-
PDF
- Split View
-
Views
-
Cite
Cite
Andrés R Latorre-Rodríguez, Emma Aschenbrenner, Sumeet K Mittal, Magnetic sphincter augmentation may limit access to magnetic resonance imaging, Diseases of the Esophagus, Volume 36, Issue 11, November 2023, doad032, https://doi.org/10.1093/dote/doad032
Close - Share Icon Share
Summary
Magnetic sphincter augmentation (MSA) is an alternative surgical treatment for gastroesophageal reflux disease; however, >1.5 T magnetic resonance imaging (MRI) is contraindicated for patients who have undergone MSA with the LINX Reflux Management System (Torax Medical, Inc. Shoreview, Minnesota, USA). This drawback can impose a barrier to access of MRI, and cases of surgical removal of the device to enable patients to undergo MRI have been reported. To evaluate access to MRI for patients with an MSA device, we conducted a structured telephone interview with all diagnostic imaging providers in Arizona in 2022. In 2022, only 54 of 110 (49.1%) locations that provide MRI services had at least one 1.5 T or lower MRI scanner. The rapid replacement of 1.5 T MRI scanners by more advanced technology may limit healthcare options and create an access barrier for patients with an MSA device.
INTRODUCTION
Gastroesophageal reflux disease (GERD) is defined as the movement of duodenogastric contents into the esophagus via the esophagogastric junction (EGJ). This often results in symptoms such as heartburn, chest pain, and dysphagia as well as undesirable esophageal and extra-esophageal complications like chronic esophagitis, Barrett’s esophagus, or silent chronic aspiration.1 Satisfactory treatment of GERD depends mainly on the patient’s condition and the reflux severity. The first step of clinical management includes acid suppression therapy. Although acid suppression, especially with proton pump inhibitors, generally provides excellent symptom control, it does not prevent retrograde flow of refluxate through the EGJ.2
Surgical treatment (i.e., mechanical control) provides definitive treatment of GERD and eliminates volume reflux symptoms as well as the need for medical management. Antireflux surgery approaches such as total (Nissen) or partial (Toupet or Dor) fundoplications aim to re-establish EGJ competence by wrapping the gastric fundus around the esophagus; these laparoscopic procedures are effective at treating GERD symptoms and complications in more than 90% of patients.3 However, traditional fundoplications are technically challenging to master, require major anatomical disruption, and may lead to postoperative dysphagia and bloating4,5; additionally, the patient loses the ability to vomit postoperatively, which may impact the endurance of the surgical wrap.6,7
For over 10 years, magnetic sphincter augmentation (MSA) has been used as an alternative surgical treatment option for GERD. In 2012, the US Food and Drug Administration (FDA) approved the first MSA device (LINX Reflux Management System, Torax Medical, Inc. Shoreview, Minnesota, USA), which comprises titanium beads that are laparoscopically implanted around the EGJ to prevent reflux episodes.8–10 The expandable ring mimics the function and pressure of the EGJ to prevent GERD while allowing the patient to retain their ability to belch and vomit.11
The original LINX device carried a restriction on performing magnetic resonance imaging (MRI) with a magnetic field strength >0.7 T.9 As the number of adults in the United States who received at least one MRI doubled between 2000 and 2016, from 6.2 to 13.9%,12 and 1.5 T MRI was rapidly adopted nationwide, the design of the device and the use of concurrent MRI was re-evaluated, resulting in a new generation of LINX, which was approved by the FDA in 2015.13
The current version of LINX is compatible with an MRI magnetic field strength of 1.5 T or less, according to the patient information packet.14 The manufacturer warns that an MRI system with a magnetic field strength >1.5 T might cause serious injury to patients or interfere with the magnetic strength and function of the MSA device. Thus, although the current LINX version is a significant advancement, the MRI contraindication is still a major drawback considering the introduction of 3 T MRI technology and the rapid phase-out of lower-magnetic-field MRI. This study aimed to provide data regarding MRI access opportunities for patients using MSA devices in Arizona, USA.
