-
PDF
- Split View
-
Views
-
Cite
Cite
Rebecca Muller, Arjun Amar, Petra Sumasgutner, Shane C McPherson, Colleen T Downs, Urbanization is associated with increased breeding rate, but decreased breeding success, in an urban population of near-threatened African Crowned Eagles, The Condor: Ornithological Applications, Volume 122, Issue 3, 4 August 2020, duaa024, https://doi.org/10.1093/condor/duaa024
Close - Share Icon Share
Abstract
Urban areas can be attractive to certain species because of increased food abundance and nesting availability, which in turn may increase productivity or breeding rates. However, there are also potential costs associated with urban living such as higher nest failure, poorer body condition, or increased prevalence of disease. These costs may result in species trading off the number of young produced against the condition of their young. African Crowned Eagles (Stephanoaetus coronatus) are a rare example of large, powerful apex predators that breed in some urban areas in Africa. In this study, we explored the breeding performance of these eagles across an urbanization gradient in KwaZulu-Natal Province, South Africa, over 7 breeding seasons. We predicted that living in an urban environment would increase productivity through an increase in breeding rate (shifting from typically biennial breeding to annual breeding). We then explored if there were any hidden costs associated with such a change in breeding strategy by examining the body condition of chicks from pairs that had successfully bred in the previous year. We found that pairs in more urban areas were more likely to breed annually, resulting in higher breeding rates, but were also less likely to successfully fledge a chick (i.e. lower breeding success). These 2 contrasting responses counteracted each other and resulted in similar productivity across the urbanization gradient. For those eagles that bred in consecutive years, annual breeding did not appear to have a negative cost on chick condition. The switch to annual breeding is thought to be a response to improved or more constant food sources in urban areas, while higher failure rates might be because of increased nest disturbances from anthropogenic sources (e.g., vegetation clearing, development of industrial areas, human and car traffic). However, although urbanization negatively affected the breeding success of African Crowned Eagles, they are able to persist and thrive in this highly transformed environment, likely through an increased breeding rate.
Resumen
Las áreas urbanas pueden ser atractivas para ciertas especies debido al aumento de la abundancia de alimento y de la disponibilidad para anidar, lo cual a su vez puede elevar las tasas de productividad o reproductivas. Sin embargo, también hay costos potenciales asociados con la vida urbana como mayor fracaso del nido, peor condición corporal o aumento de la prevalencia de enfermedades. Estos costos pueden hacer que las especies intercambien la cantidad de crías producidas en contra de la condición de sus crías. Stephanoaetus coronatus es un raro ejemplo de grandes y poderosos depredadores tope que crían en algunas áreas urbanas en África. En este estudio, exploramos el desempeño reproductivo de estas águilas a través de un gradiente de urbanización en la Provincia de KwaZulu-Natal, Sud África, a lo largo siete estaciones reproductivas. Predijimos que vivir en un ambiente urbano aumentaría la productividad a través de un aumento en la tasa reproductiva (cambiando de una reproducción típicamente bienal a una reproducción anual). Luego exploramos si hubo algunos costos ocultos asociados con tal cambio en la estrategia reproductiva, examinando la condición corporal de los polluelos de parejas que habían criado exitosamente el año previo. Encontramos que las parejas en las áreas más urbanas tuvieron mayor probabilidad de reproducirse anualmente, resultando en tasas reproductivas más altas, pero también tuvieron menos probabilidad de emplumar exitosamente un polluelo (i.e., menor éxito reproductivo). Estas dos respuestas contrastantes se contrarrestaron mutuamente y resultaron en una productividad similar a lo largo del gradiente de urbanización. Para aquellas águilas que se reproducen en años consecutivos, la reproducción anual no parece tener un costo negativo en la condición del polluelo. Se piensa que el cambio hacia la reproducción anual es una respuesta frente a fuentes de alimento mejores o más constantes en las áreas urbanas, mientras que las tasas más altas de fracaso podrían deberse al aumento de los disturbios de los nidos a partir de fuentes antropogénicas (e.g., clareo de la vegetación, desarrollo de áreas industriales, tráfico humano y de autos). Sin embargo, aunque la urbanización afectó negativamente el éxito reproductivo de S. coronatus, es capaz de persistir y prosperar en este ambiente altamente transformado probablemente a través de un aumento en la tasa reproductiva.
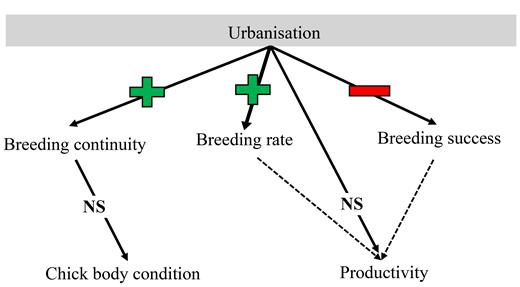
A diagram illustrating the positive effect of urbanization on breeding rate and the negative effect on breeding success of Crowned Eagles in the present study, leading to no effect on overall productivity. In addition, urbanization had a positive effect on breeding continuity, which shows a possible shift to more annualized breeding. The increase in breeding continuity apparently had no impact on chick condition. This was true for both nests that only attempted to breed in the previous year, and nests that successfully reared a chick in the previous year.
Lay Summary
• One species that is hardly recognized as an urban adapter is the Crowned Eagle in the metropoles of Durban and Pietermaritzburg, South Africa.
• We explored the breeding performance of Crowned Eagles across different levels of urbanization, and specifically teased apart breeding rate (i.e. if an eagle breeds annually or every other year) and breeding success (i.e. if they fledge a young or not in a given year).
• We showed that Crowned Eagles change their breeding strategy in urban areas by increasing their breeding rate, but found nest failures occurred more often at more urbanized sites. These contrasting responses counteracted each other and resulted in similar productivity across the urbanization gradient and highlighted the value of long-term data.
