-
PDF
- Split View
-
Views
-
Cite
Cite
Shahab Hajibandeh, Shahin Hajibandeh, Matthew McKenna, William Jones, Paul Healy, Jolene Witherspoon, Guy Blackshaw, Wyn Lewis, Antonio Foliaki, Tarig Abdelrahman, Effect of intraoperative botulinum toxin injection on delayed gastric emptying and need for endoscopic pyloric intervention following esophagectomy: a systematic review, meta-analysis, and meta-regression analysis, Diseases of the Esophagus, Volume 36, Issue 11, November 2023, doad053, https://doi.org/10.1093/dote/doad053
Close - Share Icon Share
Abstract
The aim of this study was to evaluate the effect of intraoperative botulinum toxin (BT) injection on delayed gastric emptying (DGE) and need for endoscopic pyloric intervention (NEPI) following esophagectomy. In compliance with Preferred Reporting Items for Systematic reviews and Meta-Analyses statement standards, a systematic review of studies reporting the outcomes of intraoperative BT injection in patients undergoing esophagectomy for esophageal cancer was conducted. Proportion meta-analysis model was constructed to quantify the risk of the outcomes and direct comparison meta-analysis model was constructed to compare the outcomes between BT injection and no BT injection or surgical pyloroplasty. Meta-regression was modeled to evaluate the effect of variations in different covariates among the individual studies on overall summary proportions. Nine studies enrolling 1070 patients were included. Pooled analyses showed that the risks of DGE and NEPI following intraoperative BT injection were 13.3% (95% confidence interval [CI]: 7.9–18.6%) and 15.2% (95% CI: 7.9–22.5%), respectively. There was no difference between BT injection and no BT injection in terms of DGE (odds ratio [OR]: 0.57, 95% CI: 0.20–1.61, P = 0.29) and NEPI (OR: 1.73, 95% CI: 0.42–7.12, P = 0.45). Moreover, BT injection was comparable to pyloroplasty in terms of DGE (OR: 0.85, 95% CI: 0.35–2.08, P = 0.73) and NEPI (OR: 8.20, 95% CI: 0.63–105.90, P = 0.11). Meta-regression suggested that male gender was negatively associated with the risk of DGE (coefficient: −0.007, P = 0.003). In conclusion, level 2 evidence suggests that intraoperative BT injection may not improve the risk of DGE and NEPI in patients undergoing esophagectomy. The risk of DGE seems to be higher in females and in early postoperative period. High quality randomized controlled trials with robust statistical power are required for definite conclusions. The results of the current study can be used for hypothesis synthesis and power analysis in future prospective trials.
INTRODUCTION
Esophagectomy, which is the standard of care for resectable esophageal cancer, is associated with significant risks of morbidity and mortality.1 A gastric conduit is typically fashioned for reconstruction of esophagus during esophagectomy. However, delayed gastric emptying (DGE) is a recognized complication after esophagectomy when a gastric conduit is created and can occur in 15–39% of patients.2,3 DGE is thought to be caused by damage to the vagus nerves and intramural gastric neuromuscular structure during esophagectomy which result in denervation of the gastric conduit, hence gastric conduit dysmotility and dysfunction of the pylorus.3 DGE is associated with symptoms of nausea, vomiting, and fullness and may increase the risk of aspiration pneumonia.3
In order to prevent DGE after esophagectomy, intraoperative pyloric drainage techniques including pyloroplasty, pyloromyotomy, pyloric dilation, and intraoperative botulinum toxin (BT) injection have been proposed.4 It has been shown that intraoperative pyloric intervention may reduce the risk of DGE after esophagectomy.5 The outcomes of surgical pyloroplasty have been extensively evaluated and its routine use for prevention of DGE is indeed controversial.6–8 However, the level of available evidence on role of chemical pyloroplasty using intraoperative BT injection in patients undergoing esophagectomy is poorly understood. In view of this, we aimed to conduct a systematic review and meta-analysis to evaluate the effect of intraoperative BT injection on DGE and need for endoscopic pyloric intervention (NEPI) following esophagectomy.
METHODS
This study was protocoled, conducted, and reported in compliance with Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement standards.9
Participants, interventions, comparisons, outcomes, and study design research question
The objective of the study was to evaluate the risks of DGE and NEPI following intraoperative BT injection in patients undergoing esophagectomy using a proportion meta-analysis model and to compare the risks between intraoperative BT injection and no BT injection or surgical pyloroplasty using a comparison meta-analysis model.
Eligibility criteria
Study design
All randomized controlled trials, cohort studies, case–control studies, and case series reporting the risk of DGE and NEPI following intraoperative BT injection in patients undergoing esophagectomy were considered eligible for inclusion.
Population
All adult patients (aged over 18) with resectable esophageal cancer who underwent esophagectomy were considered eligible for inclusion. In terms of pathology of esophageal cancer, both squamous cell carcinoma and adenocarcinoma were eligible. The surgical techniques of interest for esophagectomy included Ivor Lewis technique, McKeown technique, or trans-hiatal technique. Both open and minimally invasive (laparoscopic or robotic) approaches were eligible.
Intervention and comparison
Intraoperative injection of any dose of BT into pylorus was considered as intervention of interest. No BT injection or surgical pyloroplasty was considered as comparison of interest.
