-
PDF
- Split View
-
Views
-
Cite
Cite
Akikazu Yago, Yu Ohkura, Masaki Ueno, Kentoku Fujisawa, Yusuke Ogawa, Hayato Shimoyama, Shusuke Haruta, Harushi Udagawa, Importance of long-term surveillance after curative esophagectomy for esophageal squamous cell carcinoma, Diseases of the Esophagus, Volume 35, Issue 10, October 2022, doab098, https://doi.org/10.1093/dote/doab098
Close - Share Icon Share
Summary
The long-term outcomes after esophagectomy for esophageal cancer remain uncertain and the optimal surveillance strategy after curative surgery remains controversial.
In this study, the clinicopathological characteristics of patients who underwent curative thoracic esophagectomy between 1991 and 2015 at Toranomon Hospital were retrospectively analyzed and reviewed until December 2020. We evaluated the accumulated data regarding the pattern and rates of recurrence and second malignancy.
A total of 1054 patients were eligible for inclusion in the study. Of these, 97% were followed up for 5 years, and the outcomes after 25 years could be determined in 65.5%. Recurrence was diagnosed in 318 patients (30.2%), and the most common pattern was lymph node metastasis (n = 168, 52.8%). Recurrence was diagnosed within 1 year in 174 patients (54.7%) and within 3 years in 289 (90.9%). Second malignancy possibly occurred through the entire study period after esophagectomy even in early-stage cancer, keeping 2%–5% of the incidental risk. There was no significant difference in the prognosis between 3-year survivors with and without a second malignancy.
Most recurrences after resection of esophageal cancer occurred within 3 years regardless of disease stage. However, these patients have an ongoing risk of developing a second malignancy after esophagectomy. Further consideration is required regarding the efficacy of long-term surveillance.
INTRODUCTION
Esophageal cancer has the sixth highest mortality rate of all cancers worldwide.1 Although esophagectomy is the standard treatment for esophageal cancer, recurrence after curative resection is one of the most important determinants of survival.2 International guidelines recommend postoperative surveillance for most cancers, despite the lack of evidence for a survival benefit. The National Comprehensive Cancer Network guidelines3 mention that all patients should be followed up systematically, despite a lack of high-level evidence to guide development of algorithms that balance benefits and risks. Furthermore, the European Society for Medical Oncology guidelines4 state that follow-up visits should concentrate on symptoms, nutrition, and psychosocial support. According to the Japanese guidelines for treatment of esophageal cancer,5 surgeons in Japan set intervals and durations of surveillance based on their preference and institutional practice guidelines. Therefore, it is important to discuss the criteria for setting the surveillance protocol, and events that occur during the surveillance period could be helpful in this regard.
Previous reports indicate that most recurrences occur within the first few years after esophageal resection.6,7 Doctors are faced with the problem that patients with a recurrence of esophageal cancer usually cannot be cured, so it is uncertain whether aggressive follow-up significantly prolongs survival or quality of life in a palliative care setting. Furthermore, we sometimes encounter patients with recurrence beyond 5 years post-esophagectomy or remnant gastrointestinal cancer even after a long disease-free interval. The optimal follow-up interval for detection and treatment of early cancer is also unclear. Therefore, it is important to recognize the pattern and timing of onset of these recurrences and new lesions after curative esophagectomy.
Our department has maintained a robust database that includes data on many patients who have been followed up long-term over a period of 30 years. In this study, we analyzed the data we have accumulated on patients who have undergone curative surgery for esophageal cancer and been followed up during thistime.
MATERIALS AND METHODS
Population
We analyzed the clinicopathological characteristics of all patients who underwent esophagectomy between 1991 and 2015 in the Department of Gastroenterological Surgery at Toranomon Hospital, Tokyo, Japan, and reviewed them until December 2020. All patients had met the criteria for histologically proven esophageal squamous cell carcinoma and underwent standard thoracic esophagectomy with macroscopically curative resection. Staging and histopathological grading were categorized based on the Japanese Classification of Esophageal Cancer, 11th Edition.8 The patients were classified as stage 0, I, II, III, or IV (curative). Information was collected for age, sex, location, histological type, preoperative chemotherapy, method of reconstruction, postoperative complications, depth of invasion, lymph node metastasis, and distant metastasis.
