-
PDF
- Split View
-
Views
-
Cite
Cite
Tommaso Ruberto, William T Swaney, Adam R Reddon, Submissive behavior is affected by territory structure in a social fish, Current Zoology, Volume 70, Issue 6, December 2024, Pages 803–809, https://doi.org/10.1093/cz/zoae014
Close - Share Icon Share
Abstract
Group living may engender conflict over food, reproduction, or other resources and individuals must be able to manage conflict for social groups to persist. Submission signals are an adaptation for establishing and maintaining social hierarchy position, allowing a subordinate individual to avoid protracted and costly aggressive interactions with dominant individuals. In the daffodil cichlid fish (Neolamprologus pulcher), subordinates may use submission signals to resolve conflicts with dominant individuals and maintain their social status within the group. The complexity of the physical environment may affect the value of submission signals compared with fleeing or avoidance, which may require certain physical features such as shelters to be effective. We investigated how the ecological context affected the expression of submission in subordinate daffodil cichlids by examining their behavior under different arrangements of the physical environment within their territories. We altered the number of shelters provided to daffodil cichlid groups and compared the interactions between dominant and subordinate individuals under each shelter condition by scoring the social and cooperative behaviors of the group members. We found that behaviors of group members were modulated by the environment: subordinates displayed fewer submission and fleeing behaviors in more structurally complex environments and dominants were more aggressive to subordinates when more shelters were present. Our results help to elucidate the role of the physical environment in the modulation of social interactions in group-living animals and may have implications for the welfare of captively housed social cichlid groups.
For group living animals, competition for resources may be frequent and can have substantial costs (Aureli and de Waal 2000; Hardy and Briffa 2013). Managing within-group competition is therefore crucial for the stability of social groups (de Waal 1986; Aureli et al. 2002; Silk 2007; Kutsukake and Clutton-Brock 2008). Social groups may contain relatives with shared inclusive fitness interests (Hamilton 1964; Eberhard 1975; Lehmann et al. 2007), but even when there is no relatedness among the members of the group, individual fitness can be significantly enhanced by group productivity (Kokko et al. 2001). Despite such shared interests within groups, competitive interactions may be frequent and determine priority or exclusive access to disputed resources (Kaufmann 1983). Agonistic interactions are costly in terms of time, energy, and injury risk (Forkman and Haskell 2004; Beaulieu et al. 2014), which may offset the benefits of group living.
To reduce or avoid the costs of conflict, many social species exhibit submissive behaviors (Balshine et al. 2017; Fischer et al. 2024). Submissive behaviors include both avoidance behaviors such as fleeing from aggression, and submission signals which are primarily used by animals to communicate submission towards an aggressive conspecific (Reddon et al. 2022). Although avoidance and submission signals are often pooled into the same broad category of social behaviors, the evolutionary causes, and consequences of these behaviors can be distinct. Although avoidance behaviors are used to directly evade aggression and may secondarily serve as a cue of submission, submission signals are not intrinsically linked to escape but serve primarily a communicative function, for example via a shift in body coloration or a particular vocalization (Guilford and Dawkins 1995).
Social dynamics can be affected by changes in the environment (Brown 1971; Ekman 1987; Snell et al. 1988; Anholt 1990; Ward and Porter 1993; Petren and Case 1998; Cross et al. 2004; Wittemyer et al. 2005; Estevez et al. 2007; Henzi et al. 2009; de Silva et al. 2011; Godfrey et al. 2013; Pinter-Wollman et al. 2014; Smith-Aguilar et al. 2016). For example, an increase in temperature is positively correlated with aggressive behaviors in several fish species (Kvarnemo 1998; Biro and Stamps 2010; Zhao and Feng 2015; Jones et al. 2024), and in some cases may affect boldness and sociality (Angiulli et al. 2020). The availability of resources also has an influence on social behaviour. For example, Japanese medaka (Oryzias latipes) are more socially tolerant toward other group members and consume less energy in conflicts when food is abundant in their territory (Magnuson 1962). The structure of the physical environment is another ecological factor that can influence competition for resources (Bell et al. 2012). For instance, captive-bred male butterfly splitfins (Ameca splendens) allocated more time to foraging activities in unstructured aquaria compared with structured ones, and they also displayed increased aggression in structured environments compared with unstructured ones, although this effect was notable only under conditions of high population density (Kelley et al. 2006). On the other hand, zebrafish (Danio rerio) and brown trout (Salmo trutta) exhibit a decrease in aggression as environmental complexity increases (Basquill and Grant 1998; Sundbaum and Näslund 1998).