Outcome
Postoperative DGE and NEPI were considered as outcome measures of interest. The definition of DGE should have been consistent (not necessarily identical) with the criteria defined by Konradsson et al.10 which was divided into early DGE (within 14 days of surgery) and late DGE (later than 14 days after surgery).10 The criteria for early DGE included >500 mL diurnal nasogastric tube output measured on the morning of postoperative day 5 or later or >100% increased gastric tube width on frontal chest x-ray projection together with the presence of an air–fluid level.10 The criteria for late DGE included at least two of the following symptoms: early satiety/fullness, vomiting, nausea, regurgitation or inability to meet caloric need by oral intake, and delayed contrast passage on upper gastrointestinal water-soluble contrast radiogram or on timed barium swallow.10
Search methods
A comprehensive search strategy was created by two independent authors with experience in evidence synthesis using appropriate search limits, keywords, thesaurus headings, and operators (Appendix I). The search strategy had no language restrictions and was last run on 4 February 2023. The developed strategy was applied and adopted on the electronic sources listed below:
CENTRAL
Embase
MEDLINE
Scopus
CINAHL
International Standard Randomised Controlled Trial Number Registry
World Health Organization International Clinical Trials Registry
System for Information on Grey Literature
The European Association for Grey Literature Exploitation
The reference lists of relevant systematic reviews, meta-analyses, and original studies were also evaluated to identify more eligible studies.
Study selection and data extraction
Two independent authors screened the title and abstract of the identified articles and retrieved the full texts of relevant articles. The studies that met the eligibility criteria of this study were selected for inclusion. An electronic data collection proforma was created and pilot-tested based on randomly selected studies which included information on name of the first author, year of publication, name of journal, type of study design, description of included population, sample size of each study, surgical technique and approach used for esophagectomy, age, and gender of included population, dose of BT injected, and the outcome measures. The two independent authors discussed and resolved any disagreements during study selection and data extraction; a third independent author was consulted when required.
Risk-of-bias assessment
Two independent authors evaluated the methodological quality of the included studies. The Cochrane tool11 was used for assessing the risk of bias in randomized trials and the Risk Of Bias In Non-randomized Studies—of Interventions (ROBINS-I) assessment tool12 was used for assessing the risk of bias in observational studies. A separate third author was involved in case of disagreements between the first two authors.
Data analysis
OpenMeta [Analyst] software was used for proportion meta-analysis model and Review Manager 5.4 software was used for comparison meta-analysis model. A proportion meta-analysis model was constructed to quantify the risks of DGE and NEPI following intraoperative BT injection in patients undergoing esophagectomy. We integrated the quantitative risk of DGE and NEPI from individual studies and calculated numerical estimates of the overall effect. The DerSimonian–Laird random-effects method was used to calculate the weighted summary proportions under the random-effects modeling. A comparison meta-analysis model was constructed to compare the risk of DGE and NEPI between intraoperative BT injection and no BT injection or surgical pyloroplasty. The odds ratio (OR) was calculated as summary measure using random-effects modeling for comparison meta-analysis. Intention-to-treat information data from the included studies were used for data analysis and individual patients were considered as unit of analysis. Meta-regression models were constructed to assess the effect of differences in age, gender, surgical technique used, the BT dose used, and type of tumor (adenocarcinoma or squamous cell carcinoma) among the individual studies on overall summary proportions. Moreover, we conducted meta-regression to evaluate the association between DGE and NEPI. The statistical heterogeneity was measured as I2 using Cochran Q test (χ2), and it was classified as low heterogeneity when I2 was 0–25%, moderate heterogeneity when I2 was 25–75%, and high heterogeneity when I2 was 75–100%. We planned to evaluate the risk of publication bias by constructing funnel plots for the outcomes reported by at least 10 studies.
Additional analyses
We performed subgroup analyses based on the approach used for esophagectomy (open vs. minimally invasive), based on time of DGE diagnosis in relation to surgery (early vs. late), and based on clinical and radiological diagnosis of DGE. Moreover, we performed the following sensitivity analyses in order to evaluate the consistency and robustness of the results: (i) leave-one-out analysis to investigate effect of each study on the pooled risk of DGE and NEPI; (ii) separate analysis of studies with low overall risk of bias.
Summary of findings table
We evaluated the certainty of evidence based on the recommended standards and domains by the GRADE system and a summary of findings table was produced.13
RESULTS
Results of the search
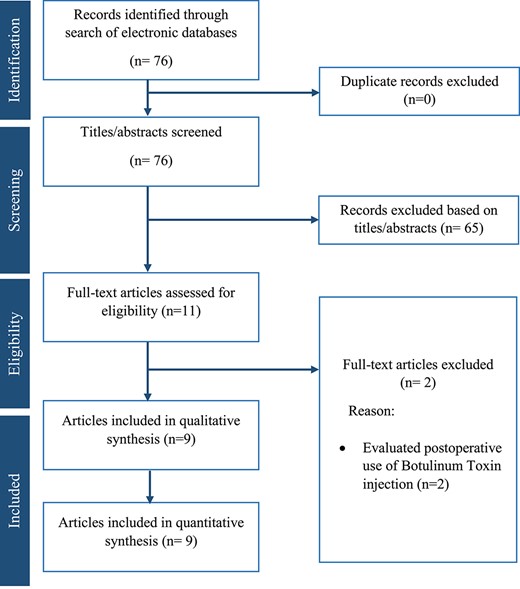
The search of electronic databases resulted in 76 articles of which 65 articles were excluded directly because they were not relevant to the subject of this study. After reviewing the full text of the remaining 11 articles, two more articles were excluded because they evaluated postoperative use of BT injection. Consequently, nine studies14–22 (eight retrospective cohorts14–19,21,22 and one randomized controlled trial20) including a total of 1070 patients were eligible for inclusion. The study flow chart is shown in Fig. 1. Among the included population, 406 received intraoperative BT injection who were compared with 318 patients who received no intraoperative intervention and 346 patients who had intraoperative surgical pyloroplasty. The baseline characteristics of the included studies are shown in Table 1.
