-
PDF
- Split View
-
Views
-
Cite
Cite
Breno Mellado, Lucas de O Carneiro, Marcelo R Nogueira, L Gerardo Herrera M, Ariovaldo P Cruz-Neto, Leandro R Monteiro, Developmental instability, body mass, and reproduction predict immunological response in short-tailed bats, Current Zoology, Volume 71, Issue 2, April 2025, Pages 162–169, https://doi.org/10.1093/cz/zoae034
Close - Share Icon Share
Abstract
Developmental instability (DI) is a phenomenon whereby organisms are unable to buffer developmental disturbances, resulting in asymmetric variation of paired traits. Previous research has demonstrated a negative relationship between DI, measured as forearm asymmetry, and survival in the bat Carollia perspicillata. This study aims to test the hypothesis that individuals with higher DI exhibit a lower immune response. We measured a delayed-type hypersensitivity to the antigen phytohemagglutinin (PHA) on 74 males and 65 females of C. perspicillata before and after the breeding season (BS). Linear models were used to predict the immunological response based on body mass (BM), forearm asymmetry, sex, BS, and testicle length. The best-fitting model accounted for 29% of the variation in immune response and included asymmetry, BM, sex, and BS as predictors. The immune response was negatively associated with asymmetry and testicle length in males but positively related to asymmetry in females. Both sexes showed a reduced immune response in the late BS. Additionally, the association between immune response and BM changed direction seasonally, with heavier individuals showing weaker responses early in the BS and stronger responses later. Individual variation in male immunity was predicted by individual attributes, whereas variation in immune response in females was mostly seasonal. Our results support the link between DI, survival, and immune response in short-tailed bats, and suggest that the immunological component measured by the PHA response may be under finer selection in males due to its stronger correlation with individual traits.
The immune system plays a crucial role in protecting organisms from various pathogens, including viruses, bacteria, and other parasites, and its effectiveness has a significant impact on an organism’s fitness (Møller and Saino 2004; Graham et al. 2010; Seppälä 2015). Activating the immune system, however, is energetically and nutritionally costly, making it difficult to sustain immunocompetence along with other energy-demanding physiological processes when resources are limited (McKean and Lazzaro 2011; Demas et al. 2012). As a result, trade-offs have been observed between immune response and life-history strategies (Lochmiller and Deerenberg 2000; Zuk and Stoehr 2002; Lee 2006).
Sexual differences in reproductive investment, as well as physiological changes during reproduction, such as increases in steroid hormone concentration, can impact the immunocompetence of individuals (Markle and Fish 2014; Foo et al. 2017; Taneja 2018) and generate sex-specific trade-offs. The immunocompetence handicap hypothesis (Folstad and Karter 1992) suggests that male secondary sexual traits are maintained by high testosterone levels at the expense of resistance to infection and parasites (Foo et al. 2017). Females usually invest more in reproduction than males, and their trade-offs are probably mediated by different physiological mechanisms (and the environment), dependent on the component of the immunological response (Klein and Flanagan 2016; Kelly et al. 2018).
The prediction of fitness variables by measures of immunological response has suggested their use as proxies of individual “quality” (Møller and Saino 2004), but the associations can vary in direction and intensity depending on the fitness and immunological components involved (Mills et al. 2010; Demas et al. 2012). Developmental instability (DI), measured as deviations from symmetry in bilateral organisms, has also been proposed as a fitness proxy (Van Dongen 2006). Higher levels of DI might be linked to a reduced ability to buffer disturbances during development and maintain critical bodily functions (Leamy and Klingenberg 2005; Cánovas et al. 2015), and a mechanism connecting DI and immunological responses has been proposed via developmental pathways, where the same set of genes are responsible for the control of organ growth, symmetry, homeostasis, and immune defense (Boone et al. 2016; Juarez-Carreño et al. 2018; Ma et al. 2019). The correlation between individual asymmetry and immune response can, therefore, arise because genotypes that are less capable of buffering environmental disturbances also develop weaker immune systems (Rantala et al. 2004; Martín and López 2006; Stephenson et al. 2020). A second possibility is that individuals with weaker immune responses are more susceptible to parasites, which can be environmental stressors and lead to increased asymmetry (Lagesen and Folstad 1998; Whitaker and Fair 2002; Bize et al. 2004).