The cooperatively breeding daffodil cichlid (Neolamprologus pulcher) is a freshwater fish species endemic to Lake Tanganyika, Africa, where it inhabits the shallow waters of the lake’s Southern coasts (Balshine et al. 1998). These small fish form permanent social groups that defend territories against conspecifics and predators (Wong and Balshine 2011; Groenewoud et al. 2016). A group of daffodil cichlids is generally composed of a dominant pair, usually the largest male and female fish, and up to 20 smaller subordinate fish that work to guard and maintain the territory, and take care of the offspring of the dominant pair (Balshine et al. 2001; Heg et al. 2005; Desjardins et al. 2008). Among daffodil cichlids, subordinate individuals may avoid aggression from dominant group members by performing cooperative behaviors such as territory maintenance (Wong and Balshine 2011), by directly fleeing from aggression (by rapidly swimming away from the aggressor), or by displaying submission signals (in the form of submissive postures or tail quivering) towards aggressing groupmates (Balshine et al. 1998, 2001; Manara et al. 2023). Disengaging from a conflict and fleeing should be the most straightforward tactic for an animal to escape aggression, however, for daffodil cichlids fleeing is not always a viable option and typically carries costs for the individual. The vital protection from predation risk provided by the social group may restrict the ability to flee from aggression by limiting how far an individual may flee away from the group territory (Wong 2010; Hick et al. 2014; Balshine et al. 2017). Subordinate daffodil cichlids that leave their group are unlikely to find new groups and must join an already established one (Jungwirth and Taborsky 2015). These dispersal events are typically preceded by a period of scouting and gradual integration into the new group prior to joining (Jungwirth et al. 2015b, 2023), as the tolerance for unfamiliar fish is low (Bergmüller et al. 2005a; Jungwirth et al. 2015a; Ligocki et al. 2015), suggesting that an ad hoc move to a new group is unlikely to succeed. Furthermore, reducing the number of shelters in wild daffodil cichlid territories results in a reduction in group size (Balshine et al. 2001), suggesting there is within-group competition for shelters, and fleeing individuals may risk losing their position in the hierarchy on returning. As a result, submission signals which allow subordinates to remain well-integrated in their current groups are thought to be a particularly important aspect of social interactions in daffodil cichlids. Fleeing and submission can be thought of as alternative tactics for avoiding aggression in daffodil cichlids (Balshine et al. 2017) with the relative frequency of each tactic forming a social stratagem, which may be affected by the social and physical environment in which the interactions are embedded. For example, a greater per capita availability of shelters or hiding places may make fleeing more successful, thereby reducing the need for submission signals, which themselves carry energetic and time costs (Grantner and Taborsky 1998; Taborsky and Grantner 1998).
