-
PDF
- Split View
-
Views
-
Cite
Cite
Carlos A. Chávez-Zichinelli, Ian Macgregor-Fors, Javier Quesada, Patricia Talamás Rohana, Marta C. Romano, Ricardo Valdéz, Jorge E. Schondube, How Stressed are Birds in an Urbanizing Landscape? Relationships Between the Physiology of Birds and Three Levels of Habitat Alteration: ¿Qué tan Estresadas Están las Aves en un Paisaje Urbanizado? Relaciones Entre la Fisiología de las Aves y Tres Niveles de Alteración de Hábitat, The Condor: Ornithological Applications, Volume 115, Issue 1, 1 February 2013, Pages 84–92, https://doi.org/10.1525/cond.2013.110201
Close - Share Icon Share
Abstract.
In this study we measured two physiological traits (levels of corticosterone and immunoglobulin) in two species of landbirds, the Canyon Towhee (Melozone fusca) and Inca Dove (Columbina inca), occupying three degrees of human alteration of a subtropical mountain landscape: forest edges, croplands, and urban sites. We found that both physiological variables differed by species and habitat condition. In both species, corticosterone concentration was significantly higher in croplands. But immunoglobulin concentration behaved differently, in C. inca being highest at urban sites, where in M. fusca it was lowest. Contrary to expectation, we only found one strong significant relationship between both physiological variables: M. fusca in urban areas. Our results suggest that 30% of the towhees captured in urban areas are under chronic stress. Results for body condition support this hypothesis, as the condition of towhees in urban areas was poorer, suggesting physiological vulnerability. Although we expected the density of both species to be high in urban areas because of the amount and predictability of resources, we found a significantly lower density of M. fusca in urban areas, suggesting that the habitat variables influencing the physiological condition of M. fusca affected its population density. In summary, our results suggest that a substantial proportion of Canyon Towhees in the urban area studied have physiological limitations, while the Inca Dove seems to have an appropriate physiological response despite low values for body condition in urban areas.
Resumen.
Medimos la respuesta fisiológica (concentraciones de corticosterona e inmunoglobulinas) de Melozone fusca y Columbina inca ante cambios en tres condiciones de hábitat dentro de un paisaje subtropical de montaña modificado por actividades humanas: borde de bosque, cultivos y zonas urbanas. Encontramos que ambas variables fisiólogicas difirieron según especie y condición de hábitat. En ambas especies, la concentración de corticosterona fue mayor en los cultivos. Mientras que la concentración de inmunoglobulinas varió por especie, con C. inca mostró valores mayores en la zona urbana, mientras que M. fusca mostró sus valores más bajos en dicha condición. Contrario a lo esperado, sólo encontramos una relación negativa para las dos variables fisiológicas para M. fusca dentro de la zona urbana, sugiriendo que 30% de los individuos de esta especie presentan estrés crónico en este hábitat. Además, M. fusca exhibió valores menores de condición corporal en la zona urbana, apoyando la hipótesis de que es fisiologicamente vulnerable en este hábitat. Aunque esperábamos que la densidad de ambas especies fuera mayor en zonas urbanas debido a la gran cantidad, y predictibilidad de recursos, encontramos una menor densidad de M. fusca, sugiriendo que las variables de hábitat que afectan la fisiología de esta especie pueden también afectar sus tamaños poblacionales. Nuestros resultados indican que una proporción importante de los individuos de M. fusca en la zona urbana tienen limitaciones fisiológicas, mientras que C. inca parece tener una respuesta fisiológica apropiada, a pesar de presenter valores menores de condición corporal dentro de la zona urbana.
Introduction
Humans have greatly altered the earth's ecosystems, and this alteration represents a threat to biodiversity and generates complex landscapes (Vitousek et al. 1997, Jetz et al. 2007). Within human-altered landscapes, wildlife confronts different types, magnitudes, intensities, spans, and frequencies of disturbance (Pickett and White 1985, White and Jentsch 2001, BenítezDíaz and Bellot-Rojas 2007). Regardless of their traits, patches of landscapes under different disturbance regimes generate stimuli stressful to organisms (Jacobs and Wingfield 2000, Breuner and Hahn 2003, Walker et al. 2005, 2008).