The Seba’s short-tailed fruit bat, Carollia perspicillata, is a model species for population ecology and evolution studies due to its high dominance and local abundance (Willig et al. 2007; Mellado et al. 2018), high roost fidelity and recapture rates (Fleming 1988; Monteiro et al. 2019; Mellado et al. 2022), and ease of captive maintenance (Rasweiler et al. 2009). A long-term population study of C. perspicillata revealed that more asymmetric bats presented lower survival probability and reproductive potential (Monteiro et al. 2019). The authors propose that the hypothesis of a systemic effect of DI reducing survival is more plausible than the idea of a direct effect of forearm asymmetry on flight aerodynamics. The observed magnitude of asymmetry would be insufficient to cause significant changes in flight manoeuverability and energy cost (Thomas 1993; Voigt 2013). Therefore, it is crucial to investigate the relationship between asymmetry and other performance measures, such as immunocompetence, to identify the possible underlying mechanisms for the systemic effect of DI on individual survival.
In this study, we examine the hypothesis that the intensity of the immune response mediates the negative association between forearm asymmetry and survival in C. perspicillata. This hypothesis assumes that the occurrence of DI correlates with reduced homeostatic mechanisms, impaired regulation of organ size, and reduced immune competence due to common developmental pathways (Juarez-Carreño et al. 2018). Furthermore, based on the assumption that there are trade-offs between reproduction and the immune response in both males and females (McKean and Lazzaro 2011), as well as seasonal effects (Martin et al. 2008), we attempt to elucidate the relationships considering the joint and partial effects of individual asymmetry, reproductive state, and seasonality on the immune response.
Materials and Methods
Study area and field procedures
The investigation was carried out at the União Biological Reserve (RBU), located in the Brazilian state of Rio de Janeiro, which comprises an area of 7,756 acres covered by low-land and sub-montane forests (ICMBIO 2008). Mellado et al. (2018) reported that C. perspicillata dominates the bat community at RBU, primarily due to the abundance of Piper shrubs in disturbed areas, which constitute a major food source for these bats (Mello et al. 2004). The bats at RBU are monitored for a long-term mark-recapture study (Monteiro et al. 2019; Mellado et al. 2022), but most animals used in this study were captured in roosts that are not regularly monitored or were captured using mist nets along trails at RBU.
In the experiments described subsequently, 139 adult bats were used (74 males and 65 females). No animals were used more than once in the experiments. The female subjects did not show signs of breeding activity (pregnancy or lactation) that could be assessed visually or by palpation (Racey 2009). The breeding season (BS) overlaps with the rainy season, which lasts from September to May, and the highest proportion of breeding females is observed between November and April (Monteiro et al. 2019). We conducted 2 experiments at the beginning of the BS (October 2018 and November 2020) and 2 at the end (May 2019 and May 2021; see Supplementary Material S1 for sample sizes in each period). BS was used in subsequent analysis as a factor with 2 levels: early and late, separating animals tested at the beginning and the end of the season.
In the field laboratory, we measured forearm lengths (right and left sides), body mass (BM), testicular length, and foot thickness. BM was measured with a 0.01 g precision scale (1002, Bel Engineering S.R.L., Italy). BM was included as a measure of body size and condition, as it was found to be more associated with fat reserves in bats than condition indices that “correct” mass using forearm length (McGuire et al. 2018; Mellado et al. 2024). Length variables were measured using 0.01 mm digital calipers (Mitutoyo Corporation, Japan, Model CD 6” PSX). We tagged the bats for individual identification using ball-chain necklaces with a numbered aluminum or plastic ring attached, after the procedures outlined by Mellado et al. (2022). During the experiment, the animals were held in a tent (4 × 4 × 2.5 m) for 2 nights and fed bananas ad libitum during the night. This project was authorized by SISBIO under license 53628-3 and the Ethics Committee on Animal Use (CEUA-UENF) under Protocol 498.
