-
PDF
- Split View
-
Views
-
Cite
Cite
Ralph Buij, Kim Kortekaas, Roderick R. D. Van Krimpen, Rien Van Wijk, Saskia Van Der Zanden, Hans H. De Iongh, Ignas M. A. Heitkönig, Geert R. De Snoo, Jan Komdeur, Breeding Performance of the Grasshopper Buzzard (Butastur rufipennis) in a Natural and A Human-Modified West African Savanna : Desempeño Reproductivo de Butastur rufipennis en una Sabana Natural y una Antrópica del Oeste de África , The Condor: Ornithological Applications, Volume 115, Issue 1, 1 February 2013, Pages 47–57, https://doi.org/10.1525/cond.2012.120049
Close - Share Icon Share
Abstract.
Few studies have examined raptor reproduction in response to land-use change in sub-Saharan Africa, hampering conservation efforts to address regional declines. To further our understanding of mechanisms underlying the dramatic declines of West African raptors, we examined the relationship between environmental conditions, nest density, and measures of reproduction in the Grasshopper Buzzard (Butastur rufipennis). Analyses were based on 244 nest sites divided between transformed and natural habitat in northern Cameroon. At the landscape scale, nest density increased with the density of preferred nest trees. Nests were more widely spaced in transformed than in natural habitat. Dispersion was adjusted to differences in availability of small mammals, which was negatively associated with distance to nearest neighbor, and in the area under cultivation, which was positively associated with distance to nearest neighbor. Productivity was positively associated with rainfall, canopy shielding the nest, availability of grasshoppers, and the nest's visibility from ground level; canopy shielding, grass cover, rainfall, and distance to nearest neighbor were positively associated with nest success. In natural habitat, losses of eggs and nestlings to natural predators were greater than in transformed habitats, while losses through human predation were small. Productivity and nest success were unaffected by land use because of the opposing effects of greater predation pressure, closer spacing of nests, and more food in natural habitat than in transformed habitat. Thus transformed habitat may provide adequate breeding habitat for the Grasshopper Buzzard, but declining rainfall and intensifying anthropogenic land use are likely to affect future reproductive output.
Resumen.
Pocos estudios han examinado la reproducción de las rapaces en respuesta a cambios de uso del suelo en África sub-sahariana, dificultando los esfuerzos de conservación que aborden las disminuciones regionales. Para profundizar nuestro entendimiento sobre los mecanismos que subyacen la disminución dramática de las rapaces en el oeste de África, examinamos la relación entre condiciones ambientales, densidad de nidos y medidas de la reproducción en Butastur rufipennis. Los análisis se basaron en 244 sitios de nidificación divididos entre hábitats transformados y naturales en el norte de Camerún. A la escala de paisaje, la densidad de nidos aumentó con la densidad de los árboles de nidificación preferidos. Los nidos se espaciaron más ampliamente en los hábitats transformados que en los naturales. La dispersión se ajustó a las diferencias en la disponibilidad de pequeños mamíferos, la que se asoció negativamente con la distancia al vecino más cercano, y a las diferencias en el área bajo cultivo, la que se asoció positivamente con la distancia al vecino más cercano. La productividad se asoció positivamente con la precipitación, la protección del nido por el dosel, la disponibilidad de saltamontes y la visibilidad del nido desde el nivel del suelo; la protección del dosel, la cobertura de pasto, la precipitación y la distancia al nido más cercano estuvieron positivamente asociados con el éxito del nido. En el hábitat natural, las pérdidas de huevos y pichones ocasionadas por los depredadores naturales fueron mayores que en el hábitat transformado, mientras que las pérdidas debidas a la depredación humana fueron pequeñas. La productividad y el éxito del nido no se vieron afectadas por el uso del suelo debido al efecto opuesto de una mayor presión de depredación, espaciado más cercano de los nidos y más alimento en el hábitat natural que en el transformado. Por lo tanto, el hábitat transformado puede brindar un hábitat reproductivo adecuado para B. rufipennis, pero la disminución en la precipitación y la intensificación en el uso antrópico del suelo es probable que afecten el resultado reproductive futuro.