INTRODUCTION
Urbanization is a major threat to biodiversity globally (Aronson et al. 2014, Sol et al. 2014), where natural environments are generally transformed into a mosaic of highly modified anthropogenic land-use areas with some remnants of green spaces. Consequently, these urban systems are usually characterized by highly fragmented and disturbed landscapes with an increase in the amount of unproductive sealed surface area and anthropogenic infrastructure (McDonnell and Pickett 1990) and a decrease in productive area and biodiversity loss (Chace and Walsh 2006).
Different avian species show variation in their tolerance to urbanization. Some more sensitive species generally disappear entirely (McKinney 2006, Sol et al. 2014), while others are able to take advantage of the opportunities that urban habitats provide, for example increased breeding or roost sites (Chace and Walsh 2006, Altwegg et al. 2014) or generally abundant anthropogenic food resources (Chace and Walsh 2006, Donázar et al. 2016, Thabethe and Downs 2018, Stofberg et al. 2019). In order to predict how urbanization will likely impact different avian species, it is important to understand how urbanization influences their respective life history strategies. For example, urban populations of many passerine species show earlier lay dates, smaller clutch sizes, lower nestling weights, and lower productivity (Chamberlain et al. 2009). Additionally, the number of avian species exhibiting a multi-brood strategy increased with urbanization, indicating that some of these species are also able to increase their breeding rate in more urban areas (Reale and Blair 2005).
Raptors may be more sensitive than other avian taxa to urbanization, because of their generally higher trophic position and slow life histories (i.e. long-lived species with low reproductive rates; K-strategists; Newton 1998). Despite this, many raptors persist in or exploit urbanized habitats around the globe, particularly medium-sized species that specialize on avian prey (Chace and Walsh 2006, Mannan and Steidl 2018), including falcons (e.g., Cade et al. 1996, Kübler et al. 2005, Altwegg et al. 2014, Sumasgutner et al. 2014b) and accipiter hawks (e.g., Boal and Mannan 1999, Suri et al. 2017). The urban success of these species is thought to be because of a combination of factors including reduced persecution, increased availability of nesting sites, and relatively high prey availability (Kettel et al. 2018). However, larger raptors such as eagles are generally absent or relatively rare in urban areas, most likely because of their larger home range sizes, specific habitat requirements, and specialist diets (Newton 1979, Chace and Walsh 2006, Isaac et al. 2014). For some raptor species that do breed in urban areas, an “ecological trap” can emerge if, for example, (1) the availability of suitable nesting sites does not match an abundance of suitable prey (Sumasgutner et al. 2014a), or (2) urban prey items transmit diseases (van Velden et al. 2017), or (3) the available habitat cannot support a species’ overall breeding requirements (Isaac et al. 2014).
Most raptor research in urban habitats has been conducted in North America and Europe (Boal and Dykstra 2018, Kettel et al. 2018). However, findings in one region may not necessarily be representative for other regions (Mannan and Steidl 2018) as they are often only relevant to a certain set of conditions in a specific system/place (Kumar et al. 2014, 2018). Although Africa and Asia are urbanizing at an unprecedented rate (Montgomery 2008, Wan and Wang 2014), relatively few studies have addressed the ecological effects of this rapid urban growth, especially in developing countries, where levels of biodiversity are generally high (Chamberlain et al. 2009, McDonnell and MacGregor-Fors 2016).
One of the few eagle species to breed in urban areas is the globally Near Threatened African Crowned Eagle (Stephanoaetus coronatus, hereafter Crowned Eagle; Taylor et al. 2015, IUCN 2018). These forest eagles are typical K-strategists with a long lifespan, long maturation period, low reproductive rate (Swartridge 2009, McPherson et al. 2016a, 2016b), and a long post-fledgling dependency period of up to 14 mo (Skorupa 1989, Shultz 2002). As with many large raptor species, Crowned Eagles exhibit a biennial breeding strategy (i.e. breeding every other year; Newton 1979), which might be strongly influenced by food supply (McIntyre and Schmidt 2012, Murgatroyd et al. 2016). Thus, in optimum conditions they have a maximum productivity of 0.5 young per pair per year (Brown 1970).
Crowned Eagles have successfully colonized the urban mosaic of major cities in KwaZulu-Natal, South Africa (McPherson et al. 2016a, 2016b, 2019), and are known from urban sites elsewhere in Africa (e.g., Kenya; Wachira 2017). Despite the challenges posed by living in an urban mosaic, this species is seemingly thriving, with up to 120 breeding territories identified in southern KwaZulu-Natal between 2012 and 2017, including the metropolitan areas of Durban and Pietermaritzburg (McPherson et al. 2016a, S. C. McPherson personal communication). The high breeding density of urban Crowned Eagles has been linked to the Durban Metropolitan Open Space System (D’MOSS) and the distribution of community conservancies and eco-estates across the urban landscape, which offer large nesting trees and an abundance of prey species (McPherson et al. 2016a, 2016b, 2019; Alexander et al. 2019a, 2019b). These environments might provide these eagles with a high availability of easy-to-catch prey species (e.g., rock hyrax [Procavia capensis] and nestling Hadada Ibis [Bostrychia hagedash]; McPherson et al. 2016a, Van der Meer et al. 2018; as opposed to their main prey in natural systems such as antelopes or monkeys; Vernon 1984, Skorupa 1989, Swatridge et al. 2014]), which may allow urban pairs to increase their breeding rate and shift from a biennial to an annual breeding strategy. Alternatively, higher mortality of young in urban habitats might also remove the constraint of the extended post-fledging dependency period, which might result in more frequent annual breeding attempts.