Wildlife respond to stressful stimuli generated by human disturbances through physiological mechanisms and behavior, which can shape the dynamics of their populations (Temple and Wiens 1989, Ricklefs and Wikelski 2002, Romero 2004, Partecke et al. 2006), and even represent evolutionary responses (Badyaev 2005). Physiological feedbacks to stressful agents include acute and chronic stress (Romero 2004, Partecke et al. 2006, Ewens 2007). Acute stress, caused by random stressful stimuli, can turn into chronic stress when the stressful agent persists for a long period (Buchanan 2000, Sapolsky et al. 2000, McEwen and Wingfield 2003). Among other effects, chronic stress often alters the immune system, lowering an individual's capability to respond to pathogens (Fowles et al. 1993, Sapolsky et al. 2000, El-Lethey et al. 2003, McEwen and Wingfield 2003).
In this study we measured two physiological traits, levels of corticosterone and immunoglobulin, in two species of landbirds, the Canyon Towhee (Melozone fusca) and Inca Dove (Columbina inca) occupying three habitat conditions along a gradient of human alteration: (1) forest edges, (2) croplands, and (3) urban sites. These habitat conditions represent contrastingly different levels of disturbance (e.g., human activities, noise). Although M. fusca and C. inca are phylogenetically distant (orders Passeriformes and Columbiformes, respectively), they are ecologically similar in size (M. fusca: length 21–25 cm; C. inca: 18–23 cm), main food sources (mostly seeds), and microhabitat needs for foraging and nesting (open areas and areas with short vegetation) (Johnson and Haight 1996, Mueller 2004). Melozone fusca is adaptable, sexually monomorphic, and widespread, associated with open spaces, and has territories not exceeding 12 ha (Johnson and Haight 1996). Columbina inca is also a sexually monomorphic and highly adaptable species that uses human-altered habitats (e.g., villages, towns, cities, farms), often in open spaces. Although little is known about its territoriality through the year, a congeneric species, C. passerina, the Common Ground-Dove, maintains territories during the nonbreeding season (Bowman 2002).
We predicted that in both species corticosterone concentrations should be low in the least disturbed habitat (i.e., forest edges), higher in more disturbed habitats (i.e., croplands, urban sites), as recorded in previous studies (e.g., Bonier et al. 2007, Fokidis and Deviche 2011). We also expected a negative correlation between immunoglobin and corticosterone concentrations in urban areas, where these species' body mass tends to be lower than expected, representing individuals with physiological alterations that could affect their health (Barnes 1998, Sapolsky et al. 2000, Dhabhar 2009). We also predicted a negative correlation between immunoglobin and corticosterone concentrations in croplands but expected the slope of this relationship to be shallower than in urban areas. Finally, if these species' population density is affected by habitat traits that alter their physiological condition, as suggested in previous studies (Eeva et al. 1997, Liker et al. 2008, Moya-Laraño et al. 2008), we expected their density to be lowest where the proportion of individuals with physiological alterations was highest.
Methods
Study Area
This study was carried out within the Cuitzeo watershed, located in northeastern Michoacán, west-central Mexico. Our sampling sites include three habitats located at similar elevations (∼1950 m above sea level) with different types and intensities of human disturbance: (1) native forest edges; (2) croplands; and (3) urban sites. Native forest edges (19° 39′ N, 101° 07′ W) constitute the ecotone between pine-oak communities and open areas generated by rocky outcrops and low levels of human disturbance (i.e., dirt roads, small fields of crops, scattered suburban settlements). Croplands (19° 48′ N, 101° 15′ W) consist of seasonal crops (e.g., corn, sorghum) and cattle pastures embedded within shrublands, which represent the early stage of ecological succession resulting from forest perturbation and/or abandonment of cropland. Urban areas include an urban park (Bosque Cuauhtemoc: 19° 42′ N, 101° 11′ W) and a suburban university campus (19° 39′ N, 101° 13′ W) located in the center and the southwestern sections of the city of Morelia, respectively. Morelia is a colonial city established during the sixteenth century that has undergone rapid and unplanned development in the last few decades (López et al. 2001, Vargas Uribe 2008). At present, Morelia covers ∼100 km2 and has a population that exceeds one million inhabitants, with little vegetation cover and few urban green areas (Vargas Uribe 2008). In general, the landscape we studied comprises the city of Morelia, small human settlements, tree plantations, croplands mixed with shrublands, and some forest patches, including pine, pineoak, and oak communities. Our sampling sites are located at least 10 km away from each other (average distance between categories of sites: urban—forest, 10.4 km; urban—cropland, 14.8 km; forest—cropland, 21.6 km). The croplands studied are located north of the city, while the forest-edge sites are located to the southeast.