Introduction
In Africa, a range of bird species have decreased in abundance as land use intensifies (Sinclair et al. 2002, Söderström et al. 2003). Raptors stand out for some of the most dramatic declines (Thiollay 2006, 2007, Ogada and Keesing 2010, Virani et al. 2011), which have been linked to land-use change, implicating livestock grazing, cultivation, pesticide use, and human disturbance in reducing prey availability, nest sites, and reproductive success (Ogada and Kibuthu 2009, Bamford et al. 2009, Virani and Harper 2009). Insectivorous raptors have suffered some of the sharpest declines (Thiollay 2006), possibly through a reduction in invertebrates' abundance due to intense grazing (Herremans and Herremans-Tonnoeyr 2000) and pesticide use (Sánchez-Zapata et al. 2007). However, knowledge of the exact mechanisms behind raptor declines in Africa remains scant, which is alarming in light of the increasing rate of land transformation due to rapidly growing human populations (UN World Population Prospects 2010).
To examine how land use might affect nest density and reproductive success, we quantified reproduction of the Grasshopper Buzzard (Butastur rufipennis) in a protected natural savanna and an unprotected transformed savanna in northern Cameroon. The Grasshopper Buzzard is a small raptor (adult body mass 310–408 g; Ferguson-Lees and Christie 2001) that breeds at the transition from the dry to the wet season in the northern part of its Sudano-Sahelian distribution, north to 18° N. During the dry season, the whole population migrates as far south as 7° N, following the decrease in grass cover due to grazing and fires (Thiollay 1978) to optimize the availability of insect prey (Thiollay and Clobert 1990). The Grasshopper Buzzard's relative abundance in West African savannas and tendency to breed in agricultural habitat make it a suitable model species for examination of the relationships between environmental conditions, nest density, and reproductive output. We hypothesized that Grasshopper Buzzards select nest trees by specific characteristics in transformed and natural habitats and that generally unfavorable food conditions and anthropogenic disturbance result in nest density and productivity being lower in transformed habitat than in natural habitat.
First, to assess how human land use influences nest availability, we examined structural characteristics of nest trees. An assessment of features of preferred nest trees is important because in agro-ecosystems trees are frequently pruned excessively (Ruelle and Bruggers 1982), potentially reducing their suitability as nest sites by increasing nests' vulnerability to predation and weather when the height of the tree or trunk (Newton 1979, Brown and Collope 2008) or canopy cover (Collias and Collias 1984, Rodríguez et al. 2006) is reduced. We predicted that the Grasshopper Buzzard should select nest trees that are less damaged, higher, sturdier, and had greater leaf cover than other trees available but not used and that these characteristics should be selected in both habitats.
Second, we examined the role of environmental factors in driving nest density. Raptors' population density may be limited by the availability of not only nest sites but food, depending on which factor is in shorter supply (Newton 1979). The availability of food to raptors is influenced by the abundance and accessibility of prey (Bechard 1982) and, for perchhunting raptors, trees (Widen 1994). In arid savannas, grass cover provides resources vital to terrestrial prey (Herremans and Herremans-Tonnoeyr 2000), but a dense grass layer may also reduce the Grasshopper Buzzard's foraging success (Thiollay and Clobert 1990). Apart from food supply, human persecution and disturbance may increase the dispersion of raptor nests (e.g., Bisson et al. 2002, Bamford et al. 2009). Although in the North Temperate Zone Sergio et al. (2003) found that raptors' response to the presence of important avian predators varied, nest dispersion may be driven by spatial avoidance of centers of activity (i.e., nest sites) of the most important avian predators (Sergio and Hiraldo 2008). The Grasshopper Buzzard, however, establishes territories at the end of the dry season (Buij et al. 2012), which precedes a seasonal influx of migratory raptors capable of killing nestlings and adults (Thiollay 1976, 1977), limiting options for spatial avoidance of avian predators. Therefore, we expected nest density to be positively associated with tree density and food availability and negatively with increasing human populations and cultivation.