Although urban areas may have higher food and nesting site availability, there may also be hidden costs to the chicks produced in these environments. For example, it is generally accepted that, given a set of resources, the number of offspring produced is inversely proportional to the investment per offspring (Smith and Fretwell 1974). Thus, if urban areas increase breeding rate, this could either lead to investing less resources per offspring, or alternatively, urban areas might provide overall more resources that could allow parents to invest similar resources per chick and thus produce offspring of similar quality irrespective of breeding rate. For example, in Arizona, although the population of Cooper’s Hawks (Accipiter cooperii) increased in urban areas, there was a greater presence of the disease trichomoniasis, which increased chick mortality (Mannan et al. 2008). Therefore, within urban areas it might be that if Crowned Eagles are breeding more frequently, they could potentially be trading off chick quantity against chick quality.
We investigated whether degree of urbanization affected either breeding rate or breeding success of Crowned Eagles. We also investigated whether any changes to breeding rate are because of urbanization increasing the number of annual breeding attempts (i.e. greater breeding continuity) and how changes in breeding rate and breeding success affect overall productivity of the species across an urbanization gradient. Furthermore, we explored whether any such changes in breeding rates may affect chick condition. If such a tradeoff between chick quantity and quality occurs, we predicted that chicks that were raised in a territory in the year following a successful breeding attempt would be in poorer condition than chicks raised following a year when there was no breeding attempt or where a breeding attempt was unsuccessful.
METHODS
Study Area and Data Collection
The study area covered ~20,000 km2 in southern KwaZulu-Natal Province, South Africa, centered on the metropoles of Durban and Pietermaritzburg, and extended to several coastal towns both north and south of Durban (Figure 1; McPherson et al. 2016a, 2016b, 2019). Crowned Eagle nesting sites were initially found by networking with interested individuals/groups (local birding experts, Birdlife and Falconry club members, and online community groups) and accessing unpublished databases from Durban Natural History Museum, eThekwini Municipality, and SABAP2, as well as by direct searching in suitable habitat or where territorial displays were observed.
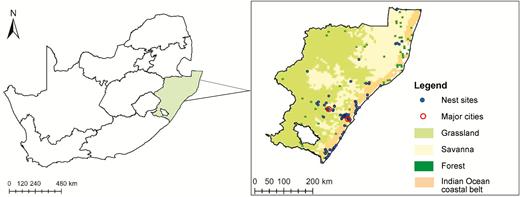
A map of South Africa, with the study area in KwaZulu-Natal Province enlarged and the biomes of the region shown. All known nest sites up until January 2017 are indicated by blue circles.
Crowned Eagle nest monitoring was conducted from August through January the following year for the years 2011 to 2017 (i.e. 7 breeding seasons), which covers their peak annual breeding period in the area (McPherson et al. 2016a, 2016b). Territories were visited regularly, at least twice in the first month, in order to assess occupation (e.g., nest building, incubation, or brooding behavior). A nest was classified as active if nest building or fresh green leaves were seen on the nest or if the adults were present in either of these first 2 nest visits. A nest was classified as having a breeding attempt if incubation or brooding behavior was seen. Nests with a breeding attempt were then monitored during 2–3 nest site visits until conclusion of the breeding event (i.e. until the chicks were around 70 ± 5 days old). Breeding success was defined as having a chick survive until banding age (70 ± 5 days old). After this age, failures in this species and most other large raptors are relatively low (Brown 1976). Nests were observed from vantage points generally 50–200 m away from the nest (see details in McPherson et al. 2016b).
Crowned Eagle chicks were banded when their estimated age was 65–75 days, a time window recommended by experts (S. Thomsett and B. Hoffman personal communication). The age estimates used in this study were ascertained by photo reference material of pulli of known age (McPherson et al. 2017) and were based on size and plumage development. During banding, chicks were weighed (with an electronic hanging scale to the nearest 5 g) and the total length and unfurled length of the eighth primary feather was taken (with a straight ruler to the nearest 1 mm). All measurements were done in accordance with the SAFRING user manual (de Beer et al. 2001).
Urbanization Score
To establish the percentage of urbanization around each nest site, we used the LandCover 2014 raster (GEOTERRAIMAGE 2015), which classifies land use into 72 different categories. We chose a circular buffer area of 10 km2 (radius = 1,784.1 m) based on the mean home range size of the species during the breeding season from 4 telemetered adults in the study area (McPherson et al. 2019). Once the percentage of each land class around each nest site had been calculated, the values for all land classes containing sealed surface (see Rose et al. 2017) were used to calculate an urban score (%) for each nest. Examples of what land classes constituted sealed surface are urban residential, industrial, townships, and mines. In territories where there was more than one nest, the mean urban score was taken to represent the territory.
Statistical Analyses
All analyses were conducted in R 3.5.1 (R Core Team 2018) with the packages lme4 (Bates et al. 2015), car (Fox and Weisberg 2018), and effects (Fox 2003). All means are presented with standard deviations (SD). Generalized linear models (GLMs) or linear mixed models (LMMs) were used to analyze the data. An initial model selection for the GLMs considered both the linear or quadratic relationship between urbanization and our response variables, as a quadratic relationship could reveal changed breeding demography at intermediate levels of urbanization. In all cases, the linear relationship had the best model fit (lowest AIC) and thus only linear relationships were considered in the final analyses.
We explored how urbanization affected several Crowned Eagle breeding parameters over 7 breeding seasons. GLMs with a binomial distribution were used to investigate the effect of urbanization on 3 key breeding parameters using the cbind function. These 3 variables were (1) breeding rates: modelled as the total number of attempts and number of non-attempts (i.e. no nest building activity [e.g., nest lining, mating behavior, incubation] at a previously occupied nest) across the years a territory was monitored; (2) breeding success: modelled as the total number of successful breeding attempts and number of failures across the years in which a territory was active; and (3) breeding continuity: modelled as the number of continuous breeding attempts (i.e. no gap between breeding attempts) and the number of noncontinuous breeding attempts (i.e. with at least 1 yr gap between breeding attempts) for the total number of years monitored. This binomial approach also accounted for differences in the number of years of data for each territory, by effectively weighting each sample according to the total number of years monitored (models 1 and 3) or total number of active years (model 2). Additionally, a different GLM was used to investigate Crowned Eagle productivity in relation to urbanization. Here the response variable was the total number of young fledged across all the years each territory was monitored. Models were fitted with a Poisson distribution, with an offset specified as the log of the number of years monitored.