Habitat Characterization
To characterize the habitat at our survey sites, we measured vegetation structure (cover and maximum height of trees, shrubs, and herbs) and human activity (passing cars, passing pedestrians) at five sites near our sites of capture and observation of birds. To assess the similarity among our sampling sites, we performed a multivariate cluster analysis, using Euclidean distance and single-linkage methods (i.e., nearest neighbors, determined by the distance of the two closest objects in a similarity matrix). We assumed that clusters formed before 50% similarity, given by the magnitude of kinship among paired-sample comparisons, represent different habitat conditions. Additionally, we evaluated differences among the measured variables in the forest edge, cropland, and urban sites. For this, we performed one-way GLM ANOVAs between each habitat trait (dependent variable) and each habitat condition (grouping variable), followed by Newman—Keuls post-hoc range tests.
Bird Captures
We netted C. inca and M. fusca in the three habitats studied from 7:00 to 11:00 during the dry season (January—May) of 2008 and 2009. We captured birds during the dry season to avoid interference with the breeding season, during which their physiological condition may be altered (Wingfield et al. 1998, Lynn and Porter 2008). We collected fecal and blood samples, which were frozen for laboratory analyses, from both species. We collected fecal samples from clean bags in which the birds were kept after capture. Blood samples were obtained from the brachial vein and collected in heparinized capillary tubes. All captured birds were weighed with a digital balance (sensitivity 0.01 g), banded, and released. To avoid pseudoreplication, we did not collect fecal or blood samples from recaptured individuals.
Physiological Analyses
Corticosterone concentration (radioimmunoassay). To asssess the physiological condition of C. inca and M. fusca at our sampling sites, we used fecal samples to determine corticosterone concentrations. We measured corticosterone in feces because they allow the integration of the stress state of individuals before capture, not the stress caused by the capture and handling of the bird (Millspaugh and Washburn 2004, Tourna and Palme 2005).
We defrosted and standardized the fecal samples to 0.2 g (wet weight) and ran the samples in duplicate. To extract corticosterone from fecal samples, we placed each sample in a vial with 1 mL of ethanol (Cavigelli 1999, Sands and Creel 2004), vortexed for 20 min and centrifuged for 20 min (10000 revolutions min-1). In most cases we used a dilution of 1:4. Afterward, we placed 100 µL of the dilution in radioimmunoassay tubes and added 100 µL of anti-corticosterone antibody (Sigma C-8784). Finally, we incorporated 50 µL of tritiated corticosterone ([1,2,6,7-3H(N), Perkin Elmer, Boston, MA). We incubated our samples for 20 hr at 4 °C, separating the unbound steroids with activated charcoal. We measured the radioactivity with a scintillation counter (Beckmann Instruments LS-6500). We validated this method for M. fusca and C. inca by analyzing the recovery of exogenous corticosterone in relation to field samples by adding exogenous corticosterone to one set of samples as control. Results of the different analyses show that the assay was applicable to samples of these species: (1) slopes from curves from fecal extracts did not differ from the corticosterone standard curve (M. fusca: F = 2.0, df = 8, P = 0.16; C. inca: F = 2.1, df = 8, P = 0.18); and (2) 94.7% of the exogenous corticosterone was recovered.
Immunoglobulin concentration (enzyme-linked immunosorbent assay—ELISA). To determine the concentration of total immunoglobulins (including IgY, IgM, and IgA) in blood samples, we used an ELISA test, as described by Martinez et al. (2003). Briefly, we extracted blood samples from capillary tubes, placed them in 0.5-mL vials, centrifuged them for 10 min, and collected blood plasma. We diluted blood plasma samples (1:4000), placed them in well plates, and incubated them at 4 °C for 18 hr. Next, we added a primary antibody (polyclonal rabbit anti-chicken IgG conjugated with peroxidase; Sigma A-9046) and incubated the samples for another 2 hr at 37 °C. Afterward, we added orthophenylendiamine as a color substrate for further identification. We read all plates for optical densities with an ELISA plate spectrophotometer (λ = 492). We ran all samples in triplicate.