Third, we examined factors potentially responsible for reproductive traits and nest success in transformed and natural habitats. Enhanced reproductive output is often associated with increased prey supply (e.g., Sergio et al. 2004, Pande et al. 2007), while the effects of rainfall on reproduction vary (e.g., Steenhof et al. 1999, Rodriguez and Bustamante 2003, Wichmann et al. 2003). While breeding, Grasshopper Buzzards consume a wide variety of vertebrate and invertebrate prey (Buij et al. 2013), the activity and availability of some of which (e.g., amphibians, insects) increase with rainfall, potentially reducing the need for long-distance foraging flights (Selås 1997). The success of raptor nests may be strongly influenced by natural predators (Sergio and Hiraldo 2008) and structural characteristics of the site that increase concealment from avian and mammalian predators (Tome 2003, Rodriguez et al. 2006). Natural predation is likely to be important in protected areas, which provide refuges for carnivores (Blaum et al. 2007) and large raptors (Thiollay 2006, 2007), whereas anthropogenic disturbance and nest harvesting are likely to be more important in agro-ecosystems (Virani and Harper 2009). Nestlings may become more conspicuous with age (Jehle et al. 2004), and larger broods may so be more vulnerable to predation. Apart from predators, intraspecific competition and aggressive interactions can profoundly depress raptors' reproductive output (Newton 1979), and these factors are likely to play a larger role in natural habitats where nests are more closely spaced than in agro-ecosystems (Thiollay 2007). We therefore expected productivity and nest success to increase with food supply and rainfall as well as with distance nesting conspecifics and inversely with the nest's detectability to predators. On the basis of generally impoverished resources and sharp declines of raptors in transformed habitat (Thiollay 2007), we expected better measures of reproduction in natural habitat.
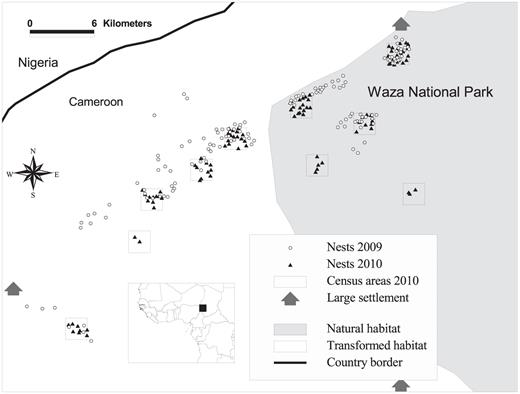
Location of the study area in northern Cameroon (inset), including the location of occupied Grasshopper Buzzard nests in 2009 and 2010 and census areas used to assess landscape correlates of nest density in 2010.
Study Area
Our study area (11° 00′ N—11° 40′ N and 14° 20′ E—15° 00′ E) was roughly equally divided between Waza National Park and the cultivated area southwest of the park in the Far North Region of Cameroon (Fig. 1). We chose the natural and transformed habitats to be adjacent to each other to ensure comparable soil type, topography, and vegetation composition, making land management the major difference. The Sudano-Sahelian climate is characterized by a dry season from November to March, followed by a wet season from April to October (annual rainfall ∼500 mm). Mean annual temperature is 28 °C and peaks from March to May when temperatures of 47 °C are reached. The study area is generally flat with some gentle slopes and three isolated inselbergs. The natural savanna encompasses woodland dominated by Sclerocarya birrea, Anogeissus leiocarpus, and Balanites aegyptiaca locally interspersed with dense clusters of Acacia seyal. The transformed habitat is characterized by a mosaic of cultivation, settlements, and woodland under severe pressure by livestock grazing and slash-and-burn agriculture. Millet and sorghum are the main crops, with some maize and onions, and pesticide use is limited to herbicides at the start of the dry season (November).