| Authors . | Year . | Country . | Journal . | Design . | Included population . | Number of patients . | ||
|---|---|---|---|---|---|---|---|---|
| . | . | . | . | . | . | BT injection group . | Pyloroplasty group . | No intervention group . |
| Marchese et al. | 2018 | UK | Journal of Gastrointestinal Surgery | Retrospective cohort | Patients with esophageal cancer undergoing esophagectomy | 30 | 30 | 30 |
| Tham et al. | 2018 | UK | Diseases of the Esophagus | Retrospective cohort | Patients with esophageal cancer undergoing esophagectomy | 65 | 0 | 163 |
| Giugliano et al. | 2017 | USA | Diseases of the Esophagus | Retrospective cohort | Patients with esophageal cancer undergoing esophagectomy | 41 | 59 | 8 |
| Stewart et al. | 2017 | USA | Annals of Surgical Oncology | Retrospective cohort | Patients with esophageal cancer undergoing esophagectomy | 35 | 0 | 36 |
| Fuchs et al. | 2016 | USA | Journal of Laparoendoscopic & Advanced Surgical Techniques | Retrospective cohort | Patients with esophageal cancer undergoing esophagectomy | 14 | 0 | 27 |
| Eldaif et al. | 2014 | USA | Annals of Thoracic Surgery | Retrospective cohort | Patients with esophageal cancer undergoing esophagectomy | 78 | 199 | 0 |
| Bagheri et al. | 2013 | Iran | Asian Cardiovascular & Thoracic Annals | Randomized controlled trial | Patients with esophageal cancer undergoing esophagectomy | 30 | 30 | 0 |
| Martin et al. | 2009 | USA | Annals of Thoracic Surgery | Retrospective cohort | Patients with esophageal cancer undergoing esophagectomy | 45 | 0 | 0 |
| Cerfolio et al. | 2009 | USA | Journal of Thoracic and Cardiovascular Surgery | Retrospective cohort | Patients with esophageal cancer undergoing esophagectomy | 68 | 28 | 54 |
| Authors . | Year . | Country . | Journal . | Design . | Included population . | Number of patients . | ||
|---|---|---|---|---|---|---|---|---|
| . | . | . | . | . | . | BT injection group . | Pyloroplasty group . | No intervention group . |
| Marchese et al. | 2018 | UK | Journal of Gastrointestinal Surgery | Retrospective cohort | Patients with esophageal cancer undergoing esophagectomy | 30 | 30 | 30 |
| Tham et al. | 2018 | UK | Diseases of the Esophagus | Retrospective cohort | Patients with esophageal cancer undergoing esophagectomy | 65 | 0 | 163 |
| Giugliano et al. | 2017 | USA | Diseases of the Esophagus | Retrospective cohort | Patients with esophageal cancer undergoing esophagectomy | 41 | 59 | 8 |
| Stewart et al. | 2017 | USA | Annals of Surgical Oncology | Retrospective cohort | Patients with esophageal cancer undergoing esophagectomy | 35 | 0 | 36 |
| Fuchs et al. | 2016 | USA | Journal of Laparoendoscopic & Advanced Surgical Techniques | Retrospective cohort | Patients with esophageal cancer undergoing esophagectomy | 14 | 0 | 27 |
| Eldaif et al. | 2014 | USA | Annals of Thoracic Surgery | Retrospective cohort | Patients with esophageal cancer undergoing esophagectomy | 78 | 199 | 0 |
| Bagheri et al. | 2013 | Iran | Asian Cardiovascular & Thoracic Annals | Randomized controlled trial | Patients with esophageal cancer undergoing esophagectomy | 30 | 30 | 0 |
| Martin et al. | 2009 | USA | Annals of Thoracic Surgery | Retrospective cohort | Patients with esophageal cancer undergoing esophagectomy | 45 | 0 | 0 |
| Cerfolio et al. | 2009 | USA | Journal of Thoracic and Cardiovascular Surgery | Retrospective cohort | Patients with esophageal cancer undergoing esophagectomy | 68 | 28 | 54 |
BT: botulinum toxin.
| Authors . | Year . | Country . | Journal . | Design . | Included population . | Number of patients . | ||
|---|---|---|---|---|---|---|---|---|
| . | . | . | . | . | . | BT injection group . | Pyloroplasty group . | No intervention group . |
| Marchese et al. | 2018 | UK | Journal of Gastrointestinal Surgery | Retrospective cohort | Patients with esophageal cancer undergoing esophagectomy | 30 | 30 | 30 |
| Tham et al. | 2018 | UK | Diseases of the Esophagus | Retrospective cohort | Patients with esophageal cancer undergoing esophagectomy | 65 | 0 | 163 |
| Giugliano et al. | 2017 | USA | Diseases of the Esophagus | Retrospective cohort | Patients with esophageal cancer undergoing esophagectomy | 41 | 59 | 8 |
| Stewart et al. | 2017 | USA | Annals of Surgical Oncology | Retrospective cohort | Patients with esophageal cancer undergoing esophagectomy | 35 | 0 | 36 |
| Fuchs et al. | 2016 | USA | Journal of Laparoendoscopic & Advanced Surgical Techniques | Retrospective cohort | Patients with esophageal cancer undergoing esophagectomy | 14 | 0 | 27 |
| Eldaif et al. | 2014 | USA | Annals of Thoracic Surgery | Retrospective cohort | Patients with esophageal cancer undergoing esophagectomy | 78 | 199 | 0 |
| Bagheri et al. | 2013 | Iran | Asian Cardiovascular & Thoracic Annals | Randomized controlled trial | Patients with esophageal cancer undergoing esophagectomy | 30 | 30 | 0 |
| Martin et al. | 2009 | USA | Annals of Thoracic Surgery | Retrospective cohort | Patients with esophageal cancer undergoing esophagectomy | 45 | 0 | 0 |