Bird Densities
To evaluate if the densities of C. inca and M. fusca were related to the habitat conditions studied, we made 10 point counts per habitat (5 min, unlimited radius) following Ralph et al. (1996), measuring the distance from each bird to the observer with a rangefinder (Bushnell Yardage Pro). Points were separated by 250 m to assure independence (Ralph et al. 1996, Huff et al. 2000); they were located within and around the areas in which birds were captured. We included in our analyses all individuals recorded of both species.
Statistical Analyses
To compare corticosterone concentrations by habitat, we performed Kruskal—Wallis tests because the data did not meet some of the criteria for parametric tests. As the data for immunoglobulin concentration did meet the criteria, we used ANOVA. In both cases (Kruskal—Wallis and ANOVA), we performed Tukey post-hoc tests. To evaluate whether corticosterone and immunoglobulin concentrations were related, we did separate linear regressions between both physiological variables for each species in each habitat. We analyzed the statistical power (post hoc) for each regression with G*Power (Faul et al. 2009). As the power of a statistical test increases, the probability of a type II error decreases, allowing us to evaluate whether our results are statistically robust regardless of sample size. Following Park (2008), we used a minimum power value resembling statistical significance of 0.80.
We set two physiological thresholds to identify those individuals with high corticosterone and low immunoglobulin concentrations, which could affect the birds' health (Buchanan 2000, Sapolsky et al. 2000, Romero 2004). Because for both species studied the standard ranges of values of both variables are unknown, we established one threshold for each variable (i.e., corticosterone and immunoglobulin concentration), calculated from the information (mean and SD values) for each species at forest edge, the least disturbed habitat. We used the mean value + SD for corticosterone concentration and the mean value — SD for immunoglobulin concentration to define a threshold for those individuals with corticosterone concentrations higher and immunoglobulin concentrations lower than expected (to which we refer as physiologically altered). We used SD because it represents the average difference between the data points and their mean (Cumming et al. 2007). In order to set our physiological results into context, we assessed changes in body condition (body mass/tarsus; Johnson et al. 1985) for both species by habitat with Kruskal—Wallis tests because our data did not satisfy some of the assumptions of parametric tests (i.e., normality, homogeneity of variance).
To calculate bird densities, we computed the number of individuals per hectare (mean ± 95% confidence intervals) in Distance 6.0 (Thomas et al. 2010). This software calculates the probability of an individual being detected at increasing distances from the observer and estimates the number of individuals within a surveyed area (Buckland et al. 2001, 2004). We report the key function/series expansion (KF/SE) to describe the coverage of our surveys and the nature of the methods used by the program to calculate bird densities. The KF is a parametric function modeling the detection function (i.e., uniform, half-normal, hazard rate, negative exponential); the SE is a mathematical representation of the functions (i.e., cosine, simple polynomial, Hermite polynomial) (Thomas et al. 2002, 2005). To determine if the computed density values for both species differed statistically by habitat, we compared their 95% confidence intervals. If confidence intervals did not overlap, we (following Payton et al. 2003) considered the data to be statistically different with an α < 0.01.
Results
Habitat Characterization and Bird Captures
The multivariate cluster analysis (Euclidean distance, single linkage), based on the variables measured at each site (Table 1), revealed that (1) survey sites in each habitat were more similar to each other than to survey sites in other habitats, with values for similarity ranging from 61 to 65%, and (2) the urban sites studied differ greatly from the sites sampled at forest edges and in croplands (29% similarity), which in turn clustered at 58% similarity (Fig. 1).
We captured a total of 119 individuals of M. fusca (43 at forest edge, 59 in cropland, 17 in urban) and 104 of C. inca (37 at forest edge, 52 in cropland, 15 in urban). Rates of recapture suggest that in our study area these species occupy well-defined nonbreeding territories. Over the two years of sampling, we recaptured an average of 18% of individuals of M. fusca captured (14% at forest edge, 24% in croplands, 12% in urban) and an average of 10% of C. inca (5% at forest edge, 17% in croplands, 0% in urban). We never recaptured a bird in a habitat other than that in which it was captured initially.
