blood flow: diminished cerebral blood flow in left middle cerebral artery area, compatible with area at risk for infarction (penumbra and potential infarct core together). (D) Computed tomography perfusion, cerebral blood volume: marked defect in relative cerebral blood volume in left middle cerebral artery, compatible with infarct core.
Diagnostic work-up
Acute ischaemic stroke patients are typically evaluated with the NIHSS score to assess and quantify the severity of neurological impairment due to the stroke in a validated manner, next to the routine clinical neurological physical examination (https://www.stroke.nih.gov/documents/NIH_Stroke_Scale_508C.pdf).
A non-contrast computed tomography (CT) of the brain is the immediate and first-line diagnostic tool in suspected AIS, mainly to rule out alternative diagnoses, in particular intracranial haemorrhage (ICH). Its secondary aim in terms of treatment is to assure safe and timely administration of recombinant tissue plasminogen activator (rtPA) when ICH is absent and the clinical picture is otherwise compatible with AIS (Figures 1 and 2). Computed tomography angiography is added to the standard imaging protocol to show large vessel occlusion possibly eligible for IAT. Further diagnostic evaluation may include CT perfusion or magnetic resonance (MR) imaging to assess infarction vs. only perfusion deficit with still salvageable brain tissue (penumbra) and determine eligibility for intravenous thrombolysis (IVT) and/or IAT beyond the conventional time windows of 4.5 and 6 h, respectively (Figures 1 and 2) (Table 1).10,11
| Therapy . | Time window . | Clinical criteria . | Imaging criteria . |
|---|---|---|---|
| IVT | <4.5 h | There should be a local protocol in every institution including the eligibility (indications and contraindications) criteria. This link could be used as a basis: https://www.ahajournals.org/doi/epub/10.1161/STR.0000000000000086 | Non-contrast CT to exclude ICH |
| IVT | >4.5 up to 9 h after known stroke onset | Known stroke symptom onset or wake up stroke | CTP or MRI core/perfusion mismatch criteria indicating salvageable brain tissue/penumbra and for whom IAT is not appropriate |
| IAT | <6 h | NIHSS ≥2 | LVO of A1, M1, M2, or basilar artery on CTA or MRA |
| IAT | 6–16 h after last known well | NIHSS ≥6. Also, see: https://journals.sagepub.com/doi/pdf/10.1177/2396987319832140 | LVO on CTA or MRA, and a perfusion mismatch indicating salvageable brain tissue/penumbra on CTP or DW/PW-MR. |
| Therapy . | Time window . | Clinical criteria . | Imaging criteria . |
|---|---|---|---|
| IVT | <4.5 h | There should be a local protocol in every institution including the eligibility (indications and contraindications) criteria. This link could be used as a basis: https://www.ahajournals.org/doi/epub/10.1161/STR.0000000000000086 | Non-contrast CT to exclude ICH |
| IVT | >4.5 up to 9 h after known stroke onset | Known stroke symptom onset or wake up stroke | CTP or MRI core/perfusion mismatch criteria indicating salvageable brain tissue/penumbra and for whom IAT is not appropriate |
| IAT | <6 h | NIHSS ≥2 | LVO of A1, M1, M2, or basilar artery on CTA or MRA |
| IAT | 6–16 h after last known well | NIHSS ≥6. Also, see: https://journals.sagepub.com/doi/pdf/10.1177/2396987319832140 | LVO on CTA or MRA, and a perfusion mismatch indicating salvageable brain tissue/penumbra on CTP or DW/PW-MR. |
CT, computed tomography; CTA, computed tomography angiography; CTP, perfusion computed tomography; DW-MRI, diffusion-weighted MRI; IAT, intra-arterial thrombectomy; ICH, intracranial haemorrhage; IVT, intravenous thrombolysis; LVO, large vessels occlusion; MRA, magnetic resonance angiography; MRI, magnetic resonance imaging.
| Therapy . | Time window . | Clinical criteria . | Imaging criteria . |
|---|---|---|---|
| IVT | <4.5 h | There should be a local protocol in every institution including the eligibility (indications and contraindications) criteria. This link could be used as a basis: https://www.ahajournals.org/doi/epub/10.1161/STR.0000000000000086 | Non-contrast CT to exclude ICH |
| IVT | >4.5 up to 9 h after known stroke onset | Known stroke symptom onset or wake up stroke | CTP or MRI core/perfusion mismatch criteria indicating salvageable brain tissue/penumbra and for whom IAT is not appropriate |
| IAT | <6 h | NIHSS ≥2 | LVO of A1, M1, M2, or basilar artery on CTA or MRA |
| IAT | 6–16 h after last known well | NIHSS ≥6. Also, see: https://journals.sagepub.com/doi/pdf/10.1177/2396987319832140 | LVO on CTA or MRA, and a perfusion mismatch indicating salvageable brain tissue/penumbra on CTP or DW/PW-MR. |
| Therapy . | Time window . | Clinical criteria . | Imaging criteria . |
|---|---|---|---|
| IVT | <4.5 h | There should be a local protocol in every institution including the eligibility (indications and contraindications) criteria. This link could be used as a basis: https://www.ahajournals.org/doi/epub/10.1161/STR.0000000000000086 | Non-contrast CT to exclude ICH |
| IVT | >4.5 up to 9 h after known stroke onset | Known stroke symptom onset or wake up stroke | CTP or MRI core/perfusion mismatch criteria indicating salvageable brain tissue/penumbra and for whom IAT is not appropriate |
| IAT | <6 h | NIHSS ≥2 | LVO of A1, M1, M2, or basilar artery on CTA or MRA |
| IAT | 6–16 h after last known well | NIHSS ≥6. Also, see: https://journals.sagepub.com/doi/pdf/10.1177/2396987319832140 | LVO on CTA or MRA, and a perfusion mismatch indicating salvageable brain tissue/penumbra on CTP or DW/PW-MR. |
CT, computed tomography; CTA, computed tomography angiography; CTP, perfusion computed tomography; DW-MRI, diffusion-weighted MRI; IAT, intra-arterial thrombectomy; ICH, intracranial haemorrhage; IVT, intravenous thrombolysis; LVO, large vessels occlusion; MRA, magnetic resonance angiography; MRI, magnetic resonance imaging.