| Cerfolio et al. | 2009 | USA | Journal of Thoracic and Cardiovascular Surgery | Retrospective cohort | Patients with esophageal cancer undergoing esophagectomy | 68 | 28 | 54 |
| Authors . | Year . | Country . | Journal . | Design . | Included population . | Number of patients . | ||
|---|---|---|---|---|---|---|---|---|
| . | . | . | . | . | . | BT injection group . | Pyloroplasty group . | No intervention group . |
| Marchese et al. | 2018 | UK | Journal of Gastrointestinal Surgery | Retrospective cohort | Patients with esophageal cancer undergoing esophagectomy | 30 | 30 | 30 |
| Tham et al. | 2018 | UK | Diseases of the Esophagus | Retrospective cohort | Patients with esophageal cancer undergoing esophagectomy | 65 | 0 | 163 |
| Giugliano et al. | 2017 | USA | Diseases of the Esophagus | Retrospective cohort | Patients with esophageal cancer undergoing esophagectomy | 41 | 59 | 8 |
| Stewart et al. | 2017 | USA | Annals of Surgical Oncology | Retrospective cohort | Patients with esophageal cancer undergoing esophagectomy | 35 | 0 | 36 |
| Fuchs et al. | 2016 | USA | Journal of Laparoendoscopic & Advanced Surgical Techniques | Retrospective cohort | Patients with esophageal cancer undergoing esophagectomy | 14 | 0 | 27 |
| Eldaif et al. | 2014 | USA | Annals of Thoracic Surgery | Retrospective cohort | Patients with esophageal cancer undergoing esophagectomy | 78 | 199 | 0 |
| Bagheri et al. | 2013 | Iran | Asian Cardiovascular & Thoracic Annals | Randomized controlled trial | Patients with esophageal cancer undergoing esophagectomy | 30 | 30 | 0 |
| Martin et al. | 2009 | USA | Annals of Thoracic Surgery | Retrospective cohort | Patients with esophageal cancer undergoing esophagectomy | 45 | 0 | 0 |
| Cerfolio et al. | 2009 | USA | Journal of Thoracic and Cardiovascular Surgery | Retrospective cohort | Patients with esophageal cancer undergoing esophagectomy | 68 | 28 | 54 |
BT: botulinum toxin.
Baseline characteristics of the included population
The mean age of the included patients who received BT injection was 64 years (95% CI: 61–66) and 75% were male. In terms of the surgical technique used for esophagectomy, 276 out of 406 (68%) underwent Ivor Lewis esophagectomy, 92 out of 406 (23%) underwent McKeown esophagectomy, and 38 out of 406 (9%) underwent trans-hiatal esophagectomy. Open approach was used in 234 out of 406 (58%) and minimally invasive approach was used in 172 out of 406 (42%). The dose of BT used ranged between 20 and 200 units; 185 out of 406 (45%) received 200 units, 187 out of 406 (46%) received 100 units, and 35 out of 406 (9%) patients received 20 units. The baseline characteristics of the included population are shown in Table 2.
Baseline characteristics of the included population who were treated with intraoperative botulinum toxin
| No of patients . | 406 . |
|---|---|
| Mean age | 64 years (95% CI: 61–66) |
| Male gender | 305 out of 406 (75%) |
| Surgical technique | |
| Ivor Lewis | 276 out of 406 (68%) |
| McKeown | 92 out of 406 (23%) |
| Trans-hiatal | 38 out of 406 (9%) |
| Approach | |
| Open | 234 out of 406 (58%) |
| Minimally invasive | 172 out of 406 (42%) |
| Botulinum toxin dose | |
| 20 Units | 35 out of 406 (9%) |
| 100 Units | 187 out of 406 (46%) |
| 200 Units | 185 out of 406 (45%) |
| No of patients . | 406 . |
|---|---|
| Mean age | 64 years (95% CI: 61–66) |
| Male gender | 305 out of 406 (75%) |
| Surgical technique | |
| Ivor Lewis | 276 out of 406 (68%) |
| McKeown | 92 out of 406 (23%) |
| Trans-hiatal | 38 out of 406 (9%) |
| Approach | |
| Open | 234 out of 406 (58%) |
| Minimally invasive | 172 out of 406 (42%) |
| Botulinum toxin dose | |
| 20 Units | 35 out of 406 (9%) |
| 100 Units | 187 out of 406 (46%) |
| 200 Units | 185 out of 406 (45%) |
Baseline characteristics of the included population who were treated with intraoperative botulinum toxin
| No of patients . | 406 . |
|---|---|
| Mean age | 64 years (95% CI: 61–66) |
| Male gender | 305 out of 406 (75%) |
| Surgical technique | |
| Ivor Lewis | 276 out of 406 (68%) |
| McKeown | 92 out of 406 (23%) |
| Trans-hiatal | 38 out of 406 (9%) |
| Approach | |
| Open | 234 out of 406 (58%) |
| Minimally invasive | 172 out of 406 (42%) |
| Botulinum toxin dose | |
| 20 Units | 35 out of 406 (9%) |
| 100 Units | 187 out of 406 (46%) |
| 200 Units | 185 out of 406 (45%) |
| No of patients . | 406 . |
|---|---|
| Mean age | 64 years (95% CI: 61–66) |
| Male gender | 305 out of 406 (75%) |
| Surgical technique | |
| Ivor Lewis | 276 out of 406 (68%) |
| McKeown | 92 out of 406 (23%) |
| Trans-hiatal | 38 out of 406 (9%) |
| Approach | |
| Open | 234 out of 406 (58%) |
| Minimally invasive | 172 out of 406 (42%) |
| Botulinum toxin dose | |
| 20 Units | 35 out of 406 (9%) |
| 100 Units | 187 out of 406 (46%) |
| 200 Units | 185 out of 406 (45%) |
Risk of bias in included studies
Fig. 2 highlights the outcomes of methodological quality assessment based on the Cochrane tool and ROBINS-I tool.