Treatment
The treatment plan was selected according to the practice guidelines for esophageal cancer in place at the time for each case.5,9 The Japanese guidelines focus on the evidence for squamous cell carcinoma of the esophagus, which is often observed in East Asia, including Japan. Patients with clinical stage 0 or I disease and those in whom endoscopic resection was contraindicated underwent straightforward esophagectomy. Patients with clinical stage II or III disease who were able to tolerate surgery underwent esophagectomy combined with perioperative chemotherapy, the strategy for which changed from adjuvant to neoadjuvant after the results of a randomized trial in Japan were published.10 Patients with clinical stage IV disease received induction chemotherapy or chemoradiotherapy first, and those who were deemed to be potentially curable by resection underwent esophagectomy.
Surgical procedure
All patients with potentially curable disease underwent curative transthoracic esophagectomy for esophageal cancer. The fields of lymph node dissection, thoracic surgical approach, and/or thoracic duct resection were selected according to tumor staging, surgical risk, and tumor location. The organ (stomach, ileocolon, or jejunum) and route of reconstruction (posterior mediastinal or retrosternal) were individualized according to the patient’s background factors.
Methods of surveillance
The study period was from January 1991 to December 2020. The duration of follow-up was defined as the interval between surgery and the last date the patient was confirmed to have been alive. In general, we aimed to follow up patients for at least 5 years and continued follow-up indefinitely for early detection if requested to do so by the patient. Follow-up evaluation included findings on clinical examination, results of laboratory investigations, and findings on imaging studies and endoscopy. All patients underwent laboratory investigations, which included tumor markers (e.g. squamous cell carcinoma antigen and cytokeratin 19 fragment), and a nutritional assessment at 3-month or 6-month intervals, the results of which were treated as an indicator of whether additional examinations were required. Computed tomography (CT) was performed at 6-month intervals with screening endoscopy annually. Bone scintigraphy, brain CT, and positron emission tomography (PET)-CT examinations were performed in patients who developed clinical symptoms that raised suspicion for the disease recurrence.
Recurrence was defined only by a reappearance of the primary esophageal cancer, which was diagnosed histologically, cytologically, and radiologically. If it could not be detected by these modalities, it was not regarded as recurrence and normal surveillance examinations continued. Recurrence was diagnosed only when an obviously abnormal lesion was confirmed. We usually performed the chemotherapy for hematogenous recurrence, whereas the resection or chemoradiotherapy for others. Second malignant disease in the remnant gastrointestinal organs, including the esophagus, stomach, and pharyngo-larynx, was not classified as recurrence and was treated as a new lesion. When detected, appropriate endoscopic or surgical treatment was provided.
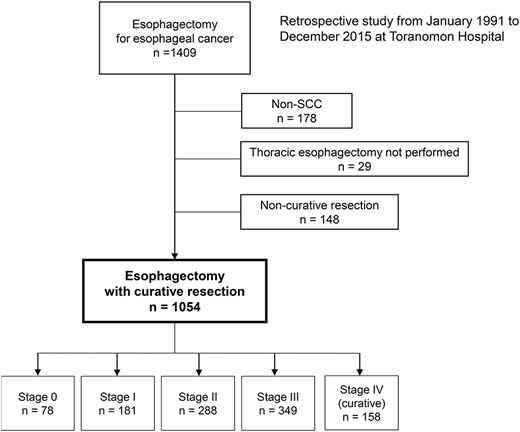
A total, of 1054 patients underwent curative esophagectomy for esophageal cancer between 1991 and 2015. The number in each stage is shown. (SCC, squamous cell carcinoma).
Evaluations and statistical analysis
The primary endpoint of the study was survival, including recurrence-free survival, disease-free survival, and overall survival times. We constructed Kaplan–Meier survival curves for each pathological stage of esophageal cancer, the annual risk of recurrence by stage, and the incidence of second malignancy excluding recurrence of primary esophageal cancer. The censored follow-up rate was defined as the percentage of censored patients excluding death up until the specified time and calculated with the potential number of patients eligible for surveillance as the denominator. The risk of recurrence in each postoperative year was calculated based on the number of patients who were recurrence-free at the start of each interval. The incidence of second malignancy was calculated based on the number of patients who were disease-free at the start of each interval. The Clopper–Pearson method was used to assess the 95% confidence intervals (CIs). A P-value of <0.05 was considered statistically significant. All statistical analyses were performed using IBM SPSS Statistics ver. 23 (IBM Corp., Armonk,NY).