Intravenous thrombolysis and intra-arterial thrombectomy
Intravenous thrombolysis and IAT are clearly time-sensitive treatments which should be applied as soon as possible to minimize the consequences of arterial occlusion. This is why AIS constitutes a true medical emergency. Intravenous thrombolysis with rtPA is the first-line therapy in all eligible AIS patients within 4.5 h after the moment of ‘last-seen-well’, independently from eligibility for IAT.7 However, recent studies have not been able to show inferiority of IAT alone in patients with large vessels occlusion, therefore it may be reasonable to withhold IVT in patients with relative contraindications who are eligible for IAT.7 In patients presenting after the 4.5 h window, further advanced imaging is helpful to select patients for IVT in the extended time window up to 12 h, such as CT angiography or MR imaging.11 When large vessels occlusion of the anterior circulation is found and the patient is within the 6-h time window after last seen normal, IAT can be performed.
The DAWN trial, which included patients with neurological deficits within 6–24 h with greater severity than expected from neuroimaging, demonstrated that IAT was associated with a reduction in disability and higher incidence of functional independence compared to medical treatment alone (48.6% vs. 13.1%). Similar results were obtained from the DEFUSE-3 trial, which demonstrated that thrombectomy between 6 and 16 h from symptoms
was associated with a highly significant benefit (as for modified Rankin Scale, mRS) compared to medical treatment alone.12 Decision on reperfusion therapies in AIS patients beyond these time-windows is summarized in Table 1 and should be guided by further advanced imaging. It is advised that each hospital has local multidisciplinary protocols in place, e.g. based on the international guidelines, to guide clinical work-flow and describe responsibilities of the various types of healthcare providers.
Importantly, both IAT and IVT can present several complications which may require ICU admission and treatment, such as artery dissection, bleeding and ICH, and contrast-encephalopathy, which should be taken in consideration in the decision to treat the patients and in case of neurological deterioration after the procedure.
Most relevant phenotypes of acute ischaemic stroke for intensivists
When, after AIS, vital functions become impaired, these should be continuously monitored in an intensive monitoring environment, typically a critical care unit and preferably before these become immediately life-threatening.5 Critical care (and emergency) physicians will encounter, and should be able to recognize and manage specific clinical phenotypes constituting patients with impaired vital functions, who are at high risk of further deteriorations (Table 2).13–16
Manifestation, recognition, diagnostics, and management of different phenotypes of stroke
| Phenotype . | Manifestation . | Recognition . | Diagnostics . | Management . |
|---|---|---|---|---|
| MCIa | Severe secondary brain swelling of infarcted area with impending brain herniation | Deterioration of consciousness, delirium, agitation, progressive hemiplegia. Unilateral pupil dilatation | Non-contrast CT scan | Surgical decompression in eligible patients, hyperosmolar therapies may be considered but rather as bridge to surgery than final therapy in most cases |
| BAO/PCSb | Disruption of vital structures for consciousness (reticular formation, thalamus) or respiration/breathing (e.g. brainstem nuclei involved in pharyngeal motor function) | Dysfunction of swallowing/coughing, progressive focal brainstem symptoms (e.g. diplopia, hemiplegia), deterioration of consciousness, headache | Non-contrast CT (but: less sensitive for posterior circulation ischaemia) or MRI, vascular imaging (CTA) | Endovascular treatment for progressive thrombosis may be considered in individual cases, neurosurgical intervention (ventricular CSF drain for obstructive hydrocephalus, decompression of cerebellum) |
| Haemorrhagic transformation | Intracranial bleeding in infarcted area | Progressive focal neurological dysfunction, acute neurological deterioration in spite initial stabilization, headache, seizures | Non-contrast CT scan | Consider reversal of anticoagulation sometimes surgical decompression is required. |
| Phenotype . | Manifestation . | Recognition . | Diagnostics . | Management . |
|---|---|---|---|---|
| MCIa | Severe secondary brain swelling of infarcted area with impending brain herniation | Deterioration of consciousness, delirium, agitation, progressive hemiplegia. Unilateral pupil dilatation | Non-contrast CT scan | Surgical decompression in eligible patients, hyperosmolar therapies may be considered but rather as bridge to surgery than final therapy in most cases |
| BAO/PCSb | Disruption of vital structures for consciousness (reticular formation, thalamus) or respiration/breathing (e.g. brainstem nuclei involved in pharyngeal motor function) | Dysfunction of swallowing/coughing, progressive focal brainstem symptoms (e.g. diplopia, hemiplegia), deterioration of consciousness, headache | Non-contrast CT (but: less sensitive for posterior circulation ischaemia) or MRI, vascular imaging (CTA) | Endovascular treatment for progressive thrombosis may be considered in individual cases, neurosurgical intervention (ventricular CSF drain for obstructive hydrocephalus, decompression of cerebellum) |
| Haemorrhagic transformation | Intracranial bleeding in infarcted area | Progressive focal neurological dysfunction, acute neurological deterioration in spite initial stabilization, headache, seizures | Non-contrast CT scan | Consider reversal of anticoagulation sometimes surgical decompression is required. |
CT, computed tomography; CSF, cerebrospinal fluid; CTA, computed tomography angiography; MRI, magnetic resonance imaging.