Asymmetry analysis
We assessed asymmetry in C. perspicillata using differences in left and right forearm length, measured between the elbow and wrist with the wing bent (see Supplementary Material S2). Compared with other body parts, forearm length is repeatable and easy to measure in live bats (Monteiro et al. 2019). The forearm is a critical structure in bat flight (Norberg 2012) and is subject to strong selective pressures, which may result in lower variability (Palmer and Strobeck 1986). Monteiro et al. (2019) validated forearm asymmetry as a proxy for DI in C. perspicillata, using a large sample (391 individuals) and providing a repeatability analysis. In this study, the animals were obtained from the same population/cohorts, and measured by the same investigators and instruments as in Monteiro et al. (2019). Therefore, we considered it unnecessary to repeat their analyses. Their results indicate that the fluctuating asymmetry (FA) observed in C. perspicillata forearms was approximately 10× larger than the FA expected based only on measurement error. The repeatability based on the intraclass correlation coefficient was RIC = 0.585 (95% CI = 0.527–0.638), whereas the hypothetical repeatability was Rhyp = 0.18 (Van Dongen 2006). The magnitude of measurement error relative to among-individual variation in DI is small enough to allow for associations between asymmetry and fitness proxies to be interpreted as DI-fitness associations, but the strength of the associations might be underestimated. We calculated individual asymmetry using the module of the difference between right and left forearm lengths |R-L| (Monteiro et al. 2019).
Immunological challenge
We measured the cell-mediated immunological response using a delayed-type hypersensitivity (DTH) test, which causes swelling at the injection site of an antigen (Demas et al. 2011). The intensity of the immune response is measured by the size of the swelling compared with a control area (Allen et al. 2009). We used the antigen phytohemagglutinin (PHA; Sigma–Aldrich L8754-50MG), a lectin derived from the bean Phaseolus vulgaris (Kennedy and Nager 2006). The immunological response to PHA is complex (Turmelle et al. 2010), and involves both adaptive and innate components (Martin et al. 2006). The swelling response to PHA might be considered evidence of an individual’s ability to mount an inflammatory response (Vinkler et al. 2010), and has been used as a measure of general health or “quality” (Martín and López 2006), or associated with sexually selected phenotypes (Vinkler et al. 2014). In bats, the PHA response was shown to be associated with environmental and physiological factors (Baker et al. 2012).
We captured the bats 1 day before the experiment and allowed them to acclimate in the housing tent. They were fed bananas ad libitum during the night. The next morning, we injected each individual with 0.03 mL of PHA, diluted in phosphate-buffered saline (PBS) solution (2.16 mg/mL) into the ventral pad of 1 randomly selected foot. This corresponded to a mean dose of 4.32 (standard deviation [SD] = 0.35) mg PHA/kg of bat BM. We used the opposite foot as a control and injected 0.03 mL of PBS solution (Martin et al. 2006; Allen et al. 2009). The individuals were then returned to the tent. There was no observable effect of foot swelling on the roosting behavior of the animals (personal observation).
We evaluated the immune response 24 h later by measuring a standardized difference in thickness between the feet, determined by the PHA index modified from Allen et al. (2009): IPHA = (TPHA−TPBS)/TPBS, where TPHA represents the thickness of the foot injected with PHA and TPBS is the thickness of the foot injected with PBS. The same researcher measured the thickness of each foot 3 times, and the index was calculated with the average of the 3 measurements. To minimize bias in swelling assessment, the measurer was blind to which foot had received the PHA injection. In the first experiment (November 2018), we evaluated the IPHA at 3, 6, 10, 24, and 36 h post-injection in 24 individuals (Supplementary Material S3). The average response stabilized approximately 24 h after injection. To minimize handling stress, we only measured the swelling 24 h after injection in all subsequent experiments.