An LMM was used to explore whether a Crowned Eagle breeding attempt or, more importantly, a breeding success in the previous year, had an influence on the body condition of chicks. For this LMM, the response variable was the condition of each chick (n = 72), where chick condition was the residual from a linear regression of weight against the length of the eighth primary feather. The explanatory variable was attempt (t − 1), where 0 = no attempt previous year and 1 = attempt the previous year; we also ran the same model but specifying success (t − 1), where 0 = no successful chick produced in the previous year and 1 = chick successfully produced in the previous year. “Year” and “Territory Identity” were included as random terms to account for the repeated measures from the same territory and from different territories in the same year. As Crowned Eagles only fledge one chick per breeding attempt we did not need to control for the number of chicks in a nest.
RESULTS
During the 7 breeding seasons from 2011 until 2017, we monitored a total of 56 different territories, which were each monitored on average for 6 ± 1.3 yr (range: 1–7 yr). The average urbanization score (%) for the monitored nests over the 7-yr study was 35.89 ± 23.77. The average urbanization score did not vary much between the years of the study, even as the number of nests being monitored increased (Table 1). The average weight for all chicks during the study was 2,588.13 ± 538.78 g, and the average length of the eighth primary feather was 194.66 ± 52.40 mm. The average chick body condition did vary across the years of the study. However, the large standard deviations for all years suggest that the variation was larger within years than between years (Table 1). The average breeding rate and breeding success for all years of the study was 0.685 ± 0.08 and 0.686 ± 0.13, respectively (Figure 2). Between the years of the study period the average breeding rate and breeding success did not show much variation (Figure 2). However, in 2012 the breeding rate was lower than in all other years, although breeding success in that year was similar to other years (Figure 2). In 2015 breeding success was lower than other years despite a similar breeding rate (Figure 2).
A summary table showing number of territories monitored each year, number of breeding attempts made, number of chicks produced, average chick body condition, and average urbanization score across all the territories monitored in that year, for each year of data collection. Body condition was calculated as the residuals from a linear regression of the eighth primary feather against weight (these measurements were gathered whenever a chick was ringed at 70 ± 5 days old).
| Year . | Number of territories monitored . | Number of breeding attempts . | Number of chicks produced . | Chick condition (Mean ± SD) . | Urbanization score (%) (Mean ± SD) . |
|---|---|---|---|---|---|
| 2011 | 37 | 27 | 22 | −74.8 ± 194.82 | 39.2 ± 21.63 |
| 2012 | 41 | 21 | 15 | −76.3 ± 201.34 | 40.6 ± 21.42 |
| 2013 | 50 | 37 | 29 | −75.5 ± 216.06 | 35.5 ± 22.90 |
| 2014 | 52 | 38 | 31 | 142.2 ± 235.17 | 35.8 ± 24.29 |
| 2015 | 55 | 39 | 19 | 218.8 ± 279.02 | 34.5 ± 24.89 |
| 2016 | 56 | 40 | 26 | −168.7 ± 323.13 | 34.0 ± 24.98 |
| 2017 | 56 | 37 | 20 | −45.3 ± 184.00 | 34.0 ± 24.98 |
| Year . | Number of territories monitored . | Number of breeding attempts . | Number of chicks produced . | Chick condition (Mean ± SD) . | Urbanization score (%) (Mean ± SD) . |
|---|---|---|---|---|---|
| 2011 | 37 | 27 | 22 | −74.8 ± 194.82 | 39.2 ± 21.63 |
| 2012 | 41 | 21 | 15 | −76.3 ± 201.34 | 40.6 ± 21.42 |
| 2013 | 50 | 37 | 29 | −75.5 ± 216.06 | 35.5 ± 22.90 |
| 2014 | 52 | 38 | 31 | 142.2 ± 235.17 | 35.8 ± 24.29 |
| 2015 | 55 | 39 | 19 | 218.8 ± 279.02 | 34.5 ± 24.89 |
| 2016 | 56 | 40 | 26 | −168.7 ± 323.13 | 34.0 ± 24.98 |
| 2017 | 56 | 37 | 20 | −45.3 ± 184.00 | 34.0 ± 24.98 |
A summary table showing number of territories monitored each year, number of breeding attempts made, number of chicks produced, average chick body condition, and average urbanization score across all the territories monitored in that year, for each year of data collection. Body condition was calculated as the residuals from a linear regression of the eighth primary feather against weight (these measurements were gathered whenever a chick was ringed at 70 ± 5 days old).