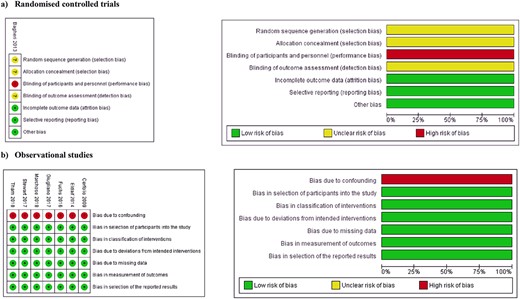
Risk of bias summary and graph showing authors’ judgments about each risk of bias item for (a) randomized controlled trials; (b) observational studies.
Outcomes of intraoperative botulinum toxin injection
Delayed gastric emptying
Analysis of 365 patients from eight studies suggested that the risk of DGE after intraoperative BT injection was 13.3% (95% CI: 7.9–18.6%) (Fig. 3a). The level of between-study statistical heterogeneity was moderate (I2 = 60%, P = 0.014). The GRADE certainty of the evidence was moderate (Supplementary Table 1). Meta-regression suggested that male gender was negatively associated with the risk of DGE (coefficient: −0.007, P = 0.003); however, there was no association between age (coefficient: 0.02, P = 0.131), BT dose (coefficient: −0.001, P = 0.197), surgical technique (coefficient: 0.0001, P = 0.907), adenocarcinoma (coefficient: −0.080, P = 0.639), or squamous cell carcinoma (coefficient: −0.037, P = 0.827), and the risk of DGE (Fig. 4a).
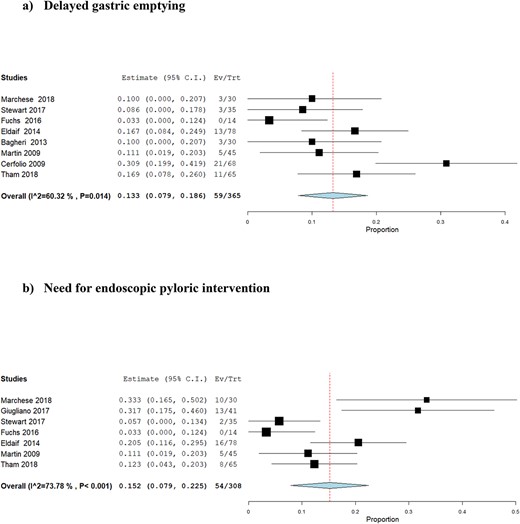
Forest plots for proportion meta-analysis of the outcomes after intraoperative botulinum toxin injection: (a) delayed gastric emptying; (b) need for endoscopic pyloric intervention.
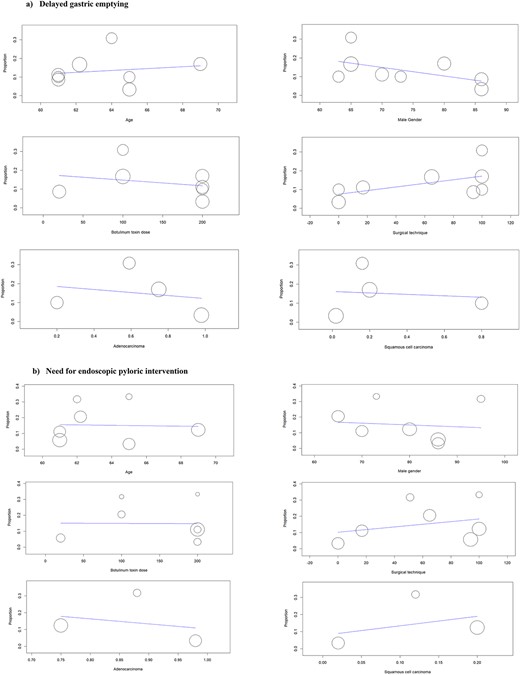
Results of meta-regression analyses for (a) delayed gastric emptying; (b) need for endoscopic pyloric intervention.
Need for endoscopic pyloric intervention
Analysis of 308 patients from seven studies suggested that the risk of NEPI after intraoperative BT injection was 15.2% (95% CI: 7.9–22.5%) (Fig. 3b). The level of between-study statistical heterogeneity was moderate (I2 = 74%, P < 0.001). The GRADE certainty of the evidence was moderate (Supplementary Table 1). Meta-regression suggested no association between age (coefficient: −0.03, P = 0.19), male gender (coefficient: −0.002, P = 0.632), BT dose (coefficient: 0.001, P = 0.191), surgical technique (coefficient: 0.002, P = 0.1), adenocarcinoma (coefficient: −0.301, P = 0.613), or squamous cell carcinoma (coefficient: 0.554, P = 0.440) and the risk of NEPI (Fig. 4b).
Association between DGE and NEPI
Meta-regression suggested no association between DGE and NEPI (coefficient: −0.080, P = 0.383) (Supplementary Fig. 1).
Intraoperative BT injection versus no BT injection
Delayed gastric emptying
Analysis of 522 patients from five studies showed no difference in the risk of DGE between BT injection and no BT injection (OR: 0.57, 95% CI: 0.20–1.61, P = 0.29). The level of between-study statistical heterogeneity was moderate (I2 = 67%, P = 0.02) (Fig. 5a). The GRADE certainty of the evidence was moderate (Supplementary Table 2).
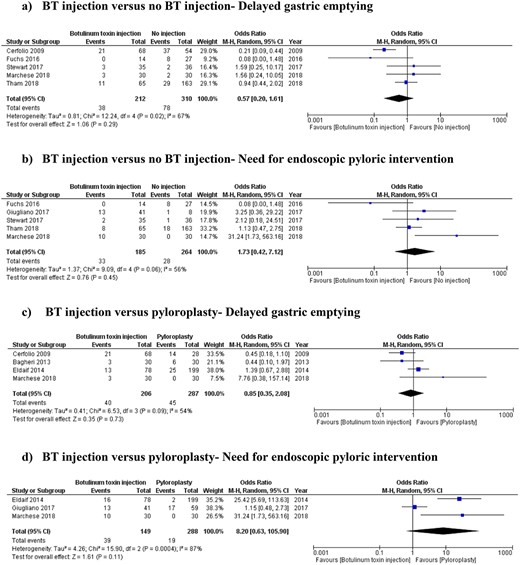
Forest plots for comparison meta-analysis model: (a) BT injection versus no BT injection—delayed gastric emptying; (b) BT injection versus no BT injection—need for endoscopic pyloric intervention; (c) BT injection versus pyloroplasty—delayed gastric emptying; (d) BT injection versus pyloroplasty—need for endoscopic pyloric intervention. BT: botulinum toxin.