A previous study reported that daffodil cichlid subordinates tend to show more submission signals toward dominant individuals when the group’s territory has fewer available shelters (Reddon et al. 2019). However, it remains unclear how changes in the physical environment, e.g., the addition or loss of territory structure, which in the wild can occur due to physical or social processes (Balshine et al. 2001), may affect the expression of submissive behavior within established daffodil cichlid groups. Here we investigated how the physical environment affects the expression of submissive behavior in daffodil cichlids. Specifically, we observed the behavior of established social groups with access to varying numbers and types of shelters using a within-groups, repeated measures design. We predicted that a greater availability of shelters would decrease the use of submission signals to de-escalate conflicts and increase the use of fleeing behavior which may be more successful with more shelter options available. We also examined how changing shelter availability affected dominant aggression toward subordinates because variation in the number of potential breeding sites may alter the value of the territory to the dominant pair and/or affect the ease with which the dominant pair can police subordinate reproduction (Bergmüller et al. 2005b). Previous work in the wild has shown that reduced shelter availability can lead to subordinate expulsions from the group, suggesting an effect on dominant behavior (Balshine et al. 2001). We also examined subordinate investment in cooperative workload in the form of digging behavior, as workload investment may also be used by subordinates to appease dominants and avoid aggression (Bergmüller and Taborsky 2005).
Materials and Methods
Study animals
The daffodil cichlids (Neolamprologus pulcher) used in this experiment were laboratory reared descendants of fish originally captured near Kasakalawe point in Lake Tanganyika (Republic of Zambia, Africa). Prior to the onset of the study, subjects were housed in mixed sex stock aquaria (105 × 43 cm and 40 cm high, 180-l), at a density of approximately 50 fish per aquarium. The stock aquaria were equipped with 2 powered filters, a heater, a thermometer, an air stone, and 3 cm of fine coral sand. These aquaria were kept at 27 ± 1 °C on a 12:12h light:dark day cycle, with 30 min of gradual brightening/dimming to simulate sunrise and sunset. The aquaria were regularly checked for water quality parameters and weekly water changes were performed. Fish were fed daily with a mix of dried prepared cichlids foods (Tetra Werke, Germany).
Focal groups
We created 10 focal social groups of 4 fish each by transferring fish from the stock aquaria into 90-l (53 × 43 cm and 38 cm high) group housing aquaria. Each group consisted of a dominant male (mean ± SE standard length, measured from the tip of the snout to the end of the caudal peduncle = 5.25 ± 0.17 cm), a dominant female (mean ± SE standard length = 4.69 ± 0.19 cm), and 2 smaller subordinates of indeterminate sex. The larger of the 2 subordinates within each group was referred to as “subordinate 1” (mean ± SE standard length = 3.31 ± 0.16 cm) and the smaller of the 2 as “subordinate 2” (mean ± SE standard length = 2.68 ± 0.10 cm). Groups were formed by first introducing the 2 subordinates into a new aquarium, and then after 24 h adding the dominant pair. Each of the group housing aquaria was furnished with 2 foam filters, a heater, a thermometer, 3 cm of fine coral sand along with 2 terracotta caves to serve as shelters and breeding substrates. New groups were observed closely after a week for excessive overt aggression or the social rejection of any group members, and unstable groups were dissolved and reformed anew with other fish from the stock aquaria whereas fish from the failed group were returned to stock housing. All groups used in this study were kept together for at least one month prior to data collection and had successfully spawned at least once. At the time of observation, all groups contained fry (< 1 cm standard length). Adult and larger juvenile daffodil cichlids in our social groups did not interact with fry, as previously reported for this species (Dey et al. 2015). The husbandry regime for the social groups was identical to that of the stock housing aquaria.
