-
PDF
- Split View
-
Views
-
Cite
Cite
James R. Wright, Christopher M. Tonra, Luke L. Powell, Prealternate molt-migration in Rusty Blackbirds and its implications for stopover biology, The Condor: Ornithological Applications, Volume 120, Issue 3, 1 August 2018, Pages 507–516, https://doi.org/10.1650/CONDOR-17-177.1
Close - Share Icon Share
Abstract
To achieve greater understanding of the full annual cycles of birds, it is critical to describe the spatial nature of little-understood phases. One of the least understood aspects of avian annual cycles is the ecology of molt: the periodic replacement of feathers. While work on the spatial nature of molt in migratory passerines has increasingly found incidences of species and populations completing molt during migration, this work has been limited entirely to prebasic flight feather molt. We examined the prevalence and progression of contour feather molt in a migratory songbird, the Rusty Blackbird (Euphagus carolinus), during spring stopover. We found that 98% of birds exhibited a partial prealternate molt during stopover, primarily in the head region. Furthermore, molt intensity peaked in the middle of the migration period and was negatively associated with fat score. This is the first evidence in the passerine literature of an obligate prealternate molt completed during migration, which is in many ways similar to the molt strategy of a variety of shorebirds (Families Charadriidae and Scolopacidae). These findings could prove crucial to understanding the constraints on spring migration in this declining species. Furthermore, we argue that molt schedules such as those of the Rusty Blackbird and shorebirds should be referred to as “prealternate molt-migration,” broadening the traditional definition of molt-migration beyond prebasic flight feather molt.
Résumé
Afin de mieux comprendre les cycles annuels complets des oiseaux, il est essentiel de décrire la nature spatiale des phases encore mal connues. L'un des aspects les moins bien compris des cycles annuels des oiseaux est l'écologie de la mue : le remplacement périodique des plumes. Alors que les travaux sur la nature spatiale de la mue chez les passereaux migrateurs ont fourni de plus en plus de preuves que des espèces et des populations complètent la mue durant la migration, ces études se sont limitées à la mue postnuptiale des plumes de vol. Nous avons examiné la prévalence et la progression de la mue des plumes de contour chez un oiseau chanteur migrateur, Euphagus carolinus, au cours d'une halte migratoire au printemps. Nous avons trouvé que 98 % des oiseaux présentaient une mue prénuptiale partielle au cours de la halte migratoire, principalement de la région de la tête. De plus, l'intensité de la mue a atteint un sommet au milieu de la période de migration et était négativement associée au taux de lipides. Ceci est la première preuve dans la littérature sur les passereaux d'une mue prénuptiale obligée complétée au cours de la migration, ce qui est similaire à plusieurs égards à la stratégie de mue d'une grande variété de limicoles (familles des Charadriidés et des Scolopacidés). Ces résultats pourraient s'avérer essentiels pour la compréhension des contraintes sur la migration printanière chez cette espèce en déclin. Par ailleurs, nous affirmons que les calendriers de mue comme ceux d'E. carolinus et les limicoles devraient être qualifiés de « mue prénuptiale-migration », ce qui élargirait la définition traditionnelle de la mue-migration au-delà de la mue postnuptiale des plumes de vol.
Mots-clés: cycles annuels, Euphagus carolinus, stratégies de mue, mue-migration, halte migratoire
Introduction
Biologists are increasingly recognizing the need to incorporate all portions of the annual cycle into animal research and conservation. However, this requires extensive research to describe the basic ecology of species, including the spatial aspects of where key stages of the cycle are completed (Marra et al. 2015). One such stage for birds is molt: the periodic replacement of feathers (Humphrey and Parkes 1959, Howell et al. 2003). Molt is an energetically expensive process (Dietz et al. 1992, Vézina et al. 2009) important to flight performance (e.g., Tucker 1991, Swaddle et al. 1996), conspecific interactions (Hill and McGraw 2006a), thermoregulation (e.g., Vézina et al. 2009), and resistance to parasites (e.g., Gunderson et al. 2008). Ecological aspects of molt, such as the acquisition of key molecules for feather color and strength (Hill and McGraw 2006b, McGraw 2007), can only be understood in the context of where these processes occur. Given the importance of molt to other parts of the life cycle through processes such as sexual selection and parasite resistance, it is vital to ecologists and managers that researchers identify where and when molt occurs.