Need for endoscopic pyloric intervention
Analysis of 449 patients from five studies showed no difference in the risk of NEPI between BT injection and no BT injection (OR: 1.73, 95% CI: 0.42–7.12, P = 0.45). The level of between-study statistical heterogeneity was moderate (I2 = 56%, P = 0.45) (Fig. 5b). The GRADE certainty of the evidence was moderate (Supplementary Table 2).
Intraoperative BT injection versus pyloroplasty
Delayed gastric emptying
Analysis of 493 patients from four studies showed no difference in the risk of DGE between BT injection and pyloroplasty (OR: 0.85, 95% CI: 0.35–2.08, P = 0.73). The level of between-study statistical heterogeneity was moderate (I2 = 54%, P = 0.0.9) (Fig. 5c). The GRADE certainty of the evidence was moderate (Supplementary Table 3).
Need for endoscopic pyloric intervention
Analysis of 437 patients from three studies showed no difference in the risk of NEPI between BT injection and pyloroplasty (OR: 8.20, 95% CI: 0.63–105.90, P = 0.11). The level of between-study statistical heterogeneity was high (I2 = 87%, P = 0.0004) (Fig. 5d). The GRADE certainty of the evidence was very low (Supplementary Table 3).
Subgroup analyses
Open approach
Subgroup analyses of patients undergoing open esophagectomy showed that the risks of DGE and NEPI after intraoperative BT injection were 16.8% (95% CI: 7.9–25.8%) and 24.8% (95% CI: 13.0–36.7%), respectively (Fig. 6a). There was no difference in the risk of DGE between BT injection and no BT injection (OR: 0.47, 95% CI: 0.07–3.31, P = 0.45) and between BT injection and pyloroplasty (OR: 0.85, 95% CI: 0.35–2.08, P = 0.73). The risk of NEPI was higher in patients who had BT injection in comparison to no BT injection (OR: 31.24, 95% CI: 1.73–563.16, P = 0.02) and pyloroplasty (OR: 26.55, 95% CI: 7.02–100.37, P < 0.00001).
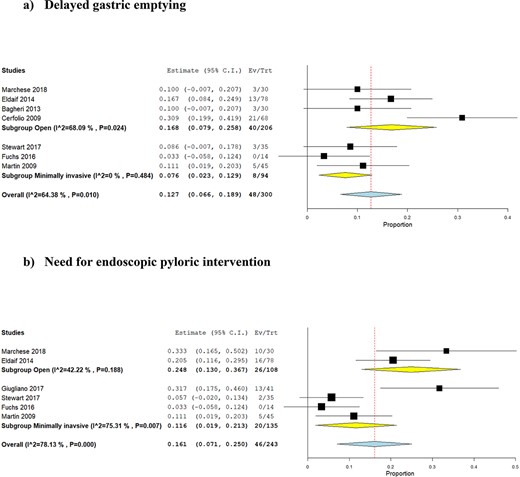
Forest plots for subgroup analyses based on open and minimally invasive approach for esophagectomy: (a) delayed gastric emptying; (b) need for endoscopic pyloric intervention.
Minimally invasive approach
Subgroup analyses of patients undergoing minimally invasive esophagectomy showed that the risks of DGE and NEPI after intraoperative BT injection were 7.6% (95% CI: 2.3–12.9%) and 11.6% (95% CI: 1.9–21.3%), respectively (Fig. 6b). There was no difference in the risk of DGE between BT injection and no BT injection (OR: 0.44, 95% CI: 0.02–9.25, P = 0.59). Moreover, there was no difference in the risk of NEPI between BT injection and no BT injection (OR: 0.96, 95% CI: 0.11–8.66, P = 0.97) and between BT injection and pyloroplasty (OR: 1.15, 95% CI: 0.48–2.73, P = 0.76).
Early and late DGE
Subgroup analyses based on the studies that reported early and delayed DGE after intraoperative BT injection showed that the risk of early DGE was 19.3%% (95% CI: 8.4–30.1%) and the risk of late DGE was 9.9% (95% CI: 5.4–14.5%). There was no difference in the risk of early DGE (OR: 0.44, 95% CI: 0.10–1.96, P = 0.28) and late DGE (OR: 0.80, 95% CI: 0.15–4.31, P = 0.79) between BT injection and no BT injection. Moreover, there was no difference in the risk of early DGE (OR: 0.45, 95% CI: 0.18–1.10, P = 0.08) and late DGE (OR: 1.18, 95% CI: 0.40–3.47, P = 0.77) between BT injection and pyloroplasty.
Clinical and radiological diagnosis of DGE
Subgroup analyses based on the studies that diagnosed DGE clinically and radiologically showed that the risk of clinically diagnosed DGE was 9.7% (95% CI: 4.0–15.4%) and the risk of radiologically diagnosed DGE was 16.9% (95% CI: 8.4–25.5%), respectively. There was no difference in the risk of clinically diagnosed between BT injection and no BT injection DGE (OR: 0.94, 95% CI: 0.42–2.06, P = 0.87) and between BT injection and pyloroplasty (OR: 1.75, 95% CI: 0.55–5.56, P = 0.34). The risk of radiologically diagnosed DGE was lower in BT injection group compared with no BT injection (OR: 0.21, 95% CI: 0.09–0.44, P < 0.0001) and pyloroplasty (OR: 0.45, 95% CI: 0.21–0.97, P = 0.04).