Malignant cerebral infarction.
Basilar artery occlusion/posterior circulation stroke.
Manifestation, recognition, diagnostics, and management of different phenotypes of stroke
| Phenotype . | Manifestation . | Recognition . | Diagnostics . | Management . |
|---|---|---|---|---|
| MCIa | Severe secondary brain swelling of infarcted area with impending brain herniation | Deterioration of consciousness, delirium, agitation, progressive hemiplegia. Unilateral pupil dilatation | Non-contrast CT scan | Surgical decompression in eligible patients, hyperosmolar therapies may be considered but rather as bridge to surgery than final therapy in most cases |
| BAO/PCSb | Disruption of vital structures for consciousness (reticular formation, thalamus) or respiration/breathing (e.g. brainstem nuclei involved in pharyngeal motor function) | Dysfunction of swallowing/coughing, progressive focal brainstem symptoms (e.g. diplopia, hemiplegia), deterioration of consciousness, headache | Non-contrast CT (but: less sensitive for posterior circulation ischaemia) or MRI, vascular imaging (CTA) | Endovascular treatment for progressive thrombosis may be considered in individual cases, neurosurgical intervention (ventricular CSF drain for obstructive hydrocephalus, decompression of cerebellum) |
| Haemorrhagic transformation | Intracranial bleeding in infarcted area | Progressive focal neurological dysfunction, acute neurological deterioration in spite initial stabilization, headache, seizures | Non-contrast CT scan | Consider reversal of anticoagulation sometimes surgical decompression is required. |
| Phenotype . | Manifestation . | Recognition . | Diagnostics . | Management . |
|---|---|---|---|---|
| MCIa | Severe secondary brain swelling of infarcted area with impending brain herniation | Deterioration of consciousness, delirium, agitation, progressive hemiplegia. Unilateral pupil dilatation | Non-contrast CT scan | Surgical decompression in eligible patients, hyperosmolar therapies may be considered but rather as bridge to surgery than final therapy in most cases |
| BAO/PCSb | Disruption of vital structures for consciousness (reticular formation, thalamus) or respiration/breathing (e.g. brainstem nuclei involved in pharyngeal motor function) | Dysfunction of swallowing/coughing, progressive focal brainstem symptoms (e.g. diplopia, hemiplegia), deterioration of consciousness, headache | Non-contrast CT (but: less sensitive for posterior circulation ischaemia) or MRI, vascular imaging (CTA) | Endovascular treatment for progressive thrombosis may be considered in individual cases, neurosurgical intervention (ventricular CSF drain for obstructive hydrocephalus, decompression of cerebellum) |
| Haemorrhagic transformation | Intracranial bleeding in infarcted area | Progressive focal neurological dysfunction, acute neurological deterioration in spite initial stabilization, headache, seizures | Non-contrast CT scan | Consider reversal of anticoagulation sometimes surgical decompression is required. |
CT, computed tomography; CSF, cerebrospinal fluid; CTA, computed tomography angiography; MRI, magnetic resonance imaging.
Malignant cerebral infarction.
Basilar artery occlusion/posterior circulation stroke.
Malignant middle cerebral artery infarction
Patients with MCA infarction may deteriorate due to progressive swelling of the infarcted area, resulting in midline shift and brain herniation syndrome.17–19 The first signs of progressive swelling may include increased agitation level, posturing, deterioration of consciousness (Glasgow Coma Scale), and unilateral fixed pupil upon imminent herniation. The time window of deterioration may span up to 5 days, but most patients with a malignant course deteriorate after 72–96 h.20 Patients should be transferred to the ICU upon the earliest signs of cerebral swelling on imaging, especially when there is midline shift, given the risk of rapid further deterioration. Early clinical signs are unreliable and often unhelpful, given that an unfavourable progressive course is often rapidly progressive with imminent herniation.5 Risk factors for malignant course of MCA infarction include younger age, higher NIHSS, large infarct volume.21 In patients treated with IAT, risk factors partly overlap, but also heavier thrombus burden, baseline Alberta Stroke Program Early CT Score (ASPECTS) ≤ 8 and unsuccessful recanalization predict a malignant course.3 ICU admission is often necessary in this setting, and when imaging shows the first signs of shift or increase in space occupation of the infarcted area, accompanying the first signs of deterioration, immediate transferal to a neurosurgical centre is recommended given the risk of herniation and possible need for decompression, in eligible patients.5 Although surgery reduces mortality significantly, the probability for unfavourable outcome is still high and should be discussed with family and considering patients’ will and expectations.