RESULTS
Patient characteristics
Figure 1 shows a flow diagram of patient enrollment in the study. In total, 1409 patients were diagnosed with esophageal cancer and underwent esophagectomy. After exclusion of patients with non-squamous cell carcinoma (n = 178), those who did not undergo standard thoracic esophagectomy (n = 29) and those in whom resection was non-curative (n = 148), 1054 patients were eligible for inclusion in the study.
Table 1 shows the demographic and clinicopathological characteristics of the 1054 patients who underwent curative esophagectomy. The median age was 62 (range, 37–88) years and 88.2% of the patients were male. The pathology was undifferentiated type in 17.0% of patients, and 22.7% received preoperative induction therapy. Severe postoperative complications (Clavien-Dindo grade ≥ IIIa) occurred in 21.5% of patients. Based on the Japanese Classification of Esophageal Cancer, 78 patients (7.4%) had stage 0 disease, 181 (17.2%) had stage I, 288 (27.3%) had stage II, 360 (34.2%) had stage III, and 147 (13.9%) had stage IV (curative).
Clinicopathological characteristics of patients who underwent curative esophagectomy
| Variable . | . | n . | % . |
|---|---|---|---|
| Median age, years (range) | 63 (37–88) | ||
| Sex | Male Female | 930 124 | 88.2 11.8 |
| Location | Ut Mt Lt Ae | 155 575 297 27 | 14.7 54.6 28.2 2.6 |
| Histological type | Differentiated Undifferentiated | 875 179 | 83.0 17.0 |
| Preoperative induction therapy | No Yes | 816 238 | 77.4 22.6 |
| Lymph node dissection | 2-field 3-field Other | 129 859 66 | 12.2 81.5 6.3 |
| Method of reconstruction | Stomach Ileocolic Jejunum | 877 173 4 | 83.2 16.4 0.4 |
| Route of reconstruction | Posterior mediastinal Retrosternal Other | 330 717 7 | 31.3 68.0 0.7 |
| Thoracic duct resection | No Yes | 330 724 | 31.3 68.7 |
| Postoperative complications | No Yes | 700 354 | 66.4 33.6 |
| Severe complications (CD grade > IIIa) | No Yes | 827 227 | 78.5 21.5 |
| Depth of invasion | T0 T1a T1b T2 T3 T4 | 16 115 331 152 410 30 | 1.5 10.9 31.4 14.4 38.9 2.8 |
| Lymph node metastasis | N0 N1 N2 N3 N4 | 456 143 221 120 114 | 43.3 13.6 21.0 11.4 10.8 |
| Distant metastasis | M0 M1 | 1025 29 | 97.2 2.8 |
| Stage | 0 I II III IV | 78 181 288 349 158 | 7.4 17.2 27.3 33.1 15.0 |
| Variable . | . | n . | % . |
|---|---|---|---|
| Median age, years (range) | 63 (37–88) | ||
| Sex | Male Female | 930 124 | 88.2 11.8 |
| Location | Ut Mt Lt Ae | 155 575 297 27 | 14.7 54.6 28.2 2.6 |
| Histological type | Differentiated Undifferentiated | 875 179 | 83.0 17.0 |
| Preoperative induction therapy | No Yes | 816 238 | 77.4 22.6 |
| Lymph node dissection | 2-field 3-field Other | 129 859 66 | 12.2 81.5 6.3 |
| Method of reconstruction | Stomach Ileocolic Jejunum | 877 173 4 | 83.2 16.4 0.4 |
| Route of reconstruction | Posterior mediastinal Retrosternal Other | 330 717 7 | 31.3 68.0 0.7 |
| Thoracic duct resection | No Yes | 330 724 | 31.3 68.7 |
| Postoperative complications | No Yes | 700 354 | 66.4 33.6 |
| Severe complications (CD grade > IIIa) | No Yes | 827 227 | 78.5 21.5 |
| Depth of invasion | T0 T1a T1b T2 T3 T4 | 16 115 331 152 410 30 | 1.5 10.9 31.4 14.4 38.9 2.8 |
| Lymph node metastasis | N0 N1 N2 N3 N4 | 456 143 221 120 114 | 43.3 13.6 21.0 11.4 10.8 |
| Distant metastasis | M0 M1 | 1025 29 | 97.2 2.8 |
| Stage | 0 I II III IV | 78 181 288 349 158 | 7.4 17.2 27.3 33.1 15.0 |
Abbreviations: Ae, abdominal esophagus; CD, Clavien-Dindo; Lt, lower thoracic esophagus; Mt, middle thoracic esophagus; Ut, upper thoracic esophagus.