| Year . | Number of territories monitored . | Number of breeding attempts . | Number of chicks produced . | Chick condition (Mean ± SD) . | Urbanization score (%) (Mean ± SD) . |
|---|---|---|---|---|---|
| 2011 | 37 | 27 | 22 | −74.8 ± 194.82 | 39.2 ± 21.63 |
| 2012 | 41 | 21 | 15 | −76.3 ± 201.34 | 40.6 ± 21.42 |
| 2013 | 50 | 37 | 29 | −75.5 ± 216.06 | 35.5 ± 22.90 |
| 2014 | 52 | 38 | 31 | 142.2 ± 235.17 | 35.8 ± 24.29 |
| 2015 | 55 | 39 | 19 | 218.8 ± 279.02 | 34.5 ± 24.89 |
| 2016 | 56 | 40 | 26 | −168.7 ± 323.13 | 34.0 ± 24.98 |
| 2017 | 56 | 37 | 20 | −45.3 ± 184.00 | 34.0 ± 24.98 |
| Year . | Number of territories monitored . | Number of breeding attempts . | Number of chicks produced . | Chick condition (Mean ± SD) . | Urbanization score (%) (Mean ± SD) . |
|---|---|---|---|---|---|
| 2011 | 37 | 27 | 22 | −74.8 ± 194.82 | 39.2 ± 21.63 |
| 2012 | 41 | 21 | 15 | −76.3 ± 201.34 | 40.6 ± 21.42 |
| 2013 | 50 | 37 | 29 | −75.5 ± 216.06 | 35.5 ± 22.90 |
| 2014 | 52 | 38 | 31 | 142.2 ± 235.17 | 35.8 ± 24.29 |
| 2015 | 55 | 39 | 19 | 218.8 ± 279.02 | 34.5 ± 24.89 |
| 2016 | 56 | 40 | 26 | −168.7 ± 323.13 | 34.0 ± 24.98 |
| 2017 | 56 | 37 | 20 | −45.3 ± 184.00 | 34.0 ± 24.98 |
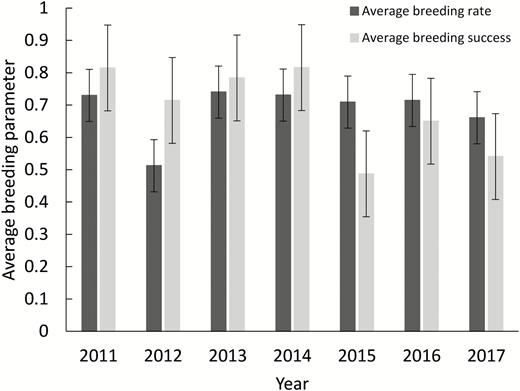
The average breeding rate (± SD) and breeding success (± SD) of all monitored territories monitored in each year of the study. Breeding rate is probability of breeding given the territory was monitored, and breeding success is probability the territory produced a fledged chick given there was a breeding attempt.
Crowned Eagles in more urban areas bred at a significantly higher rate than those in less urban areas (Table 2, Figure 3A); however, Crowned Eagle breeding success decreased significantly with increasing urbanization (Table 2, Figure 3B). The increase in breeding rate was driven by a significant increase in breeding continuity (the frequency of consecutive breeding attempts) as this increased with increasing urbanization (Table 2, Figure 4A).
Results of the GLMs and LMM showing the relationships between urbanization gradient and demographic parameters (breeding rate, breeding success, breeding continuity, productivity) and chick condition of Crowned Eagles in KwaZulu-Natal Province during the 2011–2017 breeding seasons. Quantitative variables were scaled.
| Model . | Error structure . | Estimate . | SE . | χ 2 . | P . |
|---|---|---|---|---|---|
| Breeding rate | Binomial | ||||
| Intercept | 0.323 | 0.203 | 0.112 | ||
| Urbanization | 0.014 | 0.005 | 7.628 | 0.006 | |
| Breeding success | Binomial | ||||
| Intercept | 1.315 | 0.289 | <0.001 | ||
| Urbanization | −0.014 | 0.006 | 5.641 | 0.019 | |
| Breeding continuity | Binomial | ||||
| Intercept | −0.699 | 0.223 | 0.001 | ||
| Urbanization | 0.015 | 0.005 | 9.256 | 0.002 | |
| Productivity | Poisson | ||||
| Intercept | −0.756 | 0.142 | <0.001 | ||
| Urbanization | −0.0001 | 0.003 | 0.962 | 0.962 | |
| Chick body condition | Gaussian | ||||
| Intercept | −0.055 | 0.260 | 0.045 | 0.832 | |
| Attempt (t − 1) | 0.144 | 0.124 | 1.356 | 0.244 | |
| Intercept | −0.046 | 0.249 | 0.034 | 0.853 | |
| Success (t − 1) | 0.089 | 0.126 | 0.498 | 0.480 |
| Model . | Error structure . | Estimate . | SE . | χ 2 . | P . |
|---|---|---|---|---|---|
| Breeding rate | Binomial | ||||
| Intercept | 0.323 | 0.203 | 0.112 | ||
| Urbanization | 0.014 | 0.005 | 7.628 | 0.006 | |
| Breeding success | Binomial | ||||
| Intercept | 1.315 | 0.289 | <0.001 | ||
| Urbanization | −0.014 | 0.006 | 5.641 | 0.019 | |
| Breeding continuity | Binomial | ||||
| Intercept | −0.699 | 0.223 | 0.001 | ||
| Urbanization | 0.015 | 0.005 | 9.256 | 0.002 | |
| Productivity | Poisson | ||||
| Intercept | −0.756 | 0.142 | <0.001 | ||
| Urbanization | −0.0001 | 0.003 | 0.962 | 0.962 | |
| Chick body condition | Gaussian | ||||
| Intercept | −0.055 | 0.260 | 0.045 | 0.832 | |
| Attempt (t − 1) | 0.144 | 0.124 | 1.356 | 0.244 | |
| Intercept | −0.046 | 0.249 | 0.034 | 0.853 | |
| Success (t − 1) | 0.089 | 0.126 | 0.498 | 0.480 |
Results of the GLMs and LMM showing the relationships between urbanization gradient and demographic parameters (breeding rate, breeding success, breeding continuity, productivity) and chick condition of Crowned Eagles in KwaZulu-Natal Province during the 2011–2017 breeding seasons. Quantitative variables were scaled.