Statistical analysis
Basic t-tests (Crawley 2013) were used to determine sexual dimorphism and differences between times relative to BS (before/after) in IPHA, forearm asymmetry (ForA), and BM. We fitted linear models predicting IPHA from ForA, BS, BM, and sex, as well as their interactions. Testis length (TL) was included in models fitted only with breeding males (TL > 6.0 mm shows evidence of spermatogenesis; Fleming 1988). We based model comparison and selection on Akaike’s information criterion corrected for small samples (Burnham and Anderson 2002). All linear models were checked for assumptions with diagnostic plots, and there was no need for response variable transformation or the use of generalized models. All statistical analyses were performed in the R environment (R Core Team 2023). We performed the model selection using the MuMIn package (Bartoń 2020) and visualized results using the visreg, effects, and effectsize packages (Breheny and Burchett 2017; Fox and Weisberg 2019; Ben-Shachar et al. 2020). Data sets, R scripts, and supplementary files are available at https://doi.org/10.5281/zenodo.12162350
Results
The median and mean immune response to PHA was 0.49, with a range between 0.1 and 1.29 and an interquartile interval between 0.36 and 0.62. We detected potential outliers in 2 cases where IPHA values exceeded 1.0 but these observations did not affect the model fit and were not excluded from the data set. We removed a single individual from the data set due to a problem with the injection, resulting in IPHA = 0. No sexual dimorphism was statistically supported in average IPHA (t = −0.902, df = 134.59, P = 0.3689) and average ForA (t = −0.837, df = 136.42, P = 0.4042). We did observe differences in average BM between sexes (t = −3.173, df = 136.41, P = 0.0019) and breeding period (t = −4.563, df = 136.46, P < 0.00001). The overall effect of season on average BM was an increase of 0.90 g (95% CI = 0.37–1.45 g) in late BS, whereas males were on average 0.70 g (95% CI = 0.17–1.24 g) heavier than females.
The linear models employed to predict the immunological response explained a moderate degree of variation in the IPHA (R2 ~ 0.32 for the 10 best-fitting models). Based on the information criteria, 2 models were found to be statistically equivalent (ΔAICc < 2; Supplementary Material S4). All the highly ranked models included BM, ForA, BS, and sex but differed on the inclusion of specific interactions. The only interactions that were consistent in all models were BM:BS and ForA:Sex. Among the best-fitting, statistically equivalent models, the lowest AICc was also observed in the model with the smallest number of estimated parameters (IPHA~BM*BS + ForA*Sex). The IPHA, decreased with BM in the early BS but increased with BM in late BS regardless of sex (Figure 1A). We also observed an interaction between sex and ForA in their associations with IPHA (Figure 1B). Females showed a weak but positive correlation between ForA and IPHA, whereas males exhibited a stronger negative association (Supplementary Material S5).
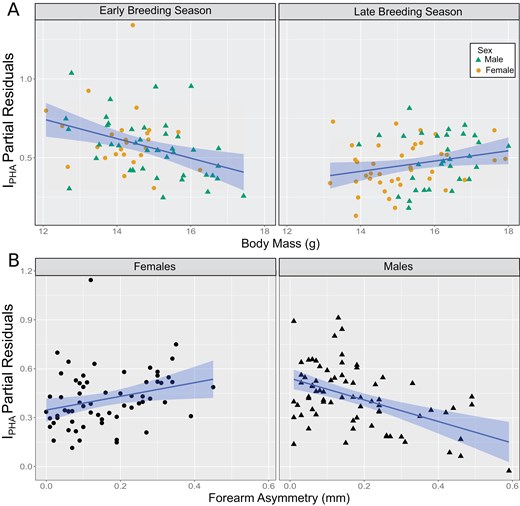
Effect plots for the linear model predicting IPHA variation for Carollia perspicillata at Reserva Biológica União, showing interactions between (A) body mass and breeding season (BS) period, and (B) forearm asymmetry and sex. Partial residuals for each predictor are shown in each plot. Predictor effects are shown as blue regression lines and 95% confidence limits (bands).