Basilar artery occlusion
Posterior circulation stroke often presents with the D’s: dysarthria, diplopia, dysphagia, dysconjugate gaze, and depressed consciousness (Figure 3). A low threshold for ICU admission should be used given the potential for rapid deterioration.5,22 The mechanism of deteriorating consciousness could be ischaemia of the reticular formation located in the dorsal brainstem, progressive swelling of concomitant cerebellar infarction compressing the reticular formation from behind and/or ischaemia/infarction involving bilateral thalamic nuclei that impairs sensory input to the brain and results in decreased responsiveness as well. Importantly, an unresponsive patient can have complete or partial locked-in syndrome, which should not be missed (infarction of ventral pons, disrupting the central motor neurons responsible for consciousness, purposeful movements of facial, axial and extremities’ muscles, with intact dorsal reticular formation, ascending connections to thalami and the cortex and frontal lobe projections; the trajectories responsible for intact consciousness), thus having an intact consciousness, but being (motorically) unresponsive, presenting as a ‘pseudo-coma’. Alternatively, even without decreased responsiveness, severe dysphagia, and disturbed swallowing may contribute to aspiration pneumonia, which may result in respiratory insufficiency.
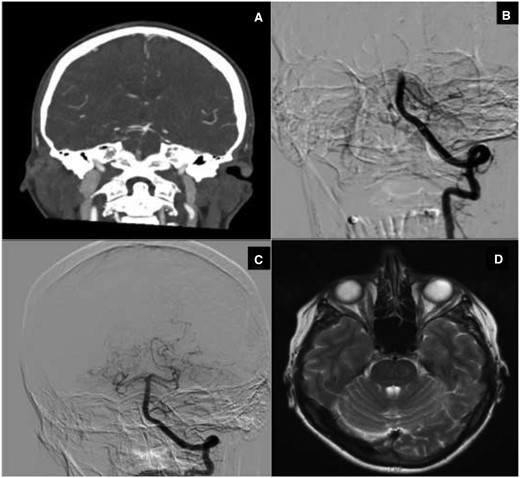
(A–D) Basilar artery thrombosis. (A) Computed tomography angiography: basilar artery occlusion with open basilar top (via collateral flow from anterior circulation). (B) Digital subtraction angiography (start intra-arterial thrombectomy procedure): mid basilar artery occlusion (open posterior inferior cerebellar artery). (C) Digital subtraction angiography (end intra-arterial thrombectomy procedure): open basilar artery. (D) Magnetic resonance imaging (T2-weighted image): no ischaemic damage brainstem at the level of the pons.
Swelling of cerebellar infarction, with risk of hydrocephalus due to compression of the forth ventricle, when present, may have a variable course,20 from rapidly progressive swelling of massive cerebellar infarction within 24–48 h to more variable or slower progression over several days, up to a week, requiring longer monitoring and higher index of suspicion for acute deteriorations in need of imminent treatment.
Haemorrhagic transformation
Post-treatment intracranial haemorrhage (ptICH) is a heterogeneous entity, as exemplified by a detailed radiographic classification described by Yaghi et al.23 Haemorrhagic transformation can occur spontaneously, after IVT, especially in patients prior on vitamin K antagonist, or remote cerebral haemorrhages could occur after IVT.
For intensivists, the more severe haemorrhages,corresponding to haemorrhages with mass effect (classified as PH-1/2 according to the European Cooperative Acute Stroke Study—ECASS classification) are most relevant,23 since these are associated with clinical deterioration that may require intensive monitoring and treatment. The risk of ptICH varies between 2% and 7%. Risk factors include age, stroke severity (both radiologically and clinically as measured with the NIHSS) and vascular risk factors in general. Of note, IAT reduces the risk of ptICH. The window of occurrence and need for monitoring of deterioration is 24 h according to the American Heart Association (AHA) recommendations, although later bleeds do occur (10–15%).23 Post-IVT CT is advised after 24 h although without clinical deterioration such imaging rarely affects clinical management. Seizures are typically more likely to occur with ptICH compared with AIS without haemorrhage, and should be considered as an indication to rule out ICH with CT.