Clinicopathological characteristics of patients who underwent curative esophagectomy
| Variable . | . | n . | % . |
|---|---|---|---|
| Median age, years (range) | 63 (37–88) | ||
| Sex | Male Female | 930 124 | 88.2 11.8 |
| Location | Ut Mt Lt Ae | 155 575 297 27 | 14.7 54.6 28.2 2.6 |
| Histological type | Differentiated Undifferentiated | 875 179 | 83.0 17.0 |
| Preoperative induction therapy | No Yes | 816 238 | 77.4 22.6 |
| Lymph node dissection | 2-field 3-field Other | 129 859 66 | 12.2 81.5 6.3 |
| Method of reconstruction | Stomach Ileocolic Jejunum | 877 173 4 | 83.2 16.4 0.4 |
| Route of reconstruction | Posterior mediastinal Retrosternal Other | 330 717 7 | 31.3 68.0 0.7 |
| Thoracic duct resection | No Yes | 330 724 | 31.3 68.7 |
| Postoperative complications | No Yes | 700 354 | 66.4 33.6 |
| Severe complications (CD grade > IIIa) | No Yes | 827 227 | 78.5 21.5 |
| Depth of invasion | T0 T1a T1b T2 T3 T4 | 16 115 331 152 410 30 | 1.5 10.9 31.4 14.4 38.9 2.8 |
| Lymph node metastasis | N0 N1 N2 N3 N4 | 456 143 221 120 114 | 43.3 13.6 21.0 11.4 10.8 |
| Distant metastasis | M0 M1 | 1025 29 | 97.2 2.8 |
| Stage | 0 I II III IV | 78 181 288 349 158 | 7.4 17.2 27.3 33.1 15.0 |
| Variable . | . | n . | % . |
|---|---|---|---|
| Median age, years (range) | 63 (37–88) | ||
| Sex | Male Female | 930 124 | 88.2 11.8 |
| Location | Ut Mt Lt Ae | 155 575 297 27 | 14.7 54.6 28.2 2.6 |
| Histological type | Differentiated Undifferentiated | 875 179 | 83.0 17.0 |
| Preoperative induction therapy | No Yes | 816 238 | 77.4 22.6 |
| Lymph node dissection | 2-field 3-field Other | 129 859 66 | 12.2 81.5 6.3 |
| Method of reconstruction | Stomach Ileocolic Jejunum | 877 173 4 | 83.2 16.4 0.4 |
| Route of reconstruction | Posterior mediastinal Retrosternal Other | 330 717 7 | 31.3 68.0 0.7 |
| Thoracic duct resection | No Yes | 330 724 | 31.3 68.7 |
| Postoperative complications | No Yes | 700 354 | 66.4 33.6 |
| Severe complications (CD grade > IIIa) | No Yes | 827 227 | 78.5 21.5 |
| Depth of invasion | T0 T1a T1b T2 T3 T4 | 16 115 331 152 410 30 | 1.5 10.9 31.4 14.4 38.9 2.8 |
| Lymph node metastasis | N0 N1 N2 N3 N4 | 456 143 221 120 114 | 43.3 13.6 21.0 11.4 10.8 |
| Distant metastasis | M0 M1 | 1025 29 | 97.2 2.8 |
| Stage | 0 I II III IV | 78 181 288 349 158 | 7.4 17.2 27.3 33.1 15.0 |
Abbreviations: Ae, abdominal esophagus; CD, Clavien-Dindo; Lt, lower thoracic esophagus; Mt, middle thoracic esophagus; Ut, upper thoracic esophagus.
Survival curves
Each survival curve based on 15 years of follow-up is shown in Figure 2. Recurrence-free survival curves according to each primary esophageal cancer stage are shown in Figure 2a, which indicates that in almost all cases, recurrence occurred within 3 years of surgery for the primary esophageal cancer regardless of disease stage, with very few cases of recurrence 5 years after surgery. Long-term survival was documented in a few patients after recurrence, reflecting interindividual variations in response to treatment. Figure 2b shows the disease-free survival curves according to stage and also reflects the incidence of other malignant diseases. This result suggests that even patients with early-stage primary esophageal cancer may have developed some type of second malignancy during long-term surveillance. Figure 2c shows the overall survival curves, which indicate a 5-year survival rate of 90.9% for stage 0 disease, 81.5% for stage I, 75.2% for stage II, 50.2% for stage III, and 35.6% for stage IV (curative) and that a proportion of patients died from other disease during our surveillance period.