Molt and plumage cycles are quite diverse among avian taxa, including very complex, age-specific plumage cycles in raptors, herons, and woodpeckers (Pyle 1997, 2008). Most birds, apart from pelagic seabirds, have 1 of 2 basic molt cycles: Complex Basic or Complex Alternate Strategies (CBS or CAS, as defined by Howell et al. 2003). For adults with these molt cycles (i.e. definitive molts), feathers are either kept throughout the year, with one annual replacement of all flight and body feathers (prebasic molt; CBS), or body feathers are additionally replaced a second time, usually prior to breeding (prealternate molt; CAS; Howell et al. 2003).
A common assumption about CBS and CAS molts is that they occur at the conclusion of stationary segments of the life cycle (i.e. at or near breeding and overwintering sites). However, there is increasing evidence that molt can also occur during migratory stopover, or after movement to specific “molting grounds,” in a variety of taxa (“molt-migration”; e.g., Salomonsen 1968, Leu and Thompson 2002, Reed et al. 2003, Pyle et al. 2009, Quinlan and Green 2011, Lourenço and Piersma 2015, Nordell et al. 2016, Pillar et al. 2016). For instance, several passerine species in western North America, such as the Bullock's Oriole (Icterus bullockii; Pillar et al. 2016) and Black-headed Grosbeak (Pheucticus melanocephalus; Siegel et al. 2016), pause their southward migration to wintering areas in the monsoon region of northwestern Mexico to complete prebasic molt. To date, however, the term “molt-migration” has been exclusively applied to prebasic flight feather molt during fall migration, or prealternate “eclipse” flight feather molt in such birds as waterfowl and grebes (Jehl 1990, P. Pyle personal communication).
While the term molt-migration has not typically been applied to the molt of shorebirds (Families Charadriidae and Scolopacidae), many species have long been known to pause their northward migration and complete prealternate molt at staging areas (e.g., Lourenço and Piersma 2015). Furthermore, a recent study has suggested that some hummingbirds (Family Trochilidae) also undergo a partial prealternate molt during fall migration (Sieburth and Pyle 2018). This raises the question of how broadly the term molt-migration should be applied, and, in turn, how pervasive molt is during prebreeding migration in other taxa. Prealternate molts are important evolutionarily and ecologically, as they usually replace cryptic basic plumage feathers with more colorful or bright feathers that often play a role in sexual selection and male–male competition (Serra et al. 2007). Despite the prevalence of this pattern in other taxa, in Passeriformes virtually all species are assumed or known to replace the body and head feathers involved in prealternate molt on the wintering grounds (e.g., Mowbray 1997, Mazerolle et al. 2005, Boone et al. 2010).
For the genus Euphagus (Rusty Blackbird [E. carolinus] and Brewer's Blackbird [E. cyanocephalus]), species accounts do not recognize the existence of an obligate prealternate molt (Avery 2013, Martin 2002). While acknowledging that some individuals replace feathers, most plumage changes from winter to breeding are attributed to feather wear. However, the existence of at least a facultative prealternate molt on the wintering grounds has been described for the Rusty Blackbird (Mettke-Hoffman et al. 2010). Rusty Blackbird annual cycles are of interest, considering that the species has been and continues to be the fastest declining songbird in North America since the mid-20th century, yet the cause of this decline remains enigmatic (Greenberg et al. 2011). Thus, it is important to understand the factors that contribute to the species' migratory decisions, such as the nature of the prealternate molt, as well as any potential carryover effects on breeding success (e.g., sexual selection).