Sensitivity analyses
Sensitivity analyses confirmed consistency of the results (Supplementary Fig. 2). Leave-one-out analysis showed that removal of one study at a time did not affect the pooled risk of risks of DGE and NEPI. Moreover, separate analysis of studies with low overall risk of bias did not affect the pooled risks of DGE and NEPI. Sensitivity analyses identified Cerfolio et al.22 as the main source of statistical heterogeneity, removal of which decreased the level of heterogeneity from moderate to low.
DISCUSSION
This systematic review and meta-analysis aimed to evaluate the effect of intraoperative BT injection on DGE and NEPI following esophagectomy. Analysis of 1070 patients from nine studies showed that the risks of DGE and NEPI after esophagectomy with intraoperative BT injection were 13.3% and 15.2%, respectively. Meta-regression suggested that male patients may be at a lower risk of DGE after intraoperative BT injection. Moreover, subgroup analyses suggested that minimally invasive approach may be associated with a lower risk of DGE and NEPI compared with open approach. Intraoperative BT injection did not reduce the risk of DGE and NEPI in comparison with no BT injection or pyloroplasty. Sensitivity analyses suggested consistency of the results. The quality and GRADE certainty of the available evidence were moderate.
Although the current study is the first meta-analysis that is specifically evaluating the outcomes of intraoperative BT injection in patients undergoing esophagectomy, the results can be compared with findings of other studies that evaluated other pyloric drainage techniques. The reported risks of DGE and NEPI found in this study in BT injection group, no BT injection group, and pyloroplasty group were consistent with risks reported in previous studies.2–8 The role of intraoperative pyloric drainage techniques in patients undergoing esophagectomy has been controversial. Loo et al.5 conducted a meta-analysis of nine studies which concluded that pyloric drainage in esophagectomy reduces the risk of DGE without affecting other postoperative outcomes.5 The pyloric intervention arm in the study by Loo et al.5 included pooled data from all techniques including pyloroplasty, pyloromyotomy, BT injection, and pyloric dilatation, hence the effect of individual drainage techniques could not be evaluated. In contrast with these findings, Arya et al.4 did not find any significant difference in the risk of DGE between pyloric drainage technique and no drainage technique. Arya et al.4 also pooled the data related to all drainage techniques together. In the absence of evident benefits associated with surgical pyloroplasty, Barone et al.8 argued that ‘the time of sunset for pyloroplasty has come’. The results of the current study suggested that intraoperative BT injection does not provide any benefits in terms of reducing the risks of DGE and NEPI after esophagectomy.
The results of the current study may have some implications. Considering that this study is the first study in literature reporting the pooled risks of DGE and NEPI after intraoperative BT injection, the provided figures can be used for hypothesis synthesis and power analysis in future prospective trials. Moreover, the estimated risks can be used as the best available evidence in communication with patients and in multidisciplinary settings when intraoperative BT injection is being considered as a treatment. Although the quality and certainty of available evidence is not high, it provides justification for not using intraoperative BT injection as a standard care in patients undergoing esophagectomy.
The risks factors for DGE after esophagectomy have been investigated by few authors. Benedix et al.23 identified female gender and anastomotic leak as risk factors for development of DGE after esophagectomy. In another study, Zhang et al.24 identified gastric size as the only predictor of DGE; use of whole stomach as an esophageal substitute was associated with a higher risk of DGE compared with a gastric tube.24 In the current study, male gender was associated with a lower risk of DGE consistent with the findings by Benedix et al.23 This may be explained by the fact that solid and liquid gastric emptying is slower in females, likely due to higher estrogen levels.25 Consistent with this, it has been shown that diabetes-related gastroparesis is more common in females than males.26 The age, dose of BT injection, and surgical technique did not affect the risk of DGE. However, the risks of DGE and NEPI seemed to be less in patients undergoing minimally invasive esophagectomy compared with those undergoing open esophagectomy. We are not able to fully explain this finding based on the available data as this is a relatively new finding and whether risk factors for development of DGE are easier to be avoided using minimally invasive techniques remains unknown. Interestingly, DGE and NEPI have not been the outcomes of interest in previous systematic reviews and meta-analyses comparing the outcomes of minimally invasive and open esophagectomies.27,28 Consequently, this association has not been established in the available literature yet and requires further research.
The included studies in this review did not provide adequate information about the outcomes of NEPI in patients with DGE, hence we could not assess whether NEPI could improve symptoms in patients who develop DGE after esophagectomy. However, successful management of DGE using endoscopic techniques have been reported by others.29,30
The available evidence is limited to results from retrospective studies with relatively small sample sizes with moderately heterogeneous baseline characteristics and outcome definitions and measurements. Although definite conclusions cannot be made, the results provided by the current study highlight areas for future research. Firstly, it highlights the need for studies with larger sample sizes and more robust statistical power; our results can be used for sample size calculation in future studies. Although we found no benefits in routine use intraoperative BT injection, future studies should investigate whether patients who are at higher risk of DGE such as female patients would benefit from routine pyloric drainage procedures. Moreover, the role of minimally invasive surgery on risk of DGE requires further evaluation as it has not been investigated adequately in the literature. Finally, although we found no association between dose of intraoperative BT injection and risk of DGE or NEPI, there have been advances in terms of techniques used for pyloric drainage and definition of DGE since the included studies in this review were completed, indicating the need for data from more recent studies.