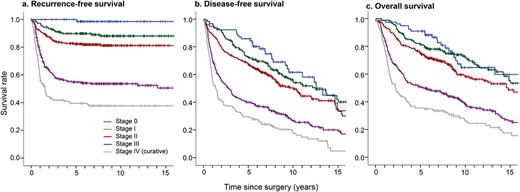
Kaplan–Meier (a) recurrence-free, (b) disease-free, and (c) overall survival curves according to disease stage.
Follow-up rates
The distribution of follow-up rates is shown in Figure 3. ‘Censored’ means the pure number of patients who were still alive at the end of follow-up, excluding the number of patients who died and including those who were lost to follow-up (i.e. those who stopped attending follow-up visits without notice) or stopped surveillance due to completion of an adequate follow-up period (including patients who went to other hospitals for further follow-up). The outcomes were confirmed in almost all patients (97.1%) with resected esophageal cancer who attended for surveillance for 5 years. In total, 61.2% of patients were followed up after their initial surgery for 5 years, 29.7% for 10 years, and 2.34% for 25 years. The already censored follow-up rate was 22.4% at 10 years, 25.7% at 15 years, 29.1% at 20 years, and 34.5% at 25 years. These figures also indicate that many patients died over time, although the cause of death was not always known.
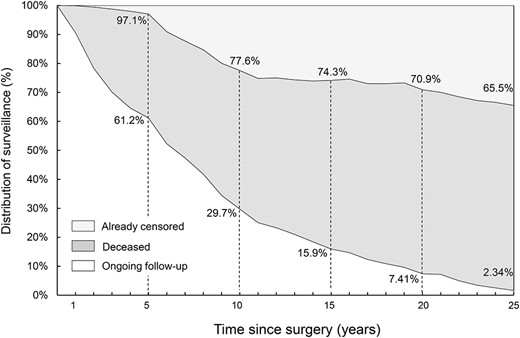
Distribution of follow-up during long-term surveillance. Almost all patients received surveillance for at least 5 years. The rate of censored follow-up increased gradually to ~30%.
Pattern of recurrence
The pattern and rate of recurrence in each postoperative period is shown in Table 2. Lymph node metastasis was the most common recurrence pattern in all study periods and occurred at a rate of ~50%. The trends for site of recurrence were similar in each period, and pulmonary metastasis was second highest with changes between each period. Of the 318 patients with recurrence, the diagnosis was made within 1 year of the primary surgery in 174 patients (54.7%), within 2 years in 256 (80.5%), and within 3 years in 289 (90.9%).
Pattern and rate of main types of recurrence during each postoperative period
| Recurrence . | Recurrences (n = 318) . | |||
|---|---|---|---|---|
| Within 1 year n = 174 . | 2–3 years n = 115 . | 4–5 years n = 17 . | Over 5 years n = 12 . | |
| Lymph node metastasis | 52.9% | 53.0% | 52.9% | 50.0% |
| Pulmonary metastasis | 10.9% | 22.6% | 35.3% | 8.3% |
| Hepatic metastasis | 10.3% | 7.0% | — | 8.3% |
| Bone metastasis | 9.2% | 6.1% | — | 8.3% |
| Other hematogenous metastasis | 8.1% | 2.6% | 5.9% | — |
| Local recurrence | 3.4% | 3.5% | — | 25.0% |
| Dissemination | 2.3% | 4.3% | — | — |
| Unknown | 2.9% | 1.7% | 5.9% | — |
| Recurrence . | Recurrences (n = 318) . | |||