Clinical management challenges for intensivists
General management of AIS patients at the ICU has recently been reviewed5 and will not be discussed in depth in this educational review. We refer to Table 3 showing a summary of important management issues to consider in critically ill AIS patients. Rather, challenges for the intensivist and/or areas of uncertainty are addressed here, especially in relation to the most relevant phenotypes of acute stroke described above.
| Organ-directed therapy . | |
|---|---|
| Haemodynamic management | |
| Avoid hypotension and aim for normovolaemia | |
| In patients that do not receive tPA and have no comorbidities requiring acute BP lowering, target a BP <220/120 mmHg | |
| Target systolic BP to <185/110 mmHg before IV tPA and for 24 h thereafter | |
| For the first 24 h post-EVT, maintain <180/105 mmHg | |
| No clear evidence exists for the preferred drug to be used to acutely control BP. We advise short-acting, easily titratable drugs initially, and oral antihypertensive on subsequent days | |
| Respiratory management | |
| Indications for intubation and mechanical ventilation include: decreased consciousness, (GCS < 9) signs of bulbar dysfunction with inability to protect airway, signs or strong suspicion of intracranial hypertension | |
| Supplemental oxygen not recommended in non-hypoxic patients | |
| Supplemental oxygen only if SpO2 < 94% | |
| Target normoxia and normocapnia | |
| Temperature management | |
| It is necessary to prompt detect, investigate and treat hyperthermia >38°C, but this advice is based on low graded evidence | |
| Pharmacological treatment can be considered as first line, and eventually active cooling therapies | |
| Therapeutic or prophylactic hypothermia is not recommended | |
| Glycaemia and nutrition | |
| Avoid or treat hypoglycaemia (<60 mg/dL) | |
| Treat hyperglycaemia to maintain blood glucose between 140 and 180 mg/dL | |
| Establish full enteral nutrition within 7 days of stroke onset | |
| Consider percutaneous gastrostomy if prolonged dysphagia (2–3 weeks) is anticipated | |
| Antiplatelet agents | |
| Aspirin (160–325 mg loading dose) or clopidogrel (300 mg loading dose) within 24 h after stroke onset. | |
| A delayed administration of 24 h is acceptable in patients receiving iv thrombolysis | |
| Dual therapy with aspirin and clopidogrel within 24 hours from stroke onset for 21 days can be beneficial | |
| Anticoagulation | |
| No benefit from urgent anticoagulation in the prevention of recurrent stroke or neurological deterioration, or to improve outcome | |
| In case of atrial fibrillation, start oral anticoagulation after 5–7 days in minor stroke and in 10–14 days after severe stroke | |
| The usefulness of anticoagulation in severe carotid stenosis of internal carotid artery, or non-occlusive extracranial intraluminal thrombus is uncertain, and anticoagulation is not recommended | |
| Other | |
| Haemodynamic management | Continuous ECG monitoring is recommended Echocardiography assessment during ICU stay might be useful
|
| Haemoglobin |
|
| Venous thromboembolism prophylaxis |
|
| Antiobiotics | Antibiotic prophylaxis is not recommended |
| Seizures management |
|
| Dysphagia | Perform early swallow test and dysphagia assessment |
| Organ-directed therapy . | |
|---|---|
| Haemodynamic management | |
| Avoid hypotension and aim for normovolaemia | |
| In patients that do not receive tPA and have no comorbidities requiring acute BP lowering, target a BP <220/120 mmHg | |
| Target systolic BP to <185/110 mmHg before IV tPA and for 24 h thereafter | |
| For the first 24 h post-EVT, maintain <180/105 mmHg | |
| No clear evidence exists for the preferred drug to be used to acutely control BP. We advise short-acting, easily titratable drugs initially, and oral antihypertensive on subsequent days | |
| Respiratory management | |
| Indications for intubation and mechanical ventilation include: decreased consciousness, (GCS < 9) signs of bulbar dysfunction with inability to protect airway, signs or strong suspicion of intracranial hypertension | |
| Supplemental oxygen not recommended in non-hypoxic patients | |
| Supplemental oxygen only if SpO2 < 94% | |
| Target normoxia and normocapnia | |
| Temperature management | |
| It is necessary to prompt detect, investigate and treat hyperthermia >38°C, but this advice is based on low graded evidence | |
| Pharmacological treatment can be considered as first line, and eventually active cooling therapies | |
| Therapeutic or prophylactic hypothermia is not recommended | |
| Glycaemia and nutrition | |
| Avoid or treat hypoglycaemia (<60 mg/dL) | |
| Treat hyperglycaemia to maintain blood glucose between 140 and 180 mg/dL | |
| Establish full enteral nutrition within 7 days of stroke onset | |
| Consider percutaneous gastrostomy if prolonged dysphagia (2–3 weeks) is anticipated | |
| Antiplatelet agents | |
| Aspirin (160–325 mg loading dose) or clopidogrel (300 mg loading dose) within 24 h after stroke onset. | |
| A delayed administration of 24 h is acceptable in patients receiving iv thrombolysis | |
| Dual therapy with aspirin and clopidogrel within 24 hours from stroke onset for 21 days can be beneficial | |
| Anticoagulation | |
| No benefit from urgent anticoagulation in the prevention of recurrent stroke or neurological deterioration, or to improve outcome | |
| In case of atrial fibrillation, start oral anticoagulation after 5–7 days in minor stroke and in 10–14 days after severe stroke | |
| The usefulness of anticoagulation in severe carotid stenosis of internal carotid artery, or non-occlusive extracranial intraluminal thrombus is uncertain, and anticoagulation is not recommended | |
| Other | |
| Haemodynamic management | Continuous ECG monitoring is recommended Echocardiography assessment during ICU stay might be useful
|
| Haemoglobin |
|
| Venous thromboembolism prophylaxis |
|
| Antiobiotics | Antibiotic prophylaxis is not recommended |
| Seizures management |
|
| Dysphagia | Perform early swallow test and dysphagia assessment |
BP, blood pressure; ECG, electrocardiography; EVT, endovascular thrombectomy; ICU, intensive care unit; IV, intravenous; NA, authors recommendations, no evidence base in AHA/ASA recommendations; SpO2, oxygen saturation; tPA, tissue plasminogen activator.