METHODS
Study design and settings
For this study, a structured interview was conducted via telephone by one of the authors (EA) to all radiology centers in Arizona, USA. The centers were previously identified through the registry of Radiology Imaging Centers (http://www.radiologyimagingcenters.com/client.list.do) and information provided by healthcare provider networks in Arizona in July of 2022. Responses were anonymized. If a center did not have any type of MRI equipment, they were excluded from the survey. Questions included in the interview are presented in Table 1. The data obtained was registered and tabulated in secure Microsoft Excel (Microsoft, Redmond, WA, USA) spreadsheets.
| 1. Does the provider agree to provide information regarding its resources and locations for research purposes? |
| 2. Does this provider have any locations in Arizona with MRI scanners? |
| 3. How many locations with MRI scanners does this provider have in the state of Arizona? |
| 4. How many of those locations are equipped with at least one 1.5 T MRI scanner? |
| 1. Does the provider agree to provide information regarding its resources and locations for research purposes? |
| 2. Does this provider have any locations in Arizona with MRI scanners? |
| 3. How many locations with MRI scanners does this provider have in the state of Arizona? |
| 4. How many of those locations are equipped with at least one 1.5 T MRI scanner? |
| 1. Does the provider agree to provide information regarding its resources and locations for research purposes? |
| 2. Does this provider have any locations in Arizona with MRI scanners? |
| 3. How many locations with MRI scanners does this provider have in the state of Arizona? |
| 4. How many of those locations are equipped with at least one 1.5 T MRI scanner? |
| 1. Does the provider agree to provide information regarding its resources and locations for research purposes? |
| 2. Does this provider have any locations in Arizona with MRI scanners? |
| 3. How many locations with MRI scanners does this provider have in the state of Arizona? |
| 4. How many of those locations are equipped with at least one 1.5 T MRI scanner? |
Definitions and analysis
Providers were defined as any legally constituted entity with facilities in Arizona that provide radiology and diagnostic imaging services to patients. The statistical analysis was limited to descriptive statistics; count and numeric proportions were used for categorical variables. The analytical software used was IBM SPSS v28.0 (IBM Corp, Armonk, NY. Released 2021).
RESULTS
All the contacted providers agreed to respond to the interview questions. A total of 286 locations for radiology and diagnostic imaging services were identified across 44 municipalities in the state of Arizona administered by seven primary providers; 110 of 286 (38.5%) were equipped with MRI scanners. Each of the seven providers had at least one location equipped with 1.5 T MRI scanners; however, only 54 of 110 (49.1%) locations had at least one 1.5 T MRI scanner. Only provider F had 1.5 T MRI scanners in all its locations. Figure 1 shows the geographical distribution of the radiology centers in the state of Arizona, and Table 2 summarizes the collected information.
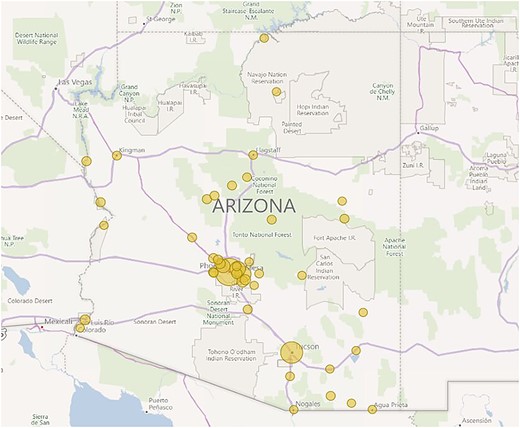
Geographical distribution of radiology centers in Arizona. Note that the Phoenix metropolitan area has a higher concentration of facilities; only 44 of 91 (48.4%) municipalities in Arizona have at least one radiology center. Created with Power BI v2.91 (Microsoft, Redmond, WA, USA).