We investigated separately the immunological response of 50 male individuals with evidence of reproductive activity (25 early BS and 25 late BS). Forearm asymmetry was included as a predictor in the top 15 best-fitting models (Supplementary Material S6). The first 2 best-fitting models are statistically equivalent (ΔAICc < 2), but only the first included TL as a predictor (IPHA ~ ForA + TL), with an adjusted R2 = 0.17. There is some uncertainty regarding the partial influence of TL (beta = −0.05, 95% CI = −0.10, 0.008), but we decided to retain it in the model due to its consistently negative beta across the model set and biological relevance of the relationship. BM and BS were not included in the best-fitting models. The plot of effects reveals a negative relationship between TL and immune response (Figure 2) that is independent and partial of asymmetry, as well as the negative relationship between forearm asymmetry and immune response observed before. The more asymmetric males and those with larger testicles showed a lower immune response.
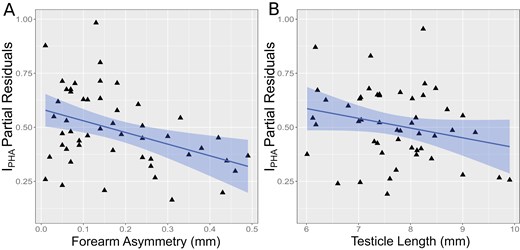
Effect plots for the linear model predicting the phytohemagglutinin index (IPHA) for male Carollia perspicillata at Reserva Biológica União, according to forearm asymmetry (A) and testicle length (B). Partial residuals for each predictor are shown in each plot. Predictor effects are shown as blue regression lines and 95% confidence limits (bands).
Discussion
This study revealed sexual dimorphism in the relationship between immune response and DI (assessed by forearm asymmetry) and a trade-off between immune response and reproduction mediated by BM. Females displayed a weak positive relationship between forearm asymmetry and IPHA, whereas males showed a consistent negative relationship. Before the BS, a stronger immune response was observed in both sexes, but heavier bats had a lower IPHA. After the BS, the immunological response was lower, but heavier animals had a higher IPHA. Thus, forearm asymmetry was a relevant predictor only for the male immunological response, along with testicle length. The IPHA decreased more in females than males after the BS, but these differences cannot be dissociated from variation in BM. Overall, sexual dimorphism was not directly observed in the magnitude of IPHA, but a strong dimorphism was observed in the predictors of immune response. Male IPHA was more associated with individual-level predictors (BM, ForA, and TL), whereas female IPHA was more associated with the period within the BS.
Immune response and DI
The positive relationship between forearm asymmetry and immune response in females is an unexpected result, which may be due to confounding effects associated with their increased energy and nutrient demands during the BS. Monteiro et al. (2019) examined the same bat population and found that more symmetrical females were more likely to have 2 pregnancies per BS. This could exhaust them in terms of energy and nutrition, potentially reducing the intensity of the PHA response. During breeding, the energy requirements of female C. perspicillata can increase by up to 1.8 times (Fleming 1988). Additionally, the costs associated with gestation are increased due to carrying extra weight during pregnancy (Stevens et al. 2013), and nursing the offspring for up to 2 months (with at least 1 month of intense lactation) (Fleming 1988). In addition to energy expenditure, nutrient, and oxidative damage costs can limit physiological processes (Hasselquist and Nilsson 2012; Schneeberger et al. 2013) and contribute to the lower immunological response in breeding, more symmetric females.
The lower cell-mediated response in symmetric females could also be associated with a trade-off with other components, such as bacterial killing ability (Allen et al. 2009), that might be more important for females due to sex-related differences in life history or behavior (Klein and Flanagan 2016). This could be the case if different patterns between sexes are associated with exposure to pathogens due to roosting behavior (Allen et al. 2009). Female C. perspicillata spend considerably less time inside crowded roosts than males (Fleming 1988; Monteiro et al. 2019). As a result, they might be less exposed to viruses or other relevant pathogens (Baker et al. 2012), and experience lower selective coefficients in immune responses (Langeloh et al. 2023). Within the limitations of our experimental design, it can be challenging to dissociate the effects of DI from the trade-off between immunity and reproduction in this case. It would be informative to measure parasite load along with other components of the immunological response, as well as discriminate between females with 1 or 2 pregnancies in a BS.