Methods
Searches for and Monitoring of Nests
From 2 to 17 May 2009 our team of 4–8 researchers on foot searched for nests in census areas totaling 14 km2 in natural habitat and 32 km2 in transformed habitat; from 3 to 17 May 2010 we searched 20 km2 in each habitat. We selected census areas by their accessibility in the wet season (<13 km from a tarred road). We recorded the coordinates of all raptor nests by GPS and noted their status. Occupied Grasshopper Buzzard nests were lined with green leaves and/or contained one or more eggs or nestlings. They were distinguished from nests of other raptors by size, shape, and characteristic features (e.g., green leaf lining; Ferguson-Lees and Christie 2001) or attending birds if present. We combined additional nest searches in the second half of May and June with visits to occupied nests. We revisited nests without eggs at 1-week intervals to determine their status and declared nests abandoned 4 weeks after discovery if no eggs or attending adults were present. To investigate the possibility of renesting we revisited nests that had failed early in the breeding season two to three times at later stages (20 May—15 June). In an attempt to minimize nest disturbance, we used a mirror on the end of a steel pole to reduce the time needed to check nest contents (∼1–2 min per nest during incubation). We revisited nests at intervals of ∼2 weeks during incubation, 7 days after hatching in 2009, and 5 days after hatching in 2010. We investigated cases of predation by intensively searching the area <100 m around the nest for clues such as clipped or bitten-off feathers, carcasses, broken branches or claw marks on the nest tree, pieces of egg shell, bite marks on eggs, and blood marks. We recorded the number and age of the eggs or nestlings, the date, the type of evidence, and a description of the remains (feathers, body parts). We recorded losses as “unknown cause” in case no remains were found or the identity of the predator could not be established.
Nest-Site Selection and Covariates of Reproductive Success
To determine potential covariates of reproductive output, we examined characteristics of the nest site and its direct surroundings and the distance to conspecifics or sources of human disturbance. Since the habitat may influence raptors' reproduction at multiple spatial scales (Penteriani and Faivre 1997), we sampled nest-site characteristics at <200 m from the nest, representing about half the mean distance to the nearest nest (NND) and core foraging area, and at <1 km, corresponding with the longest observed flight from a nest for foraging (Buij et al. 2012). For each nest, we determined the species of tree (Arbonnier 2004), its diameter at breast height (DBH), and the height of the nest and the nest tree. We categorized damage to the nest tree in four categories based on the number of cut or broken-off branches >20 cm diameter (0, 1, 2, ≥3 branches). We categorized leaf cover visually into three categories based on the percentage of leaf cover on branches (no leaves, 1–50%, 51–100%). We calculated a nest's visibility to terrestrial predators from cumulative scores (0/1) recorded in the four cardinal directions at 15 m from the base of the nest tree. We visually categorized top cover from a position directly underneath the nest, based on the percentage of canopy cover in a 4-m2 square directly over the nest (1: 0–25%; 2: 26–50%; 3: 51–75%; 4: 76–100%). We counted the number of live trees ≥8 m high, categorized by species, and felled trees <200 m from the nest. We recorded the height and cover of grass in four 25-m2 squares in the four cardinal directions at 25 m from the nest tree and averaged them for a mean score per nest tree. We assessed grazing pressure in five categories, from none (0) to severe (4), by the presence of herbivore hoofprints and reduction of the grass height through grazing (visible as bitten-off grass stems) as 0, 1–25, 26ndash;50, 51–75, and 76–100% of the surface area within 50 m of the nest. Three meteorological stations located in the north, northwest, and south of the study area provided the rainfall data throughout the nesting season. We analyzed cumulative precipitation (mm) for the months preceding (April, May) and the months following (June, July) hatching (mean hatch date in 2009 2 June; in 2010 6 June). We assessed the abundance of important prey items (Buij et al. 2013), that is, grasshoppers (>40 mm), reptiles, and amphibians along two pairs of parallel transects 6 m wide, 200 m long (total per nest: 800 m), and 25 m apart running north and south from the nest, between 07:00 and 12:00. We counted rodent burrows along 100-m sections of the transect lines, which is an effective method for estimating the relative abundance of small mammals in arid ecosystems (Vanak and Gompper 2009). The relative availability of reptiles, small mammals, and amphibians was estimated as (adapted from Thirgood et al. 2003) the total number (individuals or burrows)/(grass height × grass cover). We adopted the grasshopper-availability index proposed by Thiollay and Clobert (1990), which represents the number of grasshoppers flushed along the transect line. The percentage of agriculture and woodland <1000 m from the nest and the distance of the nest tree to the nearest village, agricultural field, tarred road, and nearest neighboring nest we determined with ArcView 3.2 (ESRI, Redlands, CA) from digitized maps and Google Earth's high-resolution global imagery.