| Provider . | Locations with MRI scanner No. (%) . | Locations equipped with at least one 1.5 T MRI scanner No. (%) . |
|---|---|---|
| A | 16 (14.5) | 3 (18.8) |
| B | 66 (60) | 30 (45.5) |
| C | 11 (10) | 8 (72.7) |
| D | 4 (3.6) | 2 (50) |
| E | 5 (4.6) | 4 (80) |
| F | 6 (5.5) | 6 (100) |
| G | 2 (1.8) | 2 (50) |
| Total | 110 (100) | 54 (49.1) |
| Provider . | Locations with MRI scanner No. (%) . | Locations equipped with at least one 1.5 T MRI scanner No. (%) . |
|---|---|---|
| A | 16 (14.5) | 3 (18.8) |
| B | 66 (60) | 30 (45.5) |
| C | 11 (10) | 8 (72.7) |
| D | 4 (3.6) | 2 (50) |
| E | 5 (4.6) | 4 (80) |
| F | 6 (5.5) | 6 (100) |
| G | 2 (1.8) | 2 (50) |
| Total | 110 (100) | 54 (49.1) |
| Provider . | Locations with MRI scanner No. (%) . | Locations equipped with at least one 1.5 T MRI scanner No. (%) . |
|---|---|---|
| A | 16 (14.5) | 3 (18.8) |
| B | 66 (60) | 30 (45.5) |
| C | 11 (10) | 8 (72.7) |
| D | 4 (3.6) | 2 (50) |
| E | 5 (4.6) | 4 (80) |
| F | 6 (5.5) | 6 (100) |
| G | 2 (1.8) | 2 (50) |
| Total | 110 (100) | 54 (49.1) |
| Provider . | Locations with MRI scanner No. (%) . | Locations equipped with at least one 1.5 T MRI scanner No. (%) . |
|---|---|---|
| A | 16 (14.5) | 3 (18.8) |
| B | 66 (60) | 30 (45.5) |
| C | 11 (10) | 8 (72.7) |
| D | 4 (3.6) | 2 (50) |
| E | 5 (4.6) | 4 (80) |
| F | 6 (5.5) | 6 (100) |
| G | 2 (1.8) | 2 (50) |
| Total | 110 (100) | 54 (49.1) |
DISCUSSION
Despite significant advances with the use of non-ferromagnetic materials such as titanium for the manufacture of biomedical devices and implants, a significant proportion of the devices that have been commercialized in recent decades continue to be labeled as ‘MR conditional’, meaning that these patients can use an MRI scanner only under specific conditions or restrictions.15 MSA devices, cochlear implants, coronary and peripheral artery stents, intrauterine devices, inferior vena cava filters, and Harrington rods are some examples of commonly used devices that fall into this safety category.16 The introduction of MRI scanners with higher magnetic field strengths is an inherent problem in research and development (R&D) of medical devices because not all medical devices or implants tested on a 1.5 T MRI scanner have been tested on a 3 T MRI scanner; thus, if a 3 T MRI scanner is the only option available at a location, patients with specific implanted devices like LINX cannot be scanned, reducing critical healthcare access.
In 2015, Torax Medical announced the approval of the new generation of LINX by the FDA,13 announcing that its product ‘...is MR conditional in MRI systems up to 1.5T, which represents about 90% of MRI systems in use in the U.S.’; however, only 8 years later, the statement does not seem to have the same validity. In our study, just less than half of facilities in Arizona were equipped with 1.5 T or less MRI scanners.
According to the Organization for Economic Cooperation and Development, the USA had 12,613 MRI scanners in 2021 (52.8% in an outpatient setting and 47.2% in a hospital setting), the second highest number of MRI scanners per capita (38 units/million inhabitants) after Japan.17 This indicates that the number of available scanners or the geographic distribution is not the problem; rather, the increasing rate of replacement of 1.5 T by 3 T MRI systems by the providers is at the root. However, there are no culprits in this situation; providers ‘upgrade’ their equipment with different objectives, e.g., to increase efficiency, improve the technical quality of images, decrease operating costs, improve return on investment, maintain cutting-edge facilities, or simply to satisfy the demands of referring physicians.