Experimental procedures
To evaluate the effect of the physical environment on daffodil cichlid agonistic behavior, we altered the number of available shelters by placing additional floating shelters (transparent green PET half-bottles affixed near the water surface) and additional substrate-level shelters (terracotta caves) in the housing tanks using a 2 × 2 design (the standard aquarium setup, extra caves, extra floating shelters, and both types of extra shelter). Both caves and floating shelters serve potential refuges from aggression, however they differ in their biological relevance: Although the caves offer a potential resource for reproduction which mimics the caves excavated among stones in the natural habitat of the species, the floating shelters are only used as a refuge for the subordinates and do not simulate structures available in the natural environment. Each social group was randomly assigned to an initial experimental condition: Standard setup (2 caves + 0 floating shelters, 2C + 0F), 2 additional caves (4C + 0F), 2 additional floating shelters (2C + 2F), and 2 additional caves with 2 additional floating shelters (4C + 2F). In each housing condition, each group was given a week of habituation and then observed for ten 30-min periods over the course of 2 weeks, resulting in a total of 300 min of observation per group per condition. The observations were taken by a stationary observer seated approximately 1.5 m from the front of the aquarium. A 10-min habituation period prior to the start of each observation allowed the fish to acclimate to the presence of the observer. The observations were performed between 10 h and 18 h and only one observation was taken per group per day. After the last observation was performed, we changed the treatment condition for the social group by adding or removing caves and/or floating shelters and we gave the group another week of habituation to the new experimental condition prior to subsequent observation. The order of presentation of the treatments was randomized for each group.
During the observations, we recorded interactions between both members of the dominant pair and each of the subordinate fish. We scored aggression received by the 2 subordinates from the dominant male and the dominant female, and the submissive responses of the subordinates to the dominants. We recorded 5 different behaviors as aggression: chases, rams, bites, head-down aggressive postures, and frontal displays. We pooled all aggressive behaviors into a single category of “aggression.” The submissive behaviors recorded were head-up postures and tail quiver displays which we pooled into a single category of “submission signals,” and any instances of fleeing (Ruberto et al. 2020; Manara et al. 2023). Finally, we recorded any instance of digging behavior performed by the subordinates, where the focal subordinate picked up sand with its mouth and carried it at least one body length before depositing it on the substrate. These territory maintenance behaviors may be used by subordinates as an additional method to appease dominants and avoid aggression (Bergmüller et al. 2005b).
Due to the SARS-CoV-2 outbreak and subsequent lockdown in the United Kingdom, our experiment was disrupted. All groups were tested for the experimental condition 2C + 2F, however, 2 groups were not tested in the conditions 2C + 0F and 4C + 0F, and 1 group was not tested in condition 4C + 2F, resulting in a sample size of 8 groups for conditions 2C + 0F and 4C + 0F and 9 for condition 4C + 2F.
Statistical analysis
We used linear mixed models (LMM) to analyze aggression received by subordinates, and the responses of subordinates to aggression across the 4 treatments. We fitted separate models to the number of aggressions received by subordinates; the number of submission signals (submissive postures + tail quivering displays) per aggression received; fleeing per aggression received; and digging performed per aggression received. In all LMMs, individual identity and group identity of the fish were included as random factors, whereas the rank of the fish (subordinate 1 or 2) and experimental housing condition were included as fixed factors. We found no significant interactions between subordinate ranks and housing conditions; therefore, we removed these interaction terms from the final models. Data that did not meet statistical assumptions (normality of residuals and homogeneity of variance across treatments) were Box–Cox transformed prior to analysis, resulting in normality of residuals. Where significant effects were found, Sidak post hoc tests for differences of means was used for pairwise comparisons. We analyzed the submission, fleeing, and digging behaviors per aggression received because each correlated strongly with dominant aggression (Ruberto et al. 2020; Manara et al. 2023) and therefore variation in aggression received could mask any effects on the subordinate choice of response to aggression. We present the raw values of submission, fleeing, and digging behavior in Supplementary Figure S1. All statistics were performed using SPSS version 26.0 (IBM) for Windows.
Ethical note
Animal housing, handling, and study protocols were approved by the Liverpool John Moores Animal Welfare and Ethics Steering Group (approval number: AR_TR/2018-4) and adhered to the guidelines of the Animal Behaviour Society and the Association for the Study of Animal Behaviour. All fish were closely monitored for social exclusion or signs of injury. All observations were drawn from stable social groups showing typical levels of agonism for daffodil cichlids (Balshine et al. 2017).