We sampled Rusty Blackbirds during their prebreeding (spring) migration to search for evidence of molt. We quantified the prevalence and intensity of contour feather molt to determine whether there was a continuation of prealternate molt beyond the wintering period in these passerine birds and, if so, the extent to which it occurred in the population (i.e. facultative vs. obligate molt). We made several predictions regarding the patterns and timing of prealternate molt. Rusty Blackbirds exhibit strong sexual dichromatism (Avery 2013), indicating a potential role for plumage in intrasexual competition or sexual selection. Since sexual dichromatism often arises from male–male competition or female preference for male plumage characteristics (Savalli 1995), we predicted that molt during migration would be more intense in male than female blackbirds. Endocrine and energetic conflicts exist between molt and breeding, such as decreased prolactin associated with molt but elevated prolactin associated with chick rearing (Williams 2012). Thus, we predicted that the intensity of molt would decline as the migratory season progressed (i.e. as breeding approached) and would be negatively associated with migratory fattening.
Methods
Study Site and Field Methods
We captured Rusty Blackbirds over the course of their spring migration (March to May) in 2016 and 2017 at a high-traffic stopover site in northern Ohio, USA. Our selection of the site was based on data from the Rusty Blackbird Spring Migration Blitz (Evans 2016), a citizen science–based effort (developed by the International Rusty Blackbird Working Group and implemented using eBird checklists) to closely monitor spring migration across the species' range from 2014 to 2016. The Blitz identified Ottawa National Wildlife Refuge (41.608°N, 83.208°W), on the southwestern shore of Lake Erie, as receiving among the highest number of migrant Rusty Blackbirds in North America. This site consists of a managed complex of wetland habitat types, ∼28 km2 in size, in a region that is dominated by agricultural development.
We captured birds passively in mist nets on 7 separate occasions from March 15 to May 5 in 2016 and on 4 occasions from March 12 to April 14 in 2017. Our capture effort depended on the ease of locating foraging flocks, but usually consisted of opening six 60-mm-mesh mist nets for 4–8 hr on capture days. We banded birds with uniquely numbered U.S. Geological Survey aluminum bands, and recorded morphometric measurements for each individual, of which mass (± 0.1 g) and wing chord (± 0.5 mm) were included in final analyses. Fat content was recorded for each bird on a scale of 0–8 (Kaiser 1993). We examined each bird for active body molt by blowing on the feathers and searching for newly emerged pinfeathers in each body patch (Berthold et al. 1970, Mettke-Hoffman et al. 2010), and recorded all patches in molt for all captured birds (17 feather patches total; Figure 1). We calculated the intensity of body molt using a variation of the Greenwood et al. (1983) method of combined contour molt index (CCMI). In brief, we estimated the percentage of feathers in active molt for a given patch of body feathers, and then summed all patch percentages and divided by 17 (the total number of patches) to produce an overall CCMI score on a scale of 0 to 100. Eye-ring and lore molt were included in the “cheek” patch, forehead included in the “crown” patch, and the nape and side of the neck were recorded together as “nape & neck.” At the outset of the 2016 field season, we had not developed the CCMI method, so CCMI scores were not recorded for the first 13 birds (15% of all captures); however, the total number of patches in molt was recorded for all captured birds. All birds were aged and sexed using plumage characteristics described by Pyle (1997) and Mettke-Hoffman et al. (2010).
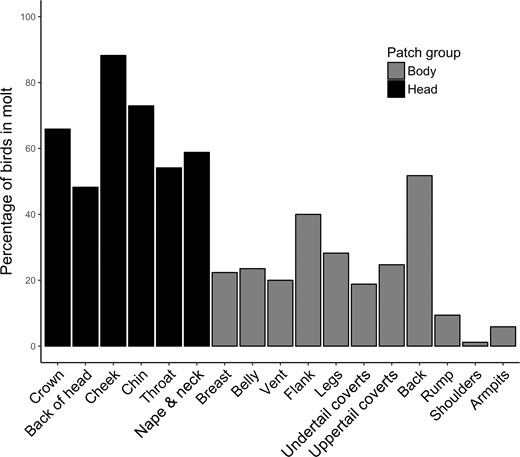
Percentage of Rusty Blackbirds molting various feather patches (n = 85 individuals) at Ottawa National Wildlife Refuge, Lucas County, Ohio, USA, in the springs of 2016 and 2017. Feather patches located on the head are represented by black bars, whereas feather patches on the body are represented by gray bars.