Physiological Analyses
Corticosterone concentration varied by species and habitat. In M. fusca it was significantly higher in croplands than at forest edges, but at urban sites it did not differ from its value in the other two habitats (H2,118 = 8.27, P = 0.01; Fig. 2). In C. inca corticosterone concentration averaged significantly higher in croplands than at forest edges or urban sites (H2,103 = 9.84, P = 0.007; Fig. 2). Immunoglobulin concentration also varied by species and habitat. In M. fusca immunoglobulin concentrations were significantly higher in croplands than at urban sites, but at forest edges they did not differ statistically from those in the other two habitats (F2,118= 6.01, P = 0.003; Fig. 2). In C. inca immunoglobulin concentrations at urban sites were significantly higher than those at forest edges, but in croplands they did not differ statistically from those in the other two habitats (F2,103= 2.46, P = 0.09; Fig. 2).
Values of habitat traits and human activity (mean ± SD) at the forest edge, cropland, and urban sites studied in and near Morelia, Mexico. Letters within parentheses following SD values represent statistical differences from Newman—Keuls post-hoc range tests.
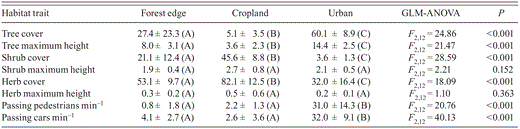
Values of habitat traits and human activity (mean ± SD) at the forest edge, cropland, and urban sites studied in and near Morelia, Mexico. Letters within parentheses following SD values represent statistical differences from Newman—Keuls post-hoc range tests.

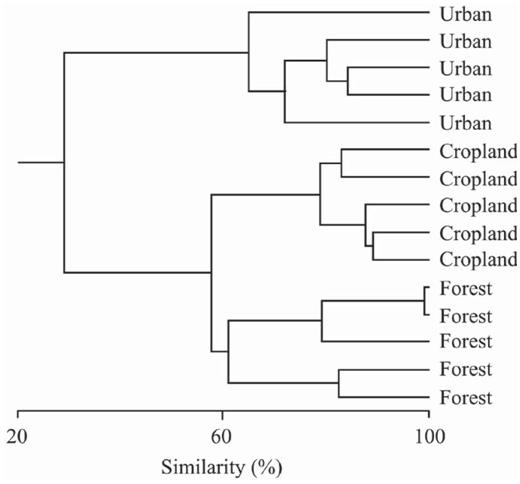
Multivariate cluster analysis (Euclidean distance—single linkage) showing similarity of traits of the three habitats sampled around Morelia, Mexico: forest edge, cropland, and urban.
In most cases, corticosterone and immunoglobulin concentrations were not coupled. With both α and statistical power considered, they only showed one strong significant (negative) relationship, for M. fusca at urban sites (r2= 0.49, P = 0.001, statistical power = 0.90). We also found a positive significant relationship between corticosterone and immunoglobulin concentrations for M. fusca in croplands. However, this relationship was weak (r2= 0.09, P = 0.01) and had a low value for statistical power (0.64). The remaining regressions for M. fusca and all regressions for C. inca were nonsignificant and showed no other nonsignificant tendencies (Table 2). When we tested for physiologically altered individuals (see Methods for criteria), we found only two individuals of C. inca, one in the croplands and one at an urban site (representing 2% and 7% of the total of that species captured in each habitat, respectively; Fig. 3). Of M. fusca, however, we found more individuals with corticosterone concentration higher and immunoglobulin concentration lower that expected, which could affect the birds' health. Although one of these individuals was from forest edge and two were from cropland, representing 2% and 3%, respectively, of the towhees captured in these habitats, we recorded five physiologically altered individuals in urban areas, representing 30% of the towhees captured in this habitat (Fig. 3).
Relationship between corticosterone and immunoglobulin concentration in Melozone fusca and Columbina inca at forest edges, in cropland, and at urban sites around Morelia, Mexico.

Relationship between corticosterone and immunoglobulin concentration in Melozone fusca and Columbina inca at forest edges, in cropland, and at urban sites around Morelia, Mexico.