Results
The total instances of aggression received by subordinates varied depending on the experimental housing condition (F3, 51.15 = 6.88, P = 0.001; Figure 1A). Post hoc tests showed that subordinates received more aggression in condition 4C + 2F when compared with each of the other conditions (4C + 2F vs. 2C + 0F: P = 0.003; 4C + 2F vs. 4C + 0F: P = 0.001; 4C + 2F vs. 2C + 2F: P = 0.009). Subordinate submission signals (F3, 53.9 = 10.99, P < 0.001; Figure 1B) and fleeing (F3, 52.68 = 6.87, P = 0.001; Figure 1C) in response to aggression also varied depending on the shelter condition. Post hoc tests showed that subordinates were less likely to use submission signals in condition 4C + 2F when compared with each of the other conditions (4C + 2F vs. 2C + 0F: P < 0.001; 4C + 2F vs. 4C + 0F: P = 0.001; 4C + 2F vs. 2C + 2F: P = 0.001), and that they fled less in condition 4C + 2F when compared with condition 4C + 0F and 2C + 2F, but not when compared with condition 2C + 0F (4C + 2F vs. 2C + 2F: P = 0.002; 4C + 2F vs. 4C + 0F: P = 0.046). Subordinates also fled more in condition 4C + 0F compared with condition 2C + 0F (P = 0.006). Digging behavior did not vary across conditions (F3, 40.86 = 1.20, P = 0.321; Figure 1D).
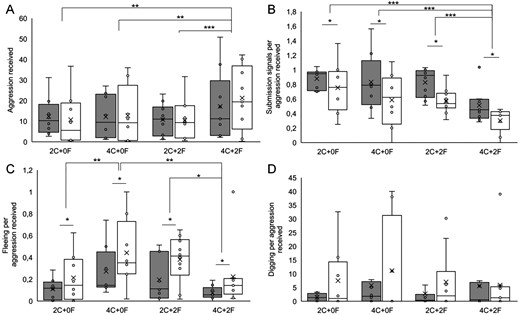
Box and whisker plots with overlaid raw data showing the aggression received by subordinate 1 (gray bars) and subordinate 2 (white bars) in the different housing conditions, and their responses to aggression: (A) aggression received across 300 min of observation, (B) submission signals per aggression received, (C) fleeing per aggression received, and (D) digging per aggression received. The 4 experimental conditions were: 2 cave shelters (2C + 0F), 4 caves (4C + 0F), 2 caves and 2 floating shelters (2C + 2F), and 4 caves with 2 floating shelters (4C + 2F). * indicates P < 0.05, ** indicates P < 0.005, *** indicates P < 0.001.
Across all experimental housing conditions, the number of aggressions received did not differ between the 2 subordinate ranks (F1, 20.39 = 0.01, P = 0.942). We did find differences between the 2 subordinate ranks in the number of submission signals (F1, 10.74 = 7.249, P = 0.021) and fleeing (F1, 20.98 = 4.66, P = 0.043) performed in response to aggression, with subordinate 1 performing more submission signals and fleeing less compared with subordinate 2. However, no differences were found between the 2 subordinate ranks in digging performed in response to aggression (F1, 11.77 = 0.01, P = 0.939).
Discussion
In this study, we investigated how the structure of the physical environment affects the expression of submissive behavior in daffodil cichlids. We predicted that submission signals would be less common relative to fleeing when more shelters to flee to were available. Fitting with this prediction, we found that fish were least likely to show submission signals in the condition with the most shelters available (4C + 2F). However, fleeing behavior showed a more complex pattern, with the 2 intermediate conditions (2C + 2F, 4C + 0F) leading to higher levels of fleeing than the conditions with the fewest (2C + 0F) or most (4C + 2F) shelters.