We examined nest-tree selection to assess whether buzzards selected specific features of trees from among those available within the range, measuring tree height, tree damage, DBH, and leaf cover of unused trees within 100 m of a random subsample of 80 nest trees occupied in 2010 (equally distributed among habitats). Unused trees were ≥8 m high and judged capable of supporting a Grasshopper Buzzard nest. We selected these trees by walking north 100 m from the nest tree and selecting the tree nearest the nest tree.
Covariates of Nest Density and Range Size
We examined the determinants of the density of Grasshopper Buzzard nests at the nest-site level, using NND as an inverse proxy measure of nest density (Newton et al. 1977), and at the landscape scale (4-km2 census areas). We examined the relationship between NND and measures of food availability, including tree density and the cover of agricultural fields within 1000 m of the nest. For analyses at the landscape scale, we measured tree density, food availability, size of the human population, and the area under cultivation in the ten census areas in 2010 with ArcView GIS 3.2 (Fig. 1) and related those to nest density. As for nest sites, we evaluated characteristics of the environment and of prey at six survey points randomly generated within each census area. The number of potentially suitable nest trees (hypothesized as all trees ≥8 m) in the census areas were counted and identified to species. We estimated the size of the human population by multiplying the number of houses by mean household size, verifying it with information from village chiefs.
Statistical Analyses
On the basis of a priori hypotheses we built models for the influence of environmental characteristics on nest density, nest success, NND, and productivity, the last two by a backward stepwise procedure. To limit pseudoreplication, nests at the same location (nest tree; n = 6) were included only once in all analyses. We considered pairs of correlated variables (r > 0.60) to be estimates of one underlying factor and excluded variables according to common sense (e.g., rainfall in June and July has no effect on clutch survival), retaining the biologically most relevant variable for analysis (Green 1979).
For analyses of covariates of NND, we employed a generalized linear model (GLM) on log-transformed NND and a normal distribution with an identity-link function and verified a model's adequacy by examination of residuals (McCullagh and Nelder 1989). Independent variables included the agricultural cover within 1000 m, tree density, grass cover, availability of grasshoppers, small mammals and reptiles, and year (amphibian availability was excluded because of limited numbers). We used Standard linear and S-curve regression to model nest density within census areas for tree density, grass cover, grasshopper, and small-mammal and reptile availability separately. Human population size and cultivation were considered only for transformed habitat. We modeled correlates of productivity with an ordinal regression model and a negative log-log link function. Besides the predictor variables listed above, we used brood size and land use to model number of fledglings, with year, top cover, and visibility as categorical factors. To test models' suitability we used tests of parallel lines.
We used MARK software (White and Burnham 1999) to estimate daily nest survival by Mayfield's method (Mayfield 1975, Johnson 1979), which has been adapted to allow for evaluation of a variety of spatial and temporal factors that might influence nest success (Dinsmore et al. 2002, Rotella et al. 2004). We carried out three different analyses, since factors affecting nest survival are likely to differ by nest stage (Rotella et al. 2004) and the age of most nests failing prematurely was unknown: (1) clutch survival with an assumption of constant daily survival rate (DSR; Dinsmore et al. 2002), i.e., a survival rate that remained constant through the egg phase, (2) a variable DSR, i.e., survival that varied with nest age, for nests with nestlings, and (3) a DSR constant for all nests. Nests for which DSR was modeled to vary with age included only those with nestlings for which body measurements were taken early in the nestling phase. A 30-day incubation period from egg laying to hatching of the last nestling and a 36-day nestling period (Buij et al. 2012) were the basis of calculations of overall nest success. We assumed nests failed at the midpoint between the date of the last visit in which eggs or young were recorded in the nest and the date of the next visit when the nest was empty and considered eggs infertile if older than 38 days, irrespective of the presence of a breeding female.