In addition to the CCMI intensity score for individuals, we also calculated the prevalence of molt by patch. Specifically, we calculated the proportion of birds actively molting each feather patch, regardless of the intensity of molt within that patch. Then, to compare the molt intensity within a patch only among birds actively molting that patch, we averaged the patch percentage ratings for all birds for which CCMI scores were recorded (n = 72); individuals that were not molting that patch were excluded from this average patch intensity calculation. Measurements of CCMI and patch percentage scores are reported as means ± SE.
Statistical Analysis
For the purposes of statistical analyses, our response variable was the square root transformation of CCMI (for normality of residuals), regressed against the independent variables of ordinal date of capture, year, sex, age, fat score, and body condition. Body condition was calculated as a scaled mass index, with wing chord used as our measure of structural body size (Peig and Green 2009). We pooled the sexes for this correction, as the slope of the body size–mass relationship did not differ between males and females (P = 0.58). To test for the effects of the independent variables on CCMI, we built multiple linear regression models using program R 3.4.1 (R Core Team 2017).
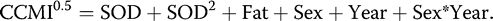
Results
We captured a total of 85 individuals over the course of 2 seasons: 56 in 2016, and 29 in 2017. This total included 37 males and 48 females. Of these 85 birds, 83 (98%) were molting body and/or head feathers. The mean ± SE score for birds for which CCMI was recorded (n = 72) was 13.5 ± 1.2 (range: 0.0–41.2). One local individual captured twice in 2016 had a CCMI score of 12.9 upon initial capture on April 12, and a score of 0.6 upon recapture 23 days later. The data from the recapture event were removed from all analyses.
Our multiple regression model explained 27% of the variation in molt intensity (adjusted R2 = 0.27, F6,65 = 5.28, P < 0.001; Appendix). Based on the quadratic term, for both males and females, molt peaked during the middle of the migration season (SOD: β = 0.007, t = 0.47, P = 0.64; SOD2: β = −0.002, t = −3.77, P < 0.001; Figure 2). The interaction between sex and year (Sex*Year: β = −2.51, t = −3.57, P = 0.001) suggested that average molt intensity may have been greater for males than for females in 2016 but not in 2017, but seasonal patterns were difficult to discern because of small sample sizes and seasonally imbalanced captures across years. Males arrived at our site earlier than females in both years; thus, the degree of overlap between migration and molt may also have differed between the sexes. More research is needed to clarify the differences in molt schedules and intensity by sex. Birds with higher fat scores tended to have less intense molt (Fat: β = −0.33, t = −0.33, P = 0.05; Figure 3), but the confidence in this relationship is weak due to small sample sizes of birds with high fat scores. In a separate linear regression, fat score was not related to SOD (βSOD = 0.01, t = 1.4, P = 0.16), so our results do not have implications for the timing of migratory fattening in relation to molt.