| Organ-directed therapy . | |
|---|---|
| Haemodynamic management | |
| Avoid hypotension and aim for normovolaemia | |
| In patients that do not receive tPA and have no comorbidities requiring acute BP lowering, target a BP <220/120 mmHg | |
| Target systolic BP to <185/110 mmHg before IV tPA and for 24 h thereafter | |
| For the first 24 h post-EVT, maintain <180/105 mmHg | |
| No clear evidence exists for the preferred drug to be used to acutely control BP. We advise short-acting, easily titratable drugs initially, and oral antihypertensive on subsequent days | |
| Respiratory management | |
| Indications for intubation and mechanical ventilation include: decreased consciousness, (GCS < 9) signs of bulbar dysfunction with inability to protect airway, signs or strong suspicion of intracranial hypertension | |
| Supplemental oxygen not recommended in non-hypoxic patients | |
| Supplemental oxygen only if SpO2 < 94% | |
| Target normoxia and normocapnia | |
| Temperature management | |
| It is necessary to prompt detect, investigate and treat hyperthermia >38°C, but this advice is based on low graded evidence | |
| Pharmacological treatment can be considered as first line, and eventually active cooling therapies | |
| Therapeutic or prophylactic hypothermia is not recommended | |
| Glycaemia and nutrition | |
| Avoid or treat hypoglycaemia (<60 mg/dL) | |
| Treat hyperglycaemia to maintain blood glucose between 140 and 180 mg/dL | |
| Establish full enteral nutrition within 7 days of stroke onset | |
| Consider percutaneous gastrostomy if prolonged dysphagia (2–3 weeks) is anticipated | |
| Antiplatelet agents | |
| Aspirin (160–325 mg loading dose) or clopidogrel (300 mg loading dose) within 24 h after stroke onset. | |
| A delayed administration of 24 h is acceptable in patients receiving iv thrombolysis | |
| Dual therapy with aspirin and clopidogrel within 24 hours from stroke onset for 21 days can be beneficial | |
| Anticoagulation | |
| No benefit from urgent anticoagulation in the prevention of recurrent stroke or neurological deterioration, or to improve outcome | |
| In case of atrial fibrillation, start oral anticoagulation after 5–7 days in minor stroke and in 10–14 days after severe stroke | |
| The usefulness of anticoagulation in severe carotid stenosis of internal carotid artery, or non-occlusive extracranial intraluminal thrombus is uncertain, and anticoagulation is not recommended | |
| Other | |
| Haemodynamic management | Continuous ECG monitoring is recommended Echocardiography assessment during ICU stay might be useful
|
| Haemoglobin |
|
| Venous thromboembolism prophylaxis |
|
| Antiobiotics | Antibiotic prophylaxis is not recommended |
| Seizures management |
|
| Dysphagia | Perform early swallow test and dysphagia assessment |
| Organ-directed therapy . | |
|---|---|
| Haemodynamic management | |
| Avoid hypotension and aim for normovolaemia | |
| In patients that do not receive tPA and have no comorbidities requiring acute BP lowering, target a BP <220/120 mmHg | |
| Target systolic BP to <185/110 mmHg before IV tPA and for 24 h thereafter | |
| For the first 24 h post-EVT, maintain <180/105 mmHg | |
| No clear evidence exists for the preferred drug to be used to acutely control BP. We advise short-acting, easily titratable drugs initially, and oral antihypertensive on subsequent days | |
| Respiratory management | |
| Indications for intubation and mechanical ventilation include: decreased consciousness, (GCS < 9) signs of bulbar dysfunction with inability to protect airway, signs or strong suspicion of intracranial hypertension | |
| Supplemental oxygen not recommended in non-hypoxic patients | |
| Supplemental oxygen only if SpO2 < 94% | |
| Target normoxia and normocapnia | |
| Temperature management | |
| It is necessary to prompt detect, investigate and treat hyperthermia >38°C, but this advice is based on low graded evidence | |
| Pharmacological treatment can be considered as first line, and eventually active cooling therapies | |
| Therapeutic or prophylactic hypothermia is not recommended | |
| Glycaemia and nutrition | |
| Avoid or treat hypoglycaemia (<60 mg/dL) | |
| Treat hyperglycaemia to maintain blood glucose between 140 and 180 mg/dL | |
| Establish full enteral nutrition within 7 days of stroke onset | |
| Consider percutaneous gastrostomy if prolonged dysphagia (2–3 weeks) is anticipated | |
| Antiplatelet agents | |
| Aspirin (160–325 mg loading dose) or clopidogrel (300 mg loading dose) within 24 h after stroke onset. | |
| A delayed administration of 24 h is acceptable in patients receiving iv thrombolysis | |
| Dual therapy with aspirin and clopidogrel within 24 hours from stroke onset for 21 days can be beneficial | |
| Anticoagulation | |
| No benefit from urgent anticoagulation in the prevention of recurrent stroke or neurological deterioration, or to improve outcome | |
| In case of atrial fibrillation, start oral anticoagulation after 5–7 days in minor stroke and in 10–14 days after severe stroke | |
| The usefulness of anticoagulation in severe carotid stenosis of internal carotid artery, or non-occlusive extracranial intraluminal thrombus is uncertain, and anticoagulation is not recommended | |
| Other | |
| Haemodynamic management | Continuous ECG monitoring is recommended Echocardiography assessment during ICU stay might be useful
|
| Haemoglobin |
|
| Venous thromboembolism prophylaxis |
|
| Antiobiotics | Antibiotic prophylaxis is not recommended |
| Seizures management |
|
| Dysphagia | Perform early swallow test and dysphagia assessment |
BP, blood pressure; ECG, electrocardiography; EVT, endovascular thrombectomy; ICU, intensive care unit; IV, intravenous; NA, authors recommendations, no evidence base in AHA/ASA recommendations; SpO2, oxygen saturation; tPA, tissue plasminogen activator.