|---|---|---|---|---|
| Within 1 year n = 174 . | 2–3 years n = 115 . | 4–5 years n = 17 . | Over 5 years n = 12 . | |
| Lymph node metastasis | 52.9% | 53.0% | 52.9% | 50.0% |
| Pulmonary metastasis | 10.9% | 22.6% | 35.3% | 8.3% |
| Hepatic metastasis | 10.3% | 7.0% | — | 8.3% |
| Bone metastasis | 9.2% | 6.1% | — | 8.3% |
| Other hematogenous metastasis | 8.1% | 2.6% | 5.9% | — |
| Local recurrence | 3.4% | 3.5% | — | 25.0% |
| Dissemination | 2.3% | 4.3% | — | — |
| Unknown | 2.9% | 1.7% | 5.9% | — |
Pattern and rate of main types of recurrence during each postoperative period
| Recurrence . | Recurrences (n = 318) . | |||
|---|---|---|---|---|
| Within 1 year n = 174 . | 2–3 years n = 115 . | 4–5 years n = 17 . | Over 5 years n = 12 . | |
| Lymph node metastasis | 52.9% | 53.0% | 52.9% | 50.0% |
| Pulmonary metastasis | 10.9% | 22.6% | 35.3% | 8.3% |
| Hepatic metastasis | 10.3% | 7.0% | — | 8.3% |
| Bone metastasis | 9.2% | 6.1% | — | 8.3% |
| Other hematogenous metastasis | 8.1% | 2.6% | 5.9% | — |
| Local recurrence | 3.4% | 3.5% | — | 25.0% |
| Dissemination | 2.3% | 4.3% | — | — |
| Unknown | 2.9% | 1.7% | 5.9% | — |
| Recurrence . | Recurrences (n = 318) . | |||
|---|---|---|---|---|
| Within 1 year n = 174 . | 2–3 years n = 115 . | 4–5 years n = 17 . | Over 5 years n = 12 . | |
| Lymph node metastasis | 52.9% | 53.0% | 52.9% | 50.0% |
| Pulmonary metastasis | 10.9% | 22.6% | 35.3% | 8.3% |
| Hepatic metastasis | 10.3% | 7.0% | — | 8.3% |
| Bone metastasis | 9.2% | 6.1% | — | 8.3% |
| Other hematogenous metastasis | 8.1% | 2.6% | 5.9% | — |
| Local recurrence | 3.4% | 3.5% | — | 25.0% |
| Dissemination | 2.3% | 4.3% | — | — |
| Unknown | 2.9% | 1.7% | 5.9% | — |
Risk of recurrence
Figure 4 shows the change in risk of recurrence in each yearly interval over 10 years of surveillance. Recurrence of stage 0 esophageal cancer was rare and the recurrence rate for early stage I disease was relatively low throughout the entire study period. Although advanced esophageal cancer had relatively high rates of recurrence in the early years of the study, almost all recurrences were found within 3 years and recurrences beyond 5 years wererare.
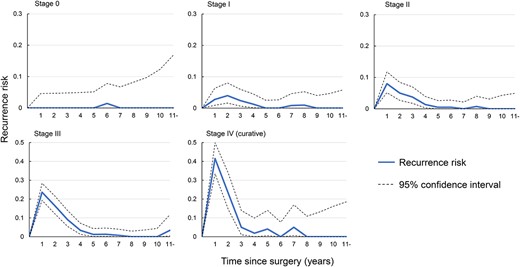
Risk of recurrence during each 1-year interval in patients who were recurrence-free at the start of each interval.
Second malignancy
Table 3 shows the sites and incidence of second malignancies. Upper gastrointestinal cancers, including remnant esophageal, gastric, and pharyngo-larynx cancer, were the most common, occurring at a rate of over 60%.