![Individual molt intensity of male (blue) and female (red) Rusty Blackbirds during the spring of 2016 (circles) and 2017 (triangles) at Ottawa National Wildlife Refuge, Lucas County, Ohio, USA. Molt intensity, as measured by the square root of the combined contour molt index (CCMI), is plotted as a function of standardized ordinal date, mean-centered by sex (mean male ordinal capture date = 89 [March 30]; mean female ordinal capture date = 105 [April 15]). The line represents the predicted quadratic relationship for both sexes and years combined and the shaded area represents the 95% confidence interval, based on the most parsimonious multiple regression model and holding fat score to its mean value.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/condor/120/3/10.1650_CONDOR-17-177.1/4/m_i0010-5422-120-3-507-f02.jpeg?Expires=1750184526&Signature=Ef7Fbufi8b9KRneWF5yOVud9PzoZZEbW~0rtZNwEJ-wS3Sm1QC-saQL4ITieZa8qgHLbCi36H2BHIATrh5cLQ0x3eMhq3oIHH65XBFQ54PUcf8jx0pyqAztSX1ZrVIlNaoqwawcmBmu21ok57p1iip2EvfJHA7wWLTbmA8~InmzYL4lm3-TA44Aq4Du36R3SUgsBwYUOF2ncvSfvlIN9Lo6cLCEEKUKZtlvW8r4sjCHtmX6BIScWVcu1oNNUY5Yann4PTfS1IERh9XgjXOqbb-XbCagimjAynYp8Yxvc5tqtFUecMknZYFIEYJhEtbXM7IbP6c2fTU6QcPVU-TAy-A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Individual molt intensity of male (blue) and female (red) Rusty Blackbirds during the spring of 2016 (circles) and 2017 (triangles) at Ottawa National Wildlife Refuge, Lucas County, Ohio, USA. Molt intensity, as measured by the square root of the combined contour molt index (CCMI), is plotted as a function of standardized ordinal date, mean-centered by sex (mean male ordinal capture date = 89 [March 30]; mean female ordinal capture date = 105 [April 15]). The line represents the predicted quadratic relationship for both sexes and years combined and the shaded area represents the 95% confidence interval, based on the most parsimonious multiple regression model and holding fat score to its mean value.
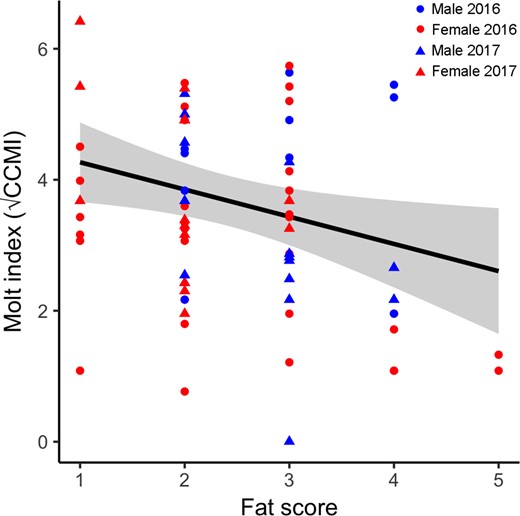
Individual molt intensity of male (blue) and female (red) Rusty Blackbirds during the spring of 2016 (circles) and 2017 (triangles) at Ottawa National Wildlife Refuge, Lucas County, Ohio, USA. Molt intensity, as measured by the square root of the combined contour molt index (CCMI), is plotted as a function of fat score (on a scale of 0–8). The line represents the predicted linear relationship for both sexes and years combined and the shaded area represents the 95% confidence interval, based on the most parsimonious multiple regression model and holding standardized ordinal date to its mean value.
Of the 17 feather patches examined, all were in active molt on at least one bird (Figure 1). The cheek patch (including eye-ring and lores) was most often in molt (88% of individuals), followed by the chin (73%) and crown (66%). When all of the head patches were grouped (crown, back of head, cheek, chin, throat, and nape & neck) and compared against the grouped body patches (breast, belly, vent, flank, legs, undertail coverts, uppertail coverts, back, rump, shoulders, and armpits), the proportion of birds molting head patches (65 ± 6%) was significantly greater than the proportion molting body patches (22 ± 4%; Kruskal-Wallis test: χ21= 10.34, P = 0.001; Figure 1). Likewise, the intensity of molt within respective patches was greater for head patches (45 ± 1%) than body patches (18 ± 2%; Kruskal-Wallis test: χ21= 11.0, P < 0.001; Figure 4). Note that Figure 1 displays the presence of patch molt among all birds, whereas Figure 4 displays the intensity of patch molt among birds molting that patch, the latter showing that more feathers were being replaced in head patches than body patches. Additionally, the quadratic relationship of total molt intensity (CCMI) with time can largely be attributed to changes in both head patch molt intensity and the number of patches in molt. These variables showed similar quadratic relationships with SOD when examined separately (Head patch intensity: βSOD2 = −0.048, t = −4.25, P < 0.001; Number of patches: βSOD2 = −0.005, t = −3.75, P < 0.001), in contrast to body patch molt intensity, which was uniformly low across the season (βSOD = 0.1, t = 0.92, P = 0.36; βSOD2 = −0.003, t = −1.34, P = 0.19). Males were growing in black feathers with no rusty tips 100% of the time when those feathers were molting, while females were growing in gray feathers with no rusty tips or narrow, barely visible rusty tips.