From the clinical perspective, sometimes the need to perform an MRI on a user of ‘MR conditional’ medical devices is imperative. Complex scenarios such as staging of neoplastic diseases, workup of metabolic diseases, or planning neurological interventions are just a few examples. In these cases, the patient is ‘between a rock and a hard place’, on the one hand with an access barrier (i.e., limited locations with 1.5 T systems) and on the other hand an opportunity barrier (i.e., access to newer systems such as 3 T MRI scanners that could grant better diagnostic performance). If an alternative diagnostic procedure cannot be used and an MRI is essential for a patient using LINX, the surgeon must remove the device; according to Ethicon Incorporated (Cincinnati, OH, USA), ~1.8% of LINX removal cases are because of the need for an MRI.18 Of note, the surgical risk is minimal for the patient.
In this R&D convergence, where the development of one technology demands the re-evaluation of another, the potential alternatives to correct or overcome this gap demand efforts from all of the stakeholders: (i) the medical device industry should re-evaluate the suitability of the device design by performing safety tests on 3 T MRI systems, obtain regulatory approval for use, and eventually, structure support programs for patients that guarantee access to nearby 1.5 T MRI scanners; (ii) the radiology centers, which are responsible for patient safety before and during the scanning, should continuously review the compatibility of the implants or medical devices with the type of available MRI scanners; (iii) the surgeon should discuss with the patient during pre-surgical counseling that the use of the LINX device may affect their ability to undergo MRI19 as well as the possible scenarios requiring removal of the device; and (iv) the physician who orders an MRI should consider the relevance of performing this exam as well as evaluate alternative diagnostic options.
Our study has some limitations, including the geographical scope. Since health systems vary from state to state (i.e. distribution of resources, number of providers, installed capacity, etc.), we recognize that the data obtained in the state of Arizona may not be applicable on the national level. Furthermore, because of the design of the structured interview, we identified only the number of providers and locations equipped with 1.5 T MRI scanners and may have missed key data points such as the number of users with biomedical devices who have required an MRI after surgery or the number of MRIs performed by each location or provider annually. However, despite these gaps, we contacted every provider in the state of Arizona, making us the first group to conduct a statewide survey to assess access to radiology services by users of ‘MR conditional’ medical devices.
CONCLUSION
Patients with an MSA device for the treatment of GERD cannot have an MRI at roughly 51% of facilities in the state of Arizona. With time and technological advances, this number will likely increase. This should be considered, and patients should be appropriately counseled before LINX implantation.
Acknowledgments/financial support
We thank Kristine Nally for editorial assistance. No outside funding was obtained for this study.
CRediT author Statement
Andrés R. Latorre-Rodríguez MD (Data curation, Formal analysis, Writing—original draft, Writing—review & editing), Emma Aschenbrenner (Data curation, Formal analysis, Writing—original draft), and Sumeet K. Mittal (Conceptualization, Formal analysis, Writing—original draft, Writing—review & editing)
Specific author contributions: Conception and design: Sumeet K. Mittal; Collection and assembly of data, data analysis, and drafting of the manuscript: Emma Aschenbrenner, Andrés R. Latorre-Rodríguez, Sumeet K. Mittal; Revision of the manuscript for critically important intellectual content: Andrés R. Latorre-Rodríguez and Sumeet K. Mittal; All the authors approved the final manuscript. Conflicts of interest: The authors declare that they have no conflict of interests.
References
Ghadimi M, Sapra A. Magnetic Resonance Imaging Contraindications. 2022 May 8. In:
OECD (2023). Magnetic resonance imaging (MRI) units (indicator), https://doi.org/10.1787/1a72e7d1-en (accessed on 19 May 2023).