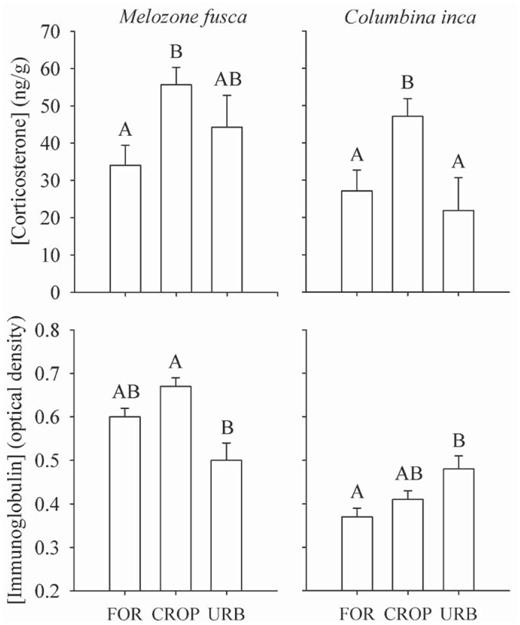
Values of corticosterone and immunoglobulin concentration (mean ± SE) for Melozone fusca and Columbina inca at forest edges, in croplands, and in urban habitat in and near Morelia, Mexico. Letters above error whiskers represent differences in Newman—Keuls post-hoc range tests (P < 0.05). FOR = forest edge, CROP = cropland, URB = urban.
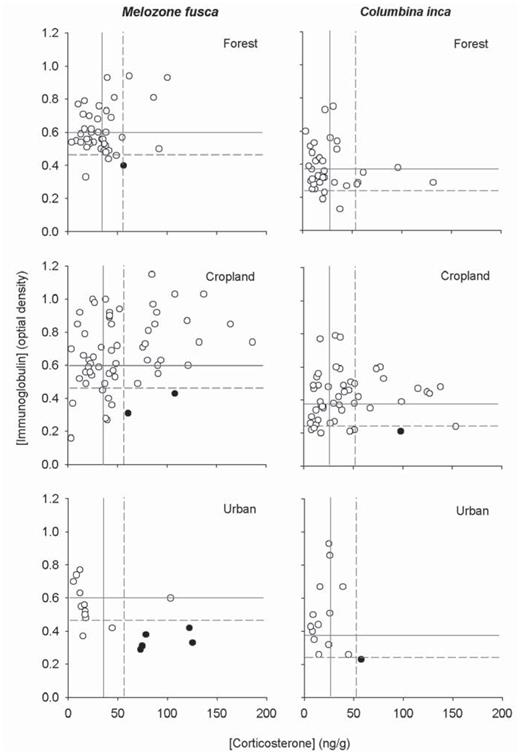
Relationship between values of corticosterone and immunoglobulin concentration fox Melozone fusca and Columbina inca at the forest edges, croplands, and urban sites studied around Morelia, Mexico. Solid gray lines represent mean values for corticosterone and immunoglobulin concentration at forest edges; dashed gray lines depict the corresponding positive and negative SD values. White circles represent individuals for which no negative ecophysiological inference can be made with respect to their values for corticosterone and immunoglobulin concentration. Black circles represent individuals with values for corticosterone higher than expected and immunoglobulin lower than expected, suggesting physiological alterations that could affect their health.
The index of body condition of M. fusca was significantly lower in urban areas than in croplands and at forest edges (H2,118 = 8.17, P = 0.01; Table 3). The index for C. inca was lower in urban areas and at forest edges, higher in croplands (H2,103 = 5.37, P = 0.06), but this difference was not significant.
Body mass (g), tarsus length (mm), and body-condition index (mm g-1) (mean ± SD) for Melozone fusca and Columbina inca at forest edges, in cropland, and at urban sites around Morelia, Mexico.
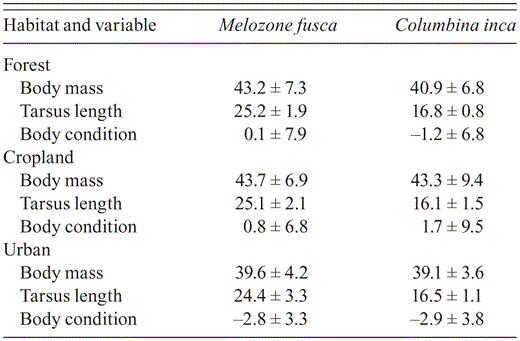
Body mass (g), tarsus length (mm), and body-condition index (mm g-1) (mean ± SD) for Melozone fusca and Columbina inca at forest edges, in cropland, and at urban sites around Morelia, Mexico.