| Model . | Error structure . | Estimate . | SE . | χ 2 . | P . |
|---|---|---|---|---|---|
| Breeding rate | Binomial | ||||
| Intercept | 0.323 | 0.203 | 0.112 | ||
| Urbanization | 0.014 | 0.005 | 7.628 | 0.006 | |
| Breeding success | Binomial | ||||
| Intercept | 1.315 | 0.289 | <0.001 | ||
| Urbanization | −0.014 | 0.006 | 5.641 | 0.019 | |
| Breeding continuity | Binomial | ||||
| Intercept | −0.699 | 0.223 | 0.001 | ||
| Urbanization | 0.015 | 0.005 | 9.256 | 0.002 | |
| Productivity | Poisson | ||||
| Intercept | −0.756 | 0.142 | <0.001 | ||
| Urbanization | −0.0001 | 0.003 | 0.962 | 0.962 | |
| Chick body condition | Gaussian | ||||
| Intercept | −0.055 | 0.260 | 0.045 | 0.832 | |
| Attempt (t − 1) | 0.144 | 0.124 | 1.356 | 0.244 | |
| Intercept | −0.046 | 0.249 | 0.034 | 0.853 | |
| Success (t − 1) | 0.089 | 0.126 | 0.498 | 0.480 |
| Model . | Error structure . | Estimate . | SE . | χ 2 . | P . |
|---|---|---|---|---|---|
| Breeding rate | Binomial | ||||
| Intercept | 0.323 | 0.203 | 0.112 | ||
| Urbanization | 0.014 | 0.005 | 7.628 | 0.006 | |
| Breeding success | Binomial | ||||
| Intercept | 1.315 | 0.289 | <0.001 | ||
| Urbanization | −0.014 | 0.006 | 5.641 | 0.019 | |
| Breeding continuity | Binomial | ||||
| Intercept | −0.699 | 0.223 | 0.001 | ||
| Urbanization | 0.015 | 0.005 | 9.256 | 0.002 | |
| Productivity | Poisson | ||||
| Intercept | −0.756 | 0.142 | <0.001 | ||
| Urbanization | −0.0001 | 0.003 | 0.962 | 0.962 | |
| Chick body condition | Gaussian | ||||
| Intercept | −0.055 | 0.260 | 0.045 | 0.832 | |
| Attempt (t − 1) | 0.144 | 0.124 | 1.356 | 0.244 | |
| Intercept | −0.046 | 0.249 | 0.034 | 0.853 | |
| Success (t − 1) | 0.089 | 0.126 | 0.498 | 0.480 |
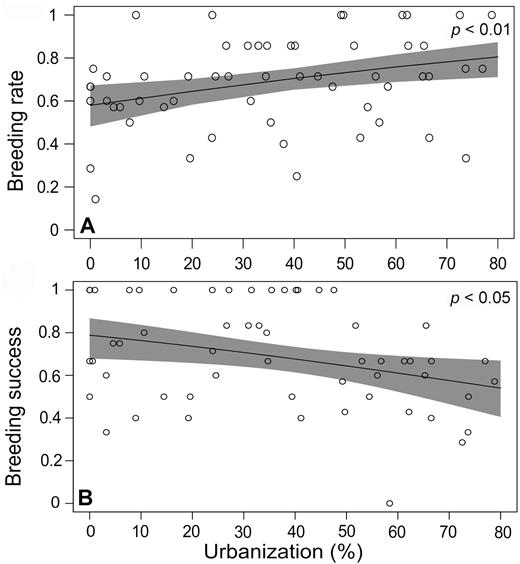
Relationship between urbanization and breeding parameters. Urbanization (%) is associated with increases in breeding rate (A) but is associated with decreases in breeding success (B) of Crowned Eagles in KwaZulu-Natal Province during the 2011–2017 breeding seasons. Breeding rate is the proportion of breeding attempts out of the years monitored for each territory, and breeding success is the number of fledged chicks out of the number of years with a breeding attempt. The line is the predicted relationship based on the output of the GLM, with 95% CIs in shaded gray. Open circles represent the observed values per territory.
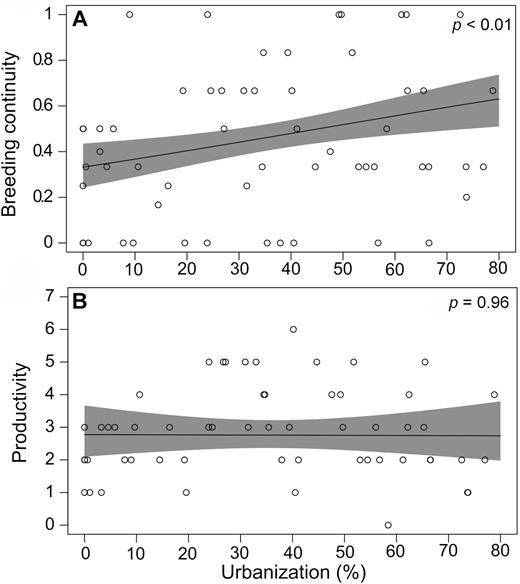
Relationship between urbanization and breeding parameters. Urbanization (%) is associated with increases in breeding continuity (A) but has no association with overall productivity (B) of Crowned Eagles in KwaZulu-Natal Province during the 2011–2017 breeding seasons. Breeding continuity is the proportion of consecutive breeding attempts (i.e. back-to-back years of breeding attempts) out of the total number of years monitored. Overall productivity is the total number of young fledged across all the years a territory was monitored. The line is the predicted relationship based on the output of the GLM, with 95% CIs in shaded gray. Open circles represent the observed values per territory.
Overall, Crowned Eagles in urban areas attempted to breed more often and had more consecutive breeding attempts (i.e. higher breeding frequency), but these attempts were less successful. As a result, overall productivity was similar across the urban gradient (Table 2, Figure 4B). No relationship was seen between Crowned Eagle chick condition and a breeding attempt the previous year (attempt [t − 1]), nor between chick condition and whether there was a successful attempt in the previous year (success [t − 1]) (Table 2, Figure 5).