Clinical deterioration usually predicts an unfavourable course and can be imminent or more gradual. After AIS, neurological deterioration may be subtle or may be caused by a systemic condition, such as infection, dehydration, or hypoglycaemia for instance. However, a high index of suspicion, and thus a low threshold for further diagnostic imaging is in place, especially in patients at risk of swelling of infarcted brain tissue and herniation syndromes after MCA infarction or cerebellar infarction as part of basilar artery occlusion (BAO). In these patients, deterioration in the Glasgow Coma Scale, or new or worse neurological deficit, should lead to consider imminent neuroimaging to rule out surgically treatable progression (i.e. brainstem compression or acute hydrocephalus). Importantly, clear criteria have not been established based on robust evidence including randomized controlled trials, and therefore clinical judgement is the only guide in these patients.
Malignant middle cerebral artery infarction
The incidence of malignant MCA infarction is expected to decrease due to IAT, but it is still an important condition to recognize and manage in a very timely manner. However, still a part of patients has contraindications to IAT or has an unsuccessful IAT.
A 2014 consensus from the AHA stated to consider patients aged <60 years, with unilateral MCA infarction within 48 h despite medical (hyperosmotic) therapy for decompressive craniectomy (DC), and to consider also DC beyond this timeframe in patients deemed eligible/salvageable (not further specified).20 Hyperosmolar therapy (hypertonic saline or mannitol) should be considered both as a bridging therapy to reduce the risk of herniation, or awaiting imminent DC. There is no robust evidence to argue for one or the other in all cases, but hypertonic saline can be applied when mannitol has insufficient effect.24 It is further suggested that hyperosmolar therapies should be titrated on clinical effect, rather than fixed osmotic values, e.g. a certain level of serum sodium, but strong evidence is lacking. Clinical decision-making in such settings requires frequent communication and re-assessments by intensivists, neurologists (or neurointensivists), and neurosurgeons. The trigger for DC should be a clear decrease in consciousness accompanying a compatible CT showing progressive swelling and/or herniation/shift, in spite of hyperosmolar therapy. A more recent European Stroke Organisation (ESO) guideline25 confirmed these recommendations, based on more recent evidence, adding that these treatment decisions should be done in close consultation with next of kin, and certifying towards family that the patient will be much more likely to survive with DC albeit with significant level of neurological impairment. Furthermore, the latter guideline indicates that DC beyond 48 h has uncertain effect but can be considered, as well as DC in patients aged >60 years. This uncertainty should be clearly discussed with family members, before DC is undertaken, considering risks and benefits especially in the latter two categories (>48 h, >60 years) with a lack of evidence supporting them, as long as uncertainty is addressed and the next of kins are motivated and feel that the choice they made is in the best interest of the patients and how he/she would have decided.
Basilar artery occlusion
Similar to the indication for DC in MCA infarction, occipital DC in CT-proven cerebellar infarction swelling should be triggered by deterioration of consciousness, despite hyperosmolar therapy, although strict criteria have not been well established. According to the ESO consensus,25 occipital DC should be considered in eligible patients. Importantly, routine occipital DC is not recommended in all patients with any degree of swelling, but it is in those with compression of the brainstem and a decrease of consciousness. Furthermore, in those not yet having clear brainstem compression on imaging from the dorsal side, but suffering from obstructive hydrocephalus causing clinical deterioration, external cerebrospinal fluid (CSF) drainage by a ventricular drain should be a first-line therapy. However, although CSF drainage may be effective to reduce compression of the brainstem and midbrain, it may also facilitate further brainstem compression by cerebellar mass from the dorsal side by alleviating the rostral counterpressure from the hydrocephalus and result in ‘reverse upward herniation from the brain stem through the tentorial hiatus’. In the latter situation, DC is still an urgent necessity. In contrast to DC in MCA infarction, occipital DC in BAO-related cerebellar infarction (might also pertain to other arteries, e.g. posterior inferior cerebellar artery), may have a very good neurological functional outcome, given that frank paresis/paralysis is usually absent, and patients can recover functionally well in spite of disturbed coordination due to the cerebellar lesion. However, this type of surgery can lead to important complications due to risk of dural sinus damage.