Bird Densities
For both M. fusca and C. inca density varied by habitat. Densities of M. fusca were significantly higher at forest edges (4.47 ha-1; 95% confidence interval 3.00–6.67; KF/ SE = uniform/cosine) and in croplands (3.59 ha-1; 95% confidence interval 2.72–4.74; KF/SE = uniform/cosine) than at urban sites (1.95 ha-1; 95% confidence interval 1.41–2.72; KF/ SE = uniform/simple polynomial). The density of C. inca, however, did not differ by habitat: forest edges, 4.02 ha-1, 95% confidence interval 2.71–6.01, KF/SE = uniform/cosine; croplands, 3.27 ha-1; 95% confidence interval 2.55–4.35, KF/ SE = uniform/cosine; urban, 4.66 ha-1; 95% confidence interval 3.09–7.03, KF/SE = uniform/cosine (Fig. 4).
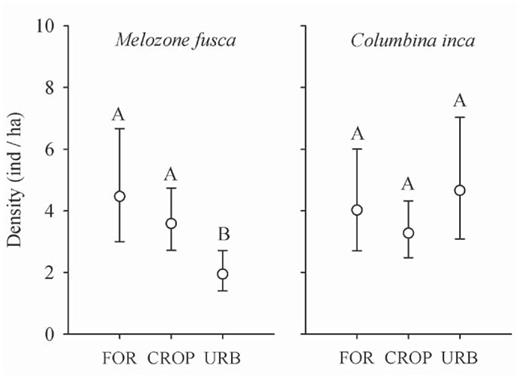
Mean density (± 95% confidence intervals) of Melozone fusca and Columbina inca at forest edges, in cropland, and at urban sites around Morelia, Mexico. Letters above CI whiskers represent statistical differences based on overlapping 95% confidence intervals (according to Payton et al. 2003). FOR = forest edge, CROP = cropland, URB = urban.
Discussion
In both the Inca Dove and Canyon Towhee, corticosterone concentrations were highest in croplands, a result different from that of Bonier et al. (2007), who found higher concentration values in urban areas than in rural areas. As our study took place during the dry season and agriculture in our study area depends basically on rainfall, crops were not available at the time of our sampling. As most of the area's croplands are managed, little space remains in which herbs can grow. We believe that the scarcity of feeding sites and food resources in croplands raised the corticosterone concentration of the species we studied. Additional physiological costs could be the result of greater predation pressure and increased competition among bird species in cropland at this critical time of the year, as recorded by other authors evaluating the effect of antagonistic relationships on stress (Romero et al. 2000, Barbosa et al. 2006, Dickens et al. 2009). Urban areas, on the other hand, tend to offer predictable and abundant food resources year round (Shochat 2004, Schoech et al. 2007, Fokidis et al. 2009), and the forest edges we studied have relatively large areas with unmanaged grasses where we have recorded numerous groups of M.fusca and C. inca feeding.
Immunoglobulin concentrations differed greatly by species and habitat. In M. fusca, immunoglobulin concentrations were higher at forest edges and croplands. A high concentration of immunoglobulin reflects two main possible scenarios: (1) strong immunocompetence, allowing the individual to respond to pathogens successfully, and (2) a response to infection (Guillette 1980, Martínez et al. 2003, Perozo et al. 2007). On the other hand, the low concentration of immunoglobulin in M. fusca in urban areas could be related to (1) an idle immune system or (2) a depression of the immune system in response to chronic stress, making the bird vulnerable to pathogens. In contrast to what we found for M. fusca, the immune condition of C. inca rose significantly from forest edges to croplands to urban sites. As suggested previously, a low concentration of immunoglobulin is often related to an idle immune system or the incapability of responding to pathogens, while a high concentration indicates strong immunocompetence and/or the successful response to pathogens.
Contrary to the expected tight relationship between the concentrations of corticosterone in feces and immunoglobulin in blood, we only found two significant relationships. The relationship between corticosterone and immunoglobulin concentrations in M. fusca in croplands was weak, with a statistical power lower than expected. For these reasons, we consider this result cautiously. On the other hand, we found a strong significant negative relationship between these variables for M. fusca in urban areas. Thus some of the population of M. fusca in urban areas had both a high corticosterone concentration and low immunoglobulin concentration, suggesting that the birds are under chronic stress (Chávez-Zichinelli et al. 2010). In such a scenario, high corticosterone concentrations for a prolonged time can reduce the number of B cells, which is subsequently translated into the reduction of immunoglobulin synthesis (Vleck et al. 2000, Royo et al. 2004, Dhabhar 2009). Our threshold analysis suggests that 30% of the towhees captured in urban areas are under such stress. Finally, our body-condition analyses support this hypothesis, as in urban areas we recorded individuals of M. fusca in poorer body condition, which are more likely to be vulnerable physiologically.