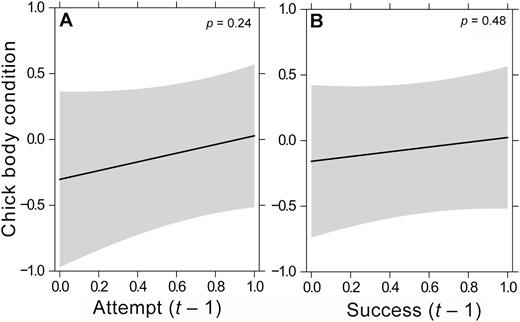
Relationship between chick body condition and (A) attempt the previous year (attempt (t − 1)) and (B) success of a breeding attempt the previous year (success (t − 1)) for Crowned Eagles in KwaZulu-Natal Province during the 2011–2017 breeding seasons, based on the predicted values of the LMM, with 95% CIs in shaded gray.
DISCUSSION
Our analyses revealed contrasting effects of urbanization on breeding rate and breeding success, which resulted in similar overall productivity across the urban gradient for Crowned Eagles. Crowned Eagle pairs nesting in more urban habitats were able to breed more often than pairs nesting in less urbanized habitats, which was achieved via a shift from a biennial toward an annual breeding strategy. However, while urbanization appeared to be beneficial for one parameter (breeding rate), there were negative effects of urbanization on breeding success, with more nest failures in more urbanized areas. These findings have implications for other studies examining the breeding productivity of raptors in relation to urbanization as few studies have attempted to tease apart the relationship between breeding rate and breeding success (Kettel et al. 2018). This is perhaps not surprising, given that among terrestrial birds, only large eagles and some vultures are known to regularly use a biennial breeding strategy (Hustler and Howells 1987, Kruger and Amar 2017). The pattern seen in our study, whereby urbanization led to improvement of one breeding parameter but a decline in another, has been observed for other urban breeding raptors. For example, Boal and Mannan (1999) found that urban Cooper’s Hawks nesting in urban habitats laid earlier and bigger clutches than non-urban pairs, but also had higher nestling mortality and thus lower nesting success. Similarly, Sumasgutner et al. (2014a) found earlier arrival dates and higher densities of Eurasian Kestrels (Falco tinnunculus) nesting in more urbanized habitat but lower breeding success of birds that bred there, which could not be explained by density dependence alone. Thus, for both these species, urban habitats were acting as ecological traps whereby they were initially attracted to the site, and started to do well, but overall they paid an unexpected cost by breeding in such habitats (Battin 2004). In contrast, however, within our system, there did not appear to be an ecological trap, since overall productivity was similar across the urbanization gradient.
As far as we are aware, the shift toward annual breeding in urban habitats has not been documented before. However, increased breeding frequency has been noted in smaller species that double brood in urban environments and are not known to do so in more natural areas. For example, Black Sparrowhawks (Accipiter melanoleucus) nesting in urban areas in South Africa have been shown to sometimes produce 2 broods (Curtis et al. 2005) in a single, albeit protracted, breeding season (Martin et al. 2014). Curtis et al. (2005) suggested that this might be a response to relatively abundant food in their urban study area (pigeons and doves in urban gardens; Suri et al. 2017), and multiple brooding has also been recorded for raptors who capitalize on periods of prolonged food abundance when they occur (e.g., Black-shouldered Kite [Elanus axillaris]; Mendelsohn 1981).
Similar to the proposed mechanism for double brooding, higher prey availability may be the mechanism driving increased breeding rate in Crowned Eagles in urban settings within our system. Kettel et al. (2018) suggested that urban areas offer ample prey for raptors, but principally for avian prey specialists. However, the review was based on studies conducted almost exclusively in northern temperate cities and does not include a single study from the African continent, whereas tropical cities, such as KwaZulu-Natal, might have entirely different prey species compositions. For example, in Durban, rock hyrax often proliferate in stone walls and culverts in suburbia (Malan et al. 2016), the relatively large Hadada Ibis (>1 kg) are common in urban areas (Singh and Downs 2016), and vervet monkey (Chlorocebus pygerythrus) populations occur at relatively high densities (Smithers 2012, Patterson et al. 2018). Additionally, fenced luxury housing estates and eco-estates often host duiker (Cephalophinae) and other small antelope—a favored prey item of Crowned Eagles (Malan et al. 2016, Alexander et al. 2019c). Thus, within our study area, prey suitable for Crowned Eagles appear diverse and abundant. Indeed, the continuous availability of certain prey species, including principal prey species such as rock hyrax, in transformed habitats is thought to be a major contributor as to why Crowned Eagles are able to persist in more transformed habitats (Reeves and Boshoff 2016).
These urban prey species, specifically rock hyrax and Hadada Ibis fledglings, are potentially easy to hunt, especially as Crowned Eagles are generally sit-and-wait predators using the green spaces in the urban mosaic (McPherson et al. 2019). This may afford greater hunting opportunities in the urban areas for breeding Crowned Eagle adults but may also influence the post-fledging dependency period of juveniles. The post-fledging dependency period is a vital period in all raptors’ lives where they learn to hunt and become independent (Amar et al. 2000, Malan and Shultz 2002). It has been suggested that juvenile Crowned Eagles might have a shorter post-fledgling dependency period because of the availability of easy to catch prey in urban areas (Malan 2005). If this dependency period was shortened, this may explain the increased breeding rates, because it may allow parents to regain their breeding condition more quickly and because they would not have a dependent juvenile (Pickford et al. 1989, Skorupa 1989). The length of the post-fledging dependency period for this species is not yet well established and is largely based on anecdotal field observations. Future research should aim to more rigorously understanding the duration of the period, how it varies with local habitat/habitat heterogeneity and prey availability, and whether variation in this period relates to annual breeding in more urbanized pairs.