| Site . | Incidental cases (n = 179/1054) . | |
|---|---|---|
| n . | % . | |
| Stomach | 33 | 18.4 |
| Remnant esophagus | 13 | 7.3 |
| Pharyngo-larynx | 67 | 37.4 |
| Colon/Rectum | 5 | 2.8 |
| Lung | 18 | 10.1 |
| Urinary | 16 | 8.9 |
| Other | 27 | 15.1 |
| Site . | Incidental cases (n = 179/1054) . | |
|---|---|---|
| n . | % . | |
| Stomach | 33 | 18.4 |
| Remnant esophagus | 13 | 7.3 |
| Pharyngo-larynx | 67 | 37.4 |
| Colon/Rectum | 5 | 2.8 |
| Lung | 18 | 10.1 |
| Urinary | 16 | 8.9 |
| Other | 27 | 15.1 |
| Site . | Incidental cases (n = 179/1054) . | |
|---|---|---|
| n . | % . | |
| Stomach | 33 | 18.4 |
| Remnant esophagus | 13 | 7.3 |
| Pharyngo-larynx | 67 | 37.4 |
| Colon/Rectum | 5 | 2.8 |
| Lung | 18 | 10.1 |
| Urinary | 16 | 8.9 |
| Other | 27 | 15.1 |
| Site . | Incidental cases (n = 179/1054) . | |
|---|---|---|
| n . | % . | |
| Stomach | 33 | 18.4 |
| Remnant esophagus | 13 | 7.3 |
| Pharyngo-larynx | 67 | 37.4 |
| Colon/Rectum | 5 | 2.8 |
| Lung | 18 | 10.1 |
| Urinary | 16 | 8.9 |
| Other | 27 | 15.1 |
Changes in the incidence of malignant disease other than recurrence of the primary in all stages are shown in Figure 5. Although keeping <5% of the incidental risk, the risk of malignant disease always remained above 2% throughout the observation period, even in patients with early-stage esophageal cancer. Figure 6 shows the survival curves for 3-year survivors without recurrence according to whether a second malignancy occurred, assuming that most recurrences of primary esophageal cancer would occur within the initial 3 years. There was no significant difference in the prognosis between these two groups, which indicates non-inferiority, although there was a slight tendency for the prognosis to be poorer in patients with a second cancer (P = 0.060).
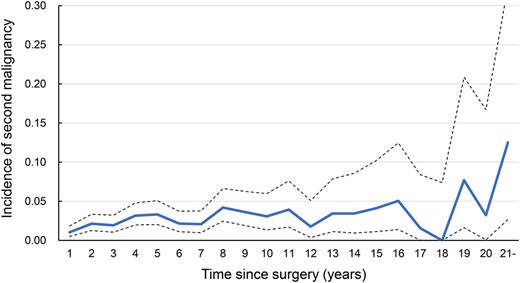
Incidence of other malignant disease during each 1-year interval in patients who were disease-free at the start of each interval.
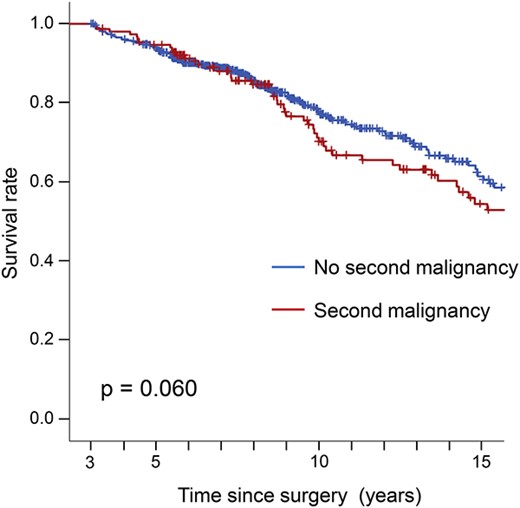
Overall survival curves according to whether or not there was a second malignancy in 3-year survivors without recurrence.
DISCUSSION
This report provides important information on long-term surveillance after resection of esophageal cancer for detection of not only recurrence of the primary cancer but also other malignant disease, mainly in the remnant upper gastrointestinal tract. Considering the mortality rate of esophageal cancer, we believe that the database maintained by our department is of considerable value.
Our clinical surveillance practice is based on the Japanese treatment guidelines for esophageal cancer.5 Both recurrence of esophageal cancer and onset of other malignant disease have the potential to be fatal and are a critical problem after curative esophagectomy. Surveillance is important because it affords an opportunity for treatment of recurrent disease or any new malignancy in the asymptomatic stage before performance status deteriorates. Therefore, we believe that annual endoscopy would be an effective screening tool for recurrence at the site of anastomosis and for detecting new lesions in the remnant aero-digestive system. However, given that the evidence may not be robust overall, controversy persists regarding the appropriate surveillance strategy in terms of how often recurrences and/or other malignant diseases occur and how long patients should be kept under surveillance. It is also unclear whether any specific follow-up strategy can provide a survival benefit.