We used the second order Akaike's information criterion corrected for small sample sizes (AICc) to evaluate nest-survival models and to select the best model fit by a forward stepwise procedure (Dinsmore et al. 2002, Rotella et al. 2004).
We examined potential differences in measures of reproduction by habitat. Clutch size and nestling number refer to the number of eggs and nestlings recorded at a nest, respectively. Date of laying we calculated by subtracting 28 days from the date of hatching of the first hatchling, corresponding with the incubation period to hatching of the first egg (Buij et al. 2012). We estimated the date of hatching from nestlings' stage of feather development (Buij et al. 2012). Fledgling number was the number of nestlings that reached 80% of the mean age at fledging (compare Steenhof and Newton 2007), i.e., ≥29 days (Buij et al. 2012), and calculated separately for all pairs and for successful pairs only. We considered a nest successful if it produced at least one fledgling. Hatching success is the nestling number divided by clutch size. Fledging and breeding success were the fledgling number divided by nestling number and clutch size, respectively.
We used paired-samples t-tests to test whether mean differences in height and DBH between nest trees and associated trees differed from zero, and Wilcoxon signed-rank sum tests to test for differences in tree damage and leaf cover. We investigated relationships with Pearson's correlations when the data did not violate the criteria of linearity, normality, and homoscedasticity; otherwise, we used Spearman's correlations. We used χ2 contingency tables to test for an interaction between habitat and nest-tree characteristics and a χ2 goodness-of-fit test to investigate whether proportional use of nest tree species differed from availability and to investigate differences in the relative frequency of loss through predation by habitat. To test for differences between natural and agricultural areas in measures of reproduction and habitat, we used Mann—Whitney U-tests. Shapiro—Wilk tests were used to test for normality, and data were log-transformed before analyses to satisfy the criterion of normality. For these analyses we used SPSS 16.0. All data are expressed as means ± SE, and statistical significance was set at α < 0.05.
Results
Nest-Site Characteristics
In total, 244 occupied nest sites were fully described over the course of the study, 131 in natural habitat and 113 in transformed habitat. Grass cover and height, and the availability of small mammals and grasshoppers, were greater around nests in natural than transformed habitat, but reptile availability was higher in transformed habitat (Table 1). Grass cover around nests was associated with small mammal (rs = 0.47, n = 244, P < 0.001), grasshopper (rs = 0.29, n = 244, P < 0.001) and reptile abundance (rs = 0.23, n = 244, P < 0.001), indicating decreasing prey populations along a gradient of increasing soil denudation. Sclerocarya birrea trees were preferred over other tree species present in the study area (χ21= 196, P < 0.001). Nest height was related to nest tree height (r = 0.49, n = 244, P < 0.001), which exceeded the height of unused trees within the range, in both habitats (Table 2). No differences between habitats in nest visibility and top cover were recorded.
Comparison of the characteristics of vegetation and prey availability at nest sites of the Grasshopper Buzzard in natural (n = 131) and transformed habitats (n = 113) in northern Cameroon. Significance of results of Mann-Whitney U-tests comparing the characteristics in the two habitats is indicated with asterisks (*P < 0.05, **P < 0.01, ***P < 0.001). See text for units of measurement and details of measures of vegetation and prey availability.

Comparison of the characteristics of vegetation and prey availability at nest sites of the Grasshopper Buzzard in natural (n = 131) and transformed habitats (n = 113) in northern Cameroon. Significance of results of Mann-Whitney U-tests comparing the characteristics in the two habitats