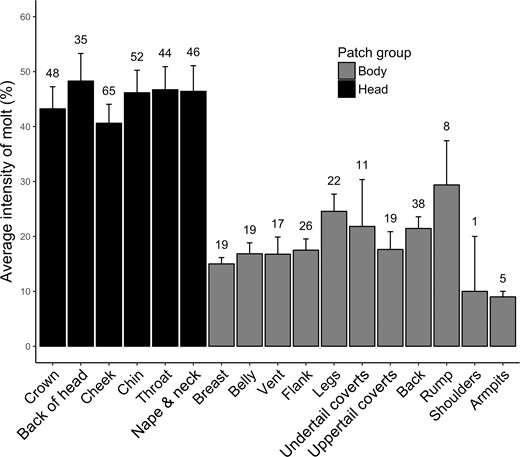
Intensity of molt within a given feather patch (calculated as an average of all patch percentage scores of birds molting that patch) for migrant Rusty Blackbirds at Ottawa National Wildlife Refuge, Lucas County, Ohio, USA, in the springs of 2016 and 2017. The sample size used to calculate the mean molt intensity for that patch is shown above each standard error bar. Feather patches located on the head are represented by black bars, whereas feather patches on the body are represented by gray bars.
Discussion
Our results provide evidence for an obligate partial prealternate molt largely completed during migration by Rusty Blackbirds. Nearly all Rusty Blackbird migrants at a major stopover site were undergoing some degree of body molt when captured, and the intensity of that molt peaked in the middle of the spring migration period for both males and females. Coupled with the finding of Mettke-Hoffman et al. (2010) that the number of birds in molt increased over the course of the wintering period, our results suggest that individuals are initiating an obligate prealternate molt late in the winter, then completing this molt during migration; however, contour feather molt should be examined at other spring stopover sites to confirm that this holds true across the species' migratory range. Furthermore, our findings contradict the long-held assumption that glossy male breeding plumage is achieved solely through feather wear (e.g., Avery 2013), as all molting males were replacing rusty-tipped feathers with black ones. Although this molt schedule is not unusual for shorebirds (Lourenço and Piersma 2015) and waterfowl (e.g., Clark et al. 2014; considered “prebasic molt” in updated terminology for waterfowl; Pyle 2005), it has not been described in the literature for Passeriformes. Nearly all migrant passerines studied to date that undergo a partial prealternate molt have been found to do so on the wintering grounds (e.g., Mowbray 1997, Mazerolle et al. 2005, Boone et al. 2010, Bulluck et al. 2017), with individuals only occasionally continuing this molt during migration (E. Johnson personal communication). However, given the paucity of studies on the spatial ecology of molt, it seems likely that this strategy occurs elsewhere in Passeriformes, and even in Icteridae.
The term “molt-migration” has very rarely, if ever, been applied to prealternate or other contour feather–only molts (P. Pyle personal communication). The reason for this is unclear, but perhaps it is simply a combination of disciplinary differences among ornithologists and a disproportionate focus on postbreeding molts. Given the role of flight in most avian daily activities and life cycles, flight feather molt is certainly critical; however, prealternate molt should not be overlooked in a comprehensive view of the role of molt in annual cycles. Prealternate molt is crucial for producing coloration important to sexual selection and intrasexual communication (Hill and McGraw 2006a). As research on molt has grown, the prevalence of molt migration has become increasingly appreciated (e.g., Jehl 1990, Pyle et al. 2009, Lourenço and Piersma 2015), thus requiring a more comprehensive view of this phenomenon that includes all phases and types of molt. For this reason, we term the molt strategy described here and in many shorebirds as “prealternate molt-migration.”