The negative relationship observed between asymmetry and immune response in males supports the hypothesis of a systemic effect on individuals with higher DI leading to differences in fitness (Lagesen and Folstad 1998; Martín and López 2006; Hammouda et al. 2012). The mechanisms underlying the association DI-fitness at the individual level are poorly understood (Van Dongen 2006). However, there is increasing evidence of systemic effects of genes associated with asymmetric phenotypes, such as heat shock proteins (Leamy et al. 2015) and Hippo signaling pathway genes (Boone et al. 2016). Such genes regulate diverse physiological processes and ensure developmental stability through the control of organ growth (Colombani et al. 2015). Dysregulation of these developmental pathways is expected to increase FA, decrease immunocompetence, increase the incidence of tumors, and affect important metabolic functions (Juarez-Carreño et al. 2018; Ma et al. 2019; Zheng and Pan 2019), thus affecting individual fitness.
Immune response and BM
The association between BM and immunological response changed from negative in the early breeding period to positive in the late breeding period. No interaction with sex was observed, but the effect was strongly associated with males. In bats, there is evidence that BM is a good proxy for an individual’s general health and energy reserves (McGuire et al. 2018), but it also incorporates variation in lean mass (Yom-Tov and Geffen 2011). A number of mechanisms might be responsible for the relationship between BM and IPHA. If there was a trade-off between immune response and growth (Lochmiller and Deerenberg 2000), a negative association between BM and IPHA would be expected. The hypothesis of trade-off with growth has little support, however, because the direction of the association changes seasonally in our study,
The immune response is usually stronger in organisms with larger BM or better body condition (Martin et al. 2008; Demas et al. 2012). Individuals with high resource levels (better body condition) do not need to compromise immune function and traits leading to direct fitness gains (Seppälä 2015). On the other hand, individuals with lower resources need to restrict their immune response to avoid compromising survival in the long-term (Houston et al. 2007). This relationship is, however, not consistent across studies with bats. Allen et al. (2009) found that variation in response to PHA was not explained by body condition in Tadarida brasiliensis. On the other hand, Christe et al. (2000) reported a positive association between response to PHA and BM in lactating Myotis myotis females (but not in non-reproductive animals), whereas Otálora-Ardila et al. (2022) found positive associations between PHA response and BM in Myotis vivesi, with a stronger effect in males. Our results are congruent with previous studies (Christe et al. 2000; Otálora-Ardila et al. 2022), with a positive association between PHA response and BM at the end of the BS and a stronger effect in males. Regardless of sex, individuals that arrive at the end of the BS with a larger BM have a higher probability of survival (Monteiro et al. 2019), which might be linked to a more effective immune system.
The negative relationship between IPHA and BM in the early period of the BS was mostly driven by males, and not reported for bats before. The lower immune response in heavier individuals might be expected if a confounding variable was not taken into account in our model. For example, in C. perspicillata males, BM is positively associated with testicle length (Monteiro et al. 2019), a variable related to increased sperm production and testosterone levels in mammals (Schulte-Hostedde and Millar 2004; Preston et al. 2012). Potential links between hormonal levels and immune response (Foo et al. 2017) might explain the influence of reproductive state on the relationships between individual attributes and immune response.