ptICH
This severe complication requires a prompt response including consideration to administer fibrinogen23 to obtain serum levels of at >150 mg/dL up to 36 h after rtPA infusion. Otherwise, correction of ptICH depends on actual coagulation parameters, e.g. thrombocytes may be given in case of platelets <50 000–100 000/microL, fresh frozen plasma or prothrombin complex. Tranexamic acid may also be given, although benefit is not beyond doubt. It is important to note that strong evidence for effective management protocols is lacking, but it is advised that in consultation with the local haematologist multidisciplinary protocols are drafted for local use to describe management of ptICH.
Airway management and intubation
A recent international consensus26 is a guidance for airway management in patients with acute brain injury including AIS. There is a strong recommendation to consider securing the airway with endotracheal intubation guided by a combination of factors: level of consciousness (especially when Glasgow Coma Scale is <9), agitation level, loss of protective airway reflexes and signs of increasing intracranial pressure or herniation. Indication for intubation should be discussion with family regarding a possible non-intubation policy (such as in elderly patients with severe comorbidity and a major cerebral damage) (Table 3).
Non-invasive modes of ventilation have no strong clinical or scientific rationale and may add the risk of aspiration due to gastric aeration due to positive pressure inducing vomiting, whereas high-flow nasal oxygen therapy may be considered as a bridge to recovery (also: no firm evidence, but deemed safer). Otherwise, mechanical ventilation settings should be approached as in most critically ill patients, with the note that effects on intracranial pressure should be taken into account and carefully weighted on a case by case basis. Of note, increasing PEEP levels when central venous pressure is close to intracranial pressure may result in intracranial hypertension.27 Regarding indication and timing of tracheostomy in patients with prolonged periods of decreased consciousness, the consensus paper reports one or several unsuccessful extubation attempts or prolonged decrease of consciousness as possible indications for tracheostomy, but did not issue recommendations on timing,26 despite early tracheostomy seems to have a beneficial effect.28
Haemodynamic management
Blood pressure is a two-sided coin in AIS patients with too low BP being a risk factor for further ischaemic damage and too high BP adding to the risk of cerebral oedema and haemorrhagic transformation of infarcted brain tissue in the acute phase after the ischaemic stroke. Upper and lower thresholds can only be broadly defined on a population level (see also Table 3) and may differ on an individual level and according to the type of stroke and treatment. However, clinical variables to guide optimal BP are not established, rendering titration of optimal individualised target BPs elusive at this time. Fluid management may be more important than previously thought, based on indirect evidence in other brain injured populations,29 and should be aimed at normal volume status (e.g. guided by mean neutral fluid balances over several days). Regarding the type of fluid and also based on indirect evidence,30 no clear evidence favouring saline vs. balanced solutions are available.
Prognostic uncertainty
Prognostication is very difficult and it is important that clinical targets of care should be defined from time to time in conjunction with the patient or (more often) with next of kin or other legal representatives. When clear targets cannot be reasonably obtained within a certain time-frame, limitations of care should be considered. In principle, it is preferable to err on the safe side with neuroprognostication (i.e. prolong treatments as long as there is neither strong confidence on a dismal prognosis nor full consensus between healthcare providers and family members on such limitations). Furthermore, it is imperative that, based on the clinical course and especially the imaging findings, an estimate is done on chances of recovery of consciousness and irreversibility of lesions.31 Indeed, it should be estimated whether imaging indicates that irreversible damage has been established in cerebral structures that are essential for consciousness (e.g. dorsal brainstem, both thalami/hemispheres), acknowledging that it may be difficult to estimate core infarction versus penumbra and completed infarction versus cerebral oedema, especially on CT in the early days after the infarction. Magnetic resonance imaging may help in the assessment of damage in selected cases, and certainly is more sensitive for subtle cerebral damage than CT for prognostic purpose.
Conclusions
Acute ischaemic stroke is an important cause of death and a major cause of permanent disability worldwide. Therapeutic salvage is possible through a number of clinical pharmacological and surgical therapies, which aim to neuronal recovery and avoidance of secondary brain damage and systemic complications.
Intensivists are fully involved in the management of the most severe cases of AIS, with evolving evidence supporting ICU treatment as important factor to impact on ultimate prognosis.
Acute ischemic stroke represents an important condition for intensivists, given the risk of clinical deterioration of these patients resulting in impaired vital functions.
Intravenous thrombolysis and intra-arterial thrombectomy are clearly time-sensitive treatments which should be applied as soon as possible to minimize the consequences of arterial occlusion.
Be aware of indication for hemicraniectomy, which should be discussed on a case by case basis, considering patients’ preferences and estimated prognosis.
Funding
None.
Conflict of interest: none declared.




Comments