We found that the aggression received by the subordinates from the dominant pair also varied depending on the experimental condition, with fish receiving more aggression in the treatment with the most shelters. This elevated aggression from the dominant pair may have affected submissive behavior in the subordinate fish, as submission signals and fleeing per aggression received were both lowest in the 4C + 2F condition. We did not find any differences in digging by the subordinate individuals across treatments despite the observed changes in aggression received by subordinates. This suggests that subordinates may not adjust their digging behavior in response to changes in dominant aggression under different environmental conditions, and thus that digging is not being used as an active form of appeasement in this context. Finally, although we found no differences between the 2 subordinate ranks in the levels of aggression received, they responded differently to aggression, with the larger subordinate submitting more but fleeing less than the smaller subordinate.
In line with the results from a previous study (Reddon et al. 2019) which compared only 2 environmental conditions that varied in the number of caves available, we found that subordinate fish in the most enriched environment tended to respond to aggression with fewer submission signals. Previous findings suggest that submission should be more common when the opportunity to flee from an aggressor is limited, either by ecological or physical restrictions such as the lack of shelters (Matsumura and Hayden 2006; Ligon 2014). As submission signals and fleeing are alternative strategies for avoiding conflict in daffodil cichlids (Balshine et al. 2017), it is plausible that the additional shelters placed in the environment provided more places to hide from aggressive dominants, reducing the need for subordinates to perform submission signals. However, surprisingly we also found that subordinates in the most enriched condition were less likely to flee than subordinates in the conditions with either only added caves or only added floating shelters and were comparable to those from the most barren condition. The decrease in both fleeing and submission per aggression received shown by subordinates in the most structurally complex condition may be driven by the heightened aggression shown by dominant pair in that condition. Increased breeding site number increases territory value for the dominant pair while also giving the territory more places to hide from predators and use as nest sites by either the dominant pair or by subordinates themselves. This increased territory value and/or difficulty in policing subordinate behavior could make dominants more aggressive toward potential reproductive competitors, as we observed, although this was only true when the floating shelters were also added.
Environmental enrichment can be beneficial for decreasing aggressive behavior and physiological stress in fish held in captivity while at the same time enhancing their well-being (Gerber et al. 2015; Näslund and Johnsson 2016). The most widely used type of environmental enrichment is physical enrichment, which involves introducing various objects like physical structures, plants, and substrates into the housing environment to increase its complexity (Johnsson et al. 2014). Several studies have examined the effect of enrichment on fish aggression, but the findings have been mixed, with some studies reporting reduction of aggression (Barley and Coleman 2010; Kadry and Barreto 2010; Torrezani et al. 2013; Bilhete and Grant 2016; Xi et al. 2017; Arechavala-Lopez et al. 2019), some instead reporting an increase of aggressive behaviors (Barreto et al. 2011; Bhat et al. 2015; Kochhann and Val 2017), and others reporting no effects (Hoelzer 1987; Kemp et al. 2005; Lachance et al. 2010). These inconsistencies may be linked to species-specific effects, developmental stage, enrichment specifics (e.g., type and number of added structures), and other methodological variations. In our experiment, we found that dominant pairs increased their level of aggression toward the subordinate fish when they were tested in the most enriched condition and therefore environmental enrichment may not be a productive approach to reducing aggression within captive housed daffodil cichlid groups.
In conclusion, we found that aggression levels of daffodil cichlid dominant breeding pairs were increased when their social groups occupied more physically complex territories. This increase in aggression in the most enriched condition was associated with changes in the behavior of subordinates, with reduced levels of both submission signals and fleeing among subordinates per aggression received. However, the digging behavior of subordinates was not affected by the physical environment. Our findings shed light on the effect of the physical environment in modulating within-group interactions of daffodil cichlids, an emerging model for the investigation of social behaviour.
Supplementary Material
Supplementary material can be found at https://dbpia.nl.go.kr/cz.
Acknowledgments
This work was supported by a Royal Society Research Grant (RGSR1191237) to AR. TR was supported by a Liverpool John Moores University Faculty of Science PhD Studentship.
Conflict of Interest
The authors declare they have no conflict of interest.