Immune response and reproduction
According to the immunocompetence handicap hypothesis (ICHH; Folstad and Karter 1992), increased testosterone levels can lead to exaggerated secondary sexual traits and are associated both with increased sperm production (Preston et al. 2012) and decreased immune function (Hosken and O’Shea 2001). Meta-analyses have partially corroborated the negative association between testosterone and immune response (Roberts et al. 2004; Foo et al. 2017), mostly based on experimental studies (not correlational), but with no indication of a universal effect. The negative association observed between TL and IPHA was independent of BM and ForA. There was some statistical uncertainty in this relationship, but if we consider it biologically relevant, the result would be congruent with the ICHH. Greiner et al. (2010) showed for C. perspicillata, that plasma testosterone levels did not predict the intensity of a DTH skin test. However, testosterone levels were lower after the test, inverting the causal relationship but still congruent with ICHH. Correlational studies are expected to generate controversial results due to the complexity of the immune response as well as uncontrolled confounding factors (Ezenwa et al. 2012; Foo et al. 2017). Therefore, further evidence would be required to confirm ICHH in C. perspicillata, such as the associations and trade-offs between different components of the immune system, parasite loads, and phenotypic and life-history variables.
The decline in immune response after the BS is consistent with a resource allocation trade-off between reproduction and immunity (Christe et al. 2000; Martin et al. 2008). As discussed before for females, the 2 pregnancies during the BS can exhaust the reserves of female C. perspicillata (Fleming 1988), reducing the survival probability of individuals that reach the end of the BS with low BM (Monteiro et al. 2019). This period of lowered immunity might coincide with the seasonality of virus shedding and the probability of zoonotic spillover (Joffrin et al. 2022). Life-history theory predicts that under conditions of insufficient food intake and energy reserves to support biological processes such as growth, maintenance, and reproduction, a competitive allocation of resources must occur (Lochmiller and Deerenberg 2000; Edward and Chapman 2011). The IPHA used in this study measures a systemic inflammatory response that can be energetically costly (Martin et al. 2003), but not necessarily so (Cutrera et al. 2014; Otálora-Ardila et al. 2016). Nevertheless, other costs, such as nutrients and oxidative stress, might be involved in the mediation of the trade-off (Hasselquist and Nilsson 2012; Schneeberger et al. 2013). It is expected that reproductive events will lead to a decrease in the immunological response (McKean and Lazzaro 2011). However, the fitness cost of this trade-off will depend on environmental variables such as roost crowding, exposure to parasites, and the opportunity for infection (Leivesley et al. 2019; Negrey et al. 2022).
In summary, the present study enhances our understanding of immune response variation in bats by revealing an association between DI and immunocompetence and a trade-off with reproduction in C. perspicillata. Males were particularly prone to individual variation predicted by DI, BM, and reproductive investment, whereas for females, the most pronounced component of immune response variation was a decrease after the BS. It is possible that the immunological component measured by the PHA response is under finer selection in males because it can be predicted more effectively by individual features. These findings support the hypothesis that DI has a systemic effect through shared developmental pathways between immunity and the control of organ growth, providing a functional link between forearm asymmetry and survival in C. perspicillata (Monteiro et al. 2019). Further research should evaluate other components of the immune response, as well as variation in parasite loads and more detailed information regarding individual reproductive investment and their fitness outcomes.
Supplementary Material
Supplementary material can be found at https://dbpia.nl.go.kr/cz. Supplementary material, scripts, and data can be found at https://doi.org/10.5281/zenodo.12162350.
Acknowledgments
We would like to thank ICMBIO staff at Rebio União for providing support and infrastructure. We also thank R.B. Lyra and J.M. Bubadué for help with fieldwork.
Funding
This work was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação de Amparo à Pesquisa do Rio de Janeiro (FAPERJ), Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP Grant nº 2014/16/320-7), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES)–Finance Code 001, and by grants to LGHM and APCN from the Visiting Research Program of the FAPESP (nº 2017-17607-6). LGHM was supported by the PASPA-DGAPA program of the Universidad Nacional Autónoma de México (nº 814-2018).
Conflict of Interest
None declared.
Authors' Contributions
The project was designed by BM, LRM, and APCN. All authors participated in the experiments, data collection and discussion of results. Statistical analyses were performed by BM and LRM. First version of the manuscript was written by BM and LRM, with input from all authors.
Ethics Statement
This project was authorized by the Ethics Committee on Animal Use (CEUA-UENF) under Protocol 498.