An alternative explanation for the increased breeding rate in more urban areas links directly to our observation of a decrease in breeding success in these habitats. In most animals, reproduction comes at a physiological cost to the adults (Gustafsson et al. 1994, Erikstad et al. 1998); thus, when early nest failures occur, parents will have invested less resources in that breeding attempt, and thus can quickly regain breeding condition before the next season. The relative higher rates of nest failures in the urban habitat could therefore lead to the higher breeding rates observed. In other eagle species, a failed attempt in one year was more often followed by a breeding attempt the following year (i.e. an annual breeding strategy) than after a successful attempt (i.e. a biennial breeding strategy; Newton 1979), as observed for Martial Eagles (Polemaetus bellicosus; Hustler and Howells 1987). In the present study, of the known nest failures, approximately half occurred during incubation and half during the chick-rearing period (C. T. Downs personal observation). However, given that we did record successful nesting in repeated years on several occasions, this is unlikely to fully explain the occurrence of annual breeding seen in this population. The increase in nest failures could also be linked to the heterogeneity of nesting sites. Breeding territories often differ substantially in quality and this can have a direct effect on the successfulness of breeding/nesting attempts (Krüger and Lindström 2001). Actual causes of nest failures of Crowned Eagles in the present study were poorly known. One potential explanation is that Crowned Eagles in urban areas are generally more vulnerable to nest disturbance because of human activities such as infrastructure development (e.g., installation and maintenance of pipelines and cabling), alien vegetation clearing, and greater human presence in urban forests (e.g., for forest resource gathering, dog walking, bird watching). Furthermore, urban nest sites may be more susceptible to the occurrence of irregular extreme weather events, such as wind and hail during thunderstorms, as well as extreme heat. These weather events may be further intensified by the urban heat island effect (McCarthy et al. 2010). Crowned Eagles mainly nest in exotic blue gum trees (Eucalyptus saligna), which are subject to government schemes of invasive tree removal (Turpie et al. 2008, McPherson et al. 2016b). Ring barking results in rapid defoliation while trees remain standing for many years, potentially compounding exposure and the effects of human disturbance and extreme weather events.
Lastly, our present study revealed no difference in Crowned Eagle chick condition between nests that previously fledged a young or not, or those where a breeding attempt was made in the previous year or not. This suggests that there is no obvious tradeoff between the number of young produced and the quality of those young, at least during this examined stage of the nestling phase (after two-thirds of the nestling phase—when chicks were banded). Based on the theory of Smith and Fretwell (1974), Crowned Eagles could produce more chicks or produce them in quicker succession either through increased resources or decreased investment per offspring. As chick body condition did not differ, it points to 2 possibilities. First, increased resources may have allowed Crowned Eagles to allocate the same level of investment per chick and they may be able to do this more often, sometimes in back-to-back breeding attempts. Second, adult Crowned Eagles may be investing more resources into producing chicks in back-to-back breeding attempts and investing less in self-maintenance. This could be impacting their own survival. Unfortunately, we do not have data on adult body condition to further explore these 2 possible impacts of the increased frequency of breeding attempts.
This study has shown the complex nature of breeding parameters in a large predatory raptor. It appears that because of the contrasting effects that urbanization has on breeding rate and breeding success, this species does not face an immediate challenge in continuing to be productive in urban areas. However, the long-term effects on the species’ persistence in urban areas are still unknown. Urbanization may yet prove to be detrimental to this species because of the decrease in breeding success seen in our study. The reproductive tradeoffs involved for birds breeding in urban areas have rarely been examined in detail, and it is still unclear how they interact with each other and what that may mean for the quality of chicks being produced. Thus, understanding such tradeoffs in this and other urban avian systems would allow us to fully appreciate how species can exist within such transformed environments.
ACKNOWLEDGMENTS
We would like to thank Victoria Country Club Estate; Zimbali Estate Management Association; Cotswold Downs Estate and Kloof Conservancy for their continued interest and support in this project. B. Padbury and numerous members of the Natal Falconers Club, R. McKibbin, D. Allan of the Durban Natural Science Museum, Birdlife Port Natal, Birdlife KZN Midlands, Kloof Conservancy, B. Coverdale of Ezemvelo KZN Wildlife, and N. Leidenberg who provided some of the nest locations. We would also like to thank our field assistants: C. Fritsch, N. Evans, M. Witteveen, L. Thompson, K. Beer, T. Caine, T. van der Meer, M. Jessen, P. Banville, T. Kunca, E. Bourret, A. White, L. Bambini, and A. Pickles. We are most grateful to the reviewers for their constructive comments that improved the manuscript.
Funding statement: Funding was provided by the National Research Foundation (ZA), D’RAP Ethekwini Municipality, University of KwaZulu-Natal, and the FitzPatrick Institute of African Ornithology at the University of Cape Town. P. Sumasgutner was supported by postdoc fellowships of the Claude Leon Foundation and the DSF-NRF Centre of Excellence. We thank the Ford Wildlife Foundation (ZA) for vehicle support.
Ethics statement: All data were obtained with techniques approved and permits obtained from Ezemvelo KZN Wildlife (OP3731/2018), SAFRING (ID numbers S. McPherson – 1619; P. Sumasgutner – 17600), and the University of KwaZulu-Natal Animal Ethics Committee (083/12/animal; 020/15/animal) and the University of Cape Town Animal Ethics Committee (2018/v21/AA).
Author contributions: CTD, AA, PS, and SM for conception of the question, supervision of research and substantial editing of the paper. SM and to a lesser extent PS and RM for data collection. RM for doing all analyses and writing the draft paper. CTD and SM for sourcing funding.
Data depository: Analyses reported in this article can be reproduced using the data provided by Muller et al. (2020).