There is some evidence that the only other Euphagus, the partially migratory Brewer's Blackbird, undergoes a similar molt of predominantly head feathers during the same time period (early spring; Martin 2002). As yet, no studies have described the nature of this molt within the context of migratory strategies. A comparison of molt strategies between migrant and resident Brewer's Blackbirds would be informative in an evolutionary and ecological context to understand the mechanisms behind Rusty Blackbird molt migration, and potentially similar strategies in other obligate migrants. Research is also warranted to identify other potential passerine systems in which prealternate molt-migration occurs. Two important groups to consider are Neotropical migrants that either relocate during the overwintering period (e.g., Ruiz-Gutierrez et al. 2016) or exhibit intratropical migrations prior to returning to the breeding grounds (Stutchbury et al. 2016). Acquisition of compounds necessary for replacement feathers (e.g., pigments), in addition to energetic resources, is one potential explanation for such within-season movements.
The driving forces of prealternate molt-migration in Rusty Blackbirds remain unclear. In shorebirds, Lourenço and Piersma (2015) suggested that molt at spring staging areas is a by-product of the long time period between departure from nonbreeding areas and arrival on the breeding grounds. Essentially, birds “top up” their plumage, potentially in a condition-dependent manner (Piersma and Jukema 1993), to maximize plumage freshness on arrival, increasing attractiveness to potential mates. A comparison of 21 shorebird species showed that most species with long migrations and breeding sites at higher latitudes followed the same molt pattern as Rusty Blackbirds, initiating prealternate molt on the wintering grounds but completing it during migration (Lourenço and Piersma 2015).
The “topping up” explanation would also appear to apply to Rusty Blackbirds. Rusty Blackbirds are not traditionally considered long-distance migrants, but migration can be as far as 5,500 km for a blackbird migrating from Louisiana to Alaska, USA, for which there is evidence based on stable isotope analysis (Hobson et al. 2010). Furthermore, Rusty Blackbird populations are present in peak numbers at Great Lakes stopover sites for >2 mo (March–May, based on eBird reports; Sullivan et al. 2009), with the earliest breeding initiated in early May (Avery 2013), and radio-tracking data suggest individual stopover lengths of ∼25 days (Wright et al. 2018). Long stopover times in this species may necessitate the “topping up” of plumage, especially the structural aspects of Rusty Blackbird plumage. Ultraviolet hue in structural plumage is at its maximum just after molt, and UV characteristics overall change with feather age, as found for Eurasian Blue Tits (Cyanistes caeruleus; Örnborg et al. 2002). However, the role of plumage in sexual selection or intrasexual communication in this species is not known, and the inconsistent sex effect in our study suggests that it may be more complicated than female-choice sexual selection. It should also be noted that the geographic location of molt could be stochastic for individuals of the same population from year to year (Pyle et al. 2009, Nordell et al. 2016), so molt may not necessarily always occur at this stopover site.
