-
PDF
- Split View
-
Views
-
Cite
Cite
Elizabeth Squirell, Gregory Rosenfeld, Brian Bressler, Susanna Meade, Natasha Klemm, Victoria Chen, Elisabet Joa, Yvette Leung, Pregnant Pause? Not for IBD Care – A single tertiary care centre prospective cohort study affirming IBD management in pregnancy, Crohn's & Colitis 360, 2025;, otaf029, https://doi.org/10.1093/crocol/otaf029
Close - Share Icon Share
Abstract
This study examined Inflammatory Bowel Disease (IBD) management and outcomes during pregnancy in a tertiary care setting, focusing on disease activity, medication use, and maternal and neonatal outcomes.
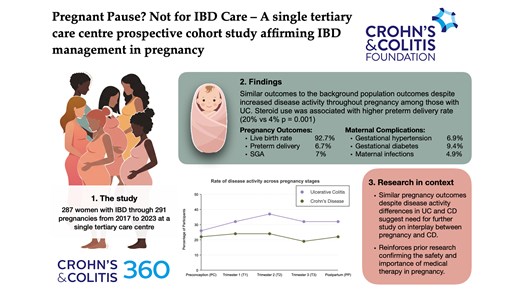
A prospective cohort study followed 287 women with IBD through 291 pregnancies from 2017 to 2023 at a single tertiary care centre, collecting data preconception, during each trimester, and postpartum.
The study observed a 92.7% live birth rate. 74% of individuals were in clinical remission preconception, and disease activity increased throughout pregnancy, particularly in UC patients (peaking at 37% in the second trimester), while remaining stable in CD patients. UC, disease duration <5 years, and preconception activity correlated with higher disease activity during pregnancy. Biologic use remained stable without significant impact on outcomes. Preterm delivery (6.7%) and small for gestational age infants (7%) rates reflected baseline population risk. Steroid use was associated with higher preterm delivery rates. Gestational hypertension (6.9%) and diabetes (9.4%) rates were similar to population norms. Maternal adverse events were higher in women 40 or older (OR 3.893).
This study reaffirms the safety of continued medical therapy for IBD throughout pregnancy in a tertiary care, prospective cohort. Increased disease activity throughout pregnancy was evident, particularly in UC. Despite higher rates of disease activity amongst those with UC, outcomes were similar in those with CD vs UC – suggesting that disease activity measures have limitations in CD and pregnancy, or there is some mild inherent risk of CD in pregnancy outcomes irrespective of disease activity.
Lay Summary
In a prospective cohort study of 291 pregnancies in women with IBD, findings demonstrated most women had healthy pregnancies, with proper medical care and monitoring. Continuing IBD medications during pregnancy was safe, though some patients experienced more active disease symptoms.