Our study has yielded important data about the pattern and rate of recurrences of primary esophageal cancer after esophagectomy and is based on a large cohort of patients. More than half of the recurrences occurred within 1 year after surgery, and almost all occurred within 3 years. These findings are consistent with previous reports showing that 57.8%–71.0% of recurrences were found within a year and 84.0%–88.9% within 2 years.2,11,12 Furthermore, the sites and timing of recurrences in this study are similar to those in previous reports. Several retrospective studies have shown that surgical resection is beneficial in terms of reducing the risk of recurrence of esophageal cancer, in particular lymph node and pulmonary metastasis.12–14 Salvage surgery for resectable metastatic lesions is also a meaningful treatment tool.15,16 Therefore, early detection of recurrence at these sites is important because it can be treated. Previous reports suggest that PET-CT and CT have different roles in detection of esophageal carcinoma and that PET-CT is more useful for detection of recurrence.17,18 Therefore, use of these modalities could be effective for early detection during surveillance.
A second primary cancer occurred in 17.0% of patients in this study, which highlights the ongoing risk of malignancy after surgery in these patients. A second primary malignancy influences the prognosis, particularly in patients with early-stage esophageal cancer.19,20 However, it is difficult to determine the exact increase in risk of a second malignancy after esophagectomy given the paucity of relevant reports. Matsubara et al.19 found that the risk of a second malignancy was three times higher in patients who developed esophageal cancer than in the general population. Furthermore, Usami et al.21 reported that early detection of a second primary malignancy in the residual cervical esophagus after thoracic esophagectomy was the most important treatment factor. A systematic review also recommended long-term yearly endoscopy for all patients who undergo esophagectomy for cancer because early detection of cancer in the gastric conduit is paramount for a good clinical outcome.22,23 The risk of squamous cell carcinoma of the head, neck, and esophagus is strongly affected by lifestyle factors, in particular consumption of alcohol and cigarette smoking,24,25 both of which increase the risk of a second primary cancer in the upper aero-digestive organs.26,27 Therefore, the importance of improving lifestyle and long-term surveillance should be emphasized.
Many of the malignant diseases that occurred during our study period were detected by surveillance endoscopy supported by additional investigations. At our hospital, the pharynx is routinely observed to ensure both sides of the piriformis are visible,28 which is helpful in detecting pharyngeal cancer at a stage when it is treatable by endoscopic submucosal dissection. We expect that improvement in early detection of other cancers may also have contributed to the survival benefit after esophagectomy, as shown in our previous reports.29,30 Our results indicate that the appearance of a second malignancy does not necessarily herald a poor prognosis, suggesting that our strategy of long-term surveillance can provide the opportunity for accurate detection and treatment of a second aero-digestive cancer. The value of this study is that we could demonstrate the incidence of these second cancers, which underscores the significance of a long follow-up program after esophagectomy for esophageal cancer.
A strength of this study is that it was conducted in a hospital department where esophagectomy has been performed in a consistent manner over many years. Moreover, more patients with esophageal cancer are treated in our department than at other hospitals in the region and are managed with a consistent follow-up program over a long period after surgery. Therefore, our database can be considered robust because of the data it contains on a large number of patients with long-term follow-up for at least 5 years. Moreover, this database allows relatively accurate identification of the time and location of recurrence of esophageal cancer and second malignany.
This study also has some limitations, despite including a large cohort of patients. First, it had a retrospective single-center design, and the findings may be different in a prospective multicenter study. This shortcoming might be offset by the fact that most of our patients return to our department if they develop any further clinical symptoms. However, some of these patients may have been treated at another hospital after the end of surveillance, which could have introduced a degree of bias in our calculations. Second, the extent to which some of the parameters analyzed in this study might have affected onset of esophageal cancer is unclear in that we included not only treatment-related factors, such as advances in surgical methods and in perioperative chemotherapy, but also patient-related factors, such as smoking and alcohol consumption. Analysis of other prognostic factors might yield different results. Third, the study focused solely on malignant disease, so we could not assess quality of life after esophagectomy. Postoperative surveillance should include an assessment of quality of life and symptoms, including reflux, stenosis, and dietary intake. We anticipate that further studies will help to identify better surveillance strategies to improve survival with an acceptable quality of life in the patients.
In conclusion, most recurrences after resection of esophageal cancer occurred within the first 3 years after surgery, regardless of disease stage. However, these patients have an ongoing risk of developing other malignant disease in the remnant gastrointestinal site. Although further research will be required to determine the impact of long-term surveillance on survival, the results of this study could help to determine the optimal postoperative surveillance strategy for patients who undergo surgery for esophageal cancer.
Ethical approval: The study was approved by the research ethics committee of Toranomon Hospital (no. 2230). All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and its later versions.
Financial disclosure: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors. The authors declare that they have no conflict of interest.