Densities of M. fusca were significantly lower at urban sites than in the other habitats. While lower densities could be related to lesser food resources, urban areas tend to favor granivores (Chace and Walsh 2006) and to offer highly predictable food resources in large quantities (Shochat 2004). However, we found the density of M. fusca to be lower in urban areas, where the proportion of physiologically altered individuals was greatest. Our results suggest that the habitat variables influencing the physiological condition of M.fusca also affect its population density. In contrast to those of M. fusca, densities of C. inca did not differ significantly by habitat. Although with C. inca we found different physiological scenarios in the three habitats studied, they do not seem to have direct relationships with the habitat traits that affect the species' density. This results could have two possible explanations: (1) that the physiological scenarios measured are not critical enough to modify the species' density, making the Inca Dove ecophysiologically capable of withstanding human disturbance of different types and different intensities, and (2) that sink-and-source dynamics of the meta-population, not evaluated in this study, maintain similar densities in all habitats where the species is present.
Our study assessed the physiological response of two sexually monomorphic landbirds in complex human-modified landscapes. The conditions of our study prevent us from being able to dissociate potential sexual effects (Bonier et al. 2007) or the effects that multiple factors in the complex landscape could have on these species. Because birds could move from the habitats sampled to other habitats in the landscape we were not able to relate the species' physiological condition with the habitat traits measured. Thus our results represent the responses of birds to landscape conditions in which human disturbance varied and indicate different responses of both species to human disturbance. Nonetheless, a difference between the two species in response to habitat could explain our ecophysiological results, as Fokidis et al. (2008) reported in their assessment of other species' physiological responses to urban and rural areas. Because we did not find a large proportion of physiologically altered Inca Doves in the habitats studied, and the species' density was similar in each, we do not have evidence that the measured habitat traits could determine its ecophysiology. In M. fusca, however, we found significantly lower densities at urban sites, where the proportion of physiologically altered individuals was greatest. Lower densities of this species could be explained by traits of urban habitat. As shrub cover provides shelter from predators and human disturbance in urban areas, the low value of shrub cover in urban areas suggests that birds that are solitary or in pairs, such as M. fusca (Johnson and Haight 1996), could be exposed to human-based stressful stimuli in this habitat. Also, tree cover and height have been identified as factors that affect the species richness of birds in urban sites (Munyenyembe et al. 1989, MacGregor-Fors 2008), including several species of granivores and omnivores in our study area (MacGregor-Fors et al. 2012). This could increase the number of antagonistic interactions between species, driving M. fusca to a physiological state less than optimal. In addition to competition, the sparse cover of herbs in urban areas decreases the species' microhabitat for foraging (Johnson and Haight 1996). Altogether, our ecophysiological results suggest that in urban habitat with extensive tree cover but little shrub and herb cover, intense human activity could depress the physiological condition of a significant proportion of the population of M. fusca below the optimum. However, we have no evidence that C. inca is physiologically affected under the habitat conditions studied. In summary, our results suggest that a higher proportion of Canyon Towhees have physiological limitations in urban areas, and possibly in croplands, whereas the Inca Dove seems to have a successful physiological response despite its low values for body condition in urban areas.
Acknowledgments
We thank Katherine Renton, Roxana Torres, and two anonymous reviewers for their helpful comments that improved our manuscript. We are also grateful to Lorena Morales-Pérez, Karla Tapia, Leticia Mirón-Melo, and Alejandro Rebollar for their help in the field. Viola Leticia Pérez-Castillo helped us with some laboratory assays. Research funds were granted to J.E.S. by the Macroproyecto: Manejo de Ecosistemas y Desarrollo Humano—Universidad Nacional Autónoma de México (SDEI—PTID—02), PAPIIT Project (IN228007-3), and Fondos Mixtos CONACYT—Gobierno del Estado de Michoacán (65503). We thank the St. Louis Audubon Society for financing some of the materials used in this study. C.A.C-Z., as part of the Graduate Program in Biological Sciences of the Universidad Nacional Autónoma de México, acknowledges the scholarship and financial support provided by the National Council of Science and Technology (CONACYT, 48612).
Literature Cited