In the context of Rusty Blackbird conservation, our findings are critical to understanding the limitations on individuals during different stages of their annual cycle, as causes of the species' decline remain enigmatic (Greenberg et al. 2011). Research on the migratory stopover ecology of this species has been very limited (but see Savard et al. 2011) in comparison with that on breeding (e.g., Matsuoka et al. 2010, Powell et al. 2010) and wintering ecology (e.g., Luscier et al. 2010, Mettke-Hofmann et al. 2015), and our study highlights the multiple roles that stopover habitats can play during Rusty Blackbird migration, including refueling and providing energetic requirements for molt. Our study site on Lake Erie hosts one of the largest concentrations of Rusty Blackbirds during spring migration (Rusty Blackbird Migration Blitz; Evans 2016), and, based on their lengthy stopovers (Wright et al. 2018), it appears that these birds are completing much of their prealternate molt here. If this molt plays a role in their breeding ecology, it is vital to protect the resources that they require to complete it in the minimum time necessary to be able to continue their migration. One hypothesis to be tested is whether stopover duration, which appears to be long in this species (Wright et al. 2018), is driven by the requirements of molt, and whether limits on available habitat or food resources could protract stopover, causing phenological delays. Our finding that birds with high fat content had lower intensity of molt could suggest that migratory fattening was occurring as molt was being completed. However, since we were often not able to reliably distinguish old feathers from recently replaced feathers (because both were black or gray), our study lacks information on the extent of molt completed upon capture (i.e. no “plumage score,” sensu Piersma and Jukema 1993). Studies that recapture individuals and examine molt over the course of stopover are needed to clarify the relationship between migratory fattening and molt.
Our results should serve as a call to action to ornithologists to increase the examination of geographic locations of not only prebasic but also prealternate and other molts (e.g., preformative molt; Howell et al. 2003). Our study examined molt of Rusty Blackbirds at a single high-use stopover site, but future studies of molt-migration should attempt to expand spatial replicates across the range of the species. Given the crucial role that all feather molt plays in avian ecology, if we wish to fully understand the full annual cycles of species (Marra et al. 2015), we must understand how they are limited, which is impossible without context. Rusty Blackbirds exhibit a molt strategy heretofore undescribed in the ornithological literature on passerines. Yet, the most parsimonious explanation for the novelty of our finding is that we have yet to adequately address this question in most taxa.
Acknowledgments
Our work would not have been possible without Dean Demarest and the International Rusty Blackbird Working Group (www.rustyblackbird.org). For their knowledge and generous assistance, we are extremely grateful to Ron Huffman, Jason Lewis, Eddy Pausch, and all the staff at Ottawa National Wildlife Refuge; Patrick Baranowski and Ohio Department of Natural Resources (ODNR) staff at Magee Marsh Wildlife Area; Mark Shieldcastle and the Black Swamp Bird Observatory; and John Simpson, Brendan Shirkey, and the staff at the Winous Point Marsh Conservancy. For assistance in the field, we thank Patricia Rodrigues, Kellene Collins, Kristie Stein, and Elizabeth Ames. We also thank the associate editor and 2 anonymous reviewers for providing helpful comments that improved the manuscript.
Funding statement: This research was funded by the Ohio Agricultural Research and Developmental Center, and grants from the U.S. Fish and Wildlife Service (F16AC00447) and Ohio Sea Grant (NA14OAR4170067) to C.M.T. Additional funds were provided to J.R.W. from a Bergstrom Memorial Research Award from the Association of Field Ornithologists, a Hesse Research Award from the American Ornithological Society, and an Ohio Avian Project Initiative award from the Kirtland Bird Club. None of our funders had any input into the content of the manuscript, nor did they require their approval of the manuscript prior to submission or publication.
Ethics statement: All field methods were approved by the Institutional Animal Care and Use Committee of The Ohio State University (protocol #201500000028).
Author contributions: J.R.W. conceived the study, acquired partial funding, collected all field data, completed statistical analysis, and contributed to manuscript writing. C.M.T. acquired funding, supervised research, and contributed to manuscript writing. L.L.P. assisted with funding proposals, contributed to project design, and helped to edit the manuscript.
Literature Cited
Appendix
Summary Output of Final Multiple Regression Model for Square Root CCMI (Combined Contour Molt Index) of Rusty Blackbirds during Spring Migration at Western Lake Erie, Ohio, USA, from Program R
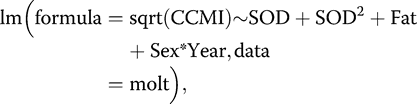
Coefficients:
Author notes
Current address: Department of Biosciences, Durham University, Durham, UK