The results of the current study should be interpreted taking into account the following strengths and limitations. The strengths of the current study include (i) use of objective and systematic approach in evidence synthesis compliant with PRISMA standards; (ii) evaluation of effect of different covariates on the results using meta-regression technique; (iii) conducting appropriate subgroup analyses; (iv) use of GRADE system to evaluate certainty of available evidence; and (v) consistency of results through sensitivity analyses. The limitations for the current study include (i) retrospective nature of most of the included studies and inevitable risk of selection bias; (ii) only nine studies were included, hence likelihood of type 2 error cannot be excluded; (iii) moderate statistical between-study heterogeneity; nevertheless, sensitivity analyses identified the study by Cerfolio et al.22 as the source of heterogeneity, removal of which did not change the overall conclusions; (iv) moderate methodological quality of the included studies; (v) inability to assess the risk of publication bias as the number of included studies was less than 10; (vi) the predefined protocol of the study was not registered in a publicly available registry; however, we declare that there were no deviations from the predefined protocol of the review; (vii) the definition of DGE was based on consistency (not necessarily identical) with criteria defined by Konradsson et al.10 in order to achieve consistency in reporting. However, the included studies were published before the study by Konradsson et al.10 and the definitions of DGE may have not been absolutely identical to criteria defined by Konradsson et al.10 In order to address potential inconsistencies, we conducted subgroup analyses based on clinical versus radiological diagnosis of DGE and based on early versus late DGE.
CONCLUSIONS
Level 2 evidence suggests that intraoperative BT injection may not improve the risk of DGE and NEPI in patients undergoing esophagectomy. The risk of DGE seems to be higher in females and in early postoperative period. High quality randomized controlled trials with robust statistical power are required for definite conclusions. The results of the current study can be used for hypothesis synthesis and power analysis in future prospective trials.
Compliance with ethical standards
Ethical approval
Considering the nature of this study, ethical approval was not required.
Human and animal rights
This study is a systematic review with meta-analysis of outcomes which does not include research directly involving human or animal participation.
Informed consent
Considering the nature of this study, informed consent was not required.
CRediT author statement
Shahab Hajibandeh (Conceptualization, Data curation, Formal analysis, Methodology, Project administration, Validation, Writing—original draft, Writing—review & editing), Shahin Hajibandeh (Data curation, Formal analysis, Methodology, Writing—original draft, Writing—review & editing), Matthew McKenna (Methodology, Validation, Visualization, Writing—review & editing), William Jones (Methodology, Validation, Visualization, Writing—original draft, Writing—review & editing), Paul Healy (Methodology, Validation, Visualization, Writing—review & editing), Jolene Witherspoon (Methodology, Supervision, Validation, Visualization, Writing—review & editing), Guy Blackshaw (Methodology, Supervision, Validation, Visualization, Writing—review & editing), Wyn Lewis (Methodology, Supervision, Validation, Visualization, Writing—review & editing), Antonio Foliaki (Methodology, Supervision, Validation, Visualization, Writing—review & editing), Tariq Abdelrahman (Conceptualization, Methodology, Supervision, Validation, Visualization, Writing—review & editing).
Data availability
The data and materials related to this study will be available upon reasonable request from the corresponding author.
Specific author contributions: Conception: Tarig Abdelrahman; Design: Shahab Hajibandeh; Data collection: Shahab Hajibandeh, Shahin Hajibandeh; Analysis and interpretation: Shahab Hajibandeh, Shahin Hajibandeh; Writing the article: All authors; Critical revision of the article: All authors; Final approval of the article: All authors.
Financial support: None.
Conflicts of interest: The authors declare that they have no conflict of interest.
References
| Search . | Search description . | Action . |
|---|---|---|
| Search #1 | MeSH term: [botulinum toxin] | Explode all trees |
| Search #2 | botulinum near 2 toxin | T, A, K |
| Search #3 | botox | T, A, K |
| Search #4 | #1 OR # 2 OR #3 | Combined with OR |
| Search #5 | MeSH term: [esophagectomy] | Explode all trees |
| Search #6 | esophagectom* | T, A, K |
| Search #7 | oesophagectom* | T, A, K |
| Search #8 | #5 OR #6 OR #7 | Combined with OR |
| Search #9 | #4 AND #8 | Combined with AND |
| Search . | Search description . | Action . |
|---|---|---|
| Search #1 | MeSH term: [botulinum toxin] | Explode all trees |
| Search #2 | botulinum near 2 toxin | T, A, K |
| Search #3 | botox | T, A, K |
| Search #4 | #1 OR # 2 OR #3 | Combined with OR |
| Search #5 | MeSH term: [esophagectomy] | Explode all trees |
| Search #6 | esophagectom* | T, A, K |
| Search #7 | oesophagectom* | T, A, K |
| Search #8 | #5 OR #6 OR #7 | Combined with OR |
| Search #9 | #4 AND #8 | Combined with AND |
T, A, K: titles, abstracts, keywords.
| Search . | Search description . | Action . |
|---|---|---|
| Search #1 | MeSH term: [botulinum toxin] | Explode all trees |
| Search #2 | botulinum near 2 toxin | T, A, K |
| Search #3 | botox | T, A, K |
| Search #4 | #1 OR # 2 OR #3 | Combined with OR |
| Search #5 | MeSH term: [esophagectomy] | Explode all trees |
| Search #6 | esophagectom* | T, A, K |
| Search #7 | oesophagectom* | T, A, K |
| Search #8 | #5 OR #6 OR #7 | Combined with OR |
| Search #9 | #4 AND #8 | Combined with AND |
| Search . | Search description . | Action . |
|---|---|---|
| Search #1 | MeSH term: [botulinum toxin] | Explode all trees |
| Search #2 | botulinum near 2 toxin | T, A, K |
| Search #3 | botox | T, A, K |
| Search #4 | #1 OR # 2 OR #3 | Combined with OR |
| Search #5 | MeSH term: [esophagectomy] | Explode all trees |
| Search #6 | esophagectom* | T, A, K |
| Search #7 | oesophagectom* | T, A, K |
| Search #8 | #5 OR #6 OR #7 | Combined with OR |
| Search #9 | #4 AND #8 | Combined with AND |
T, A, K: titles, abstracts, keywords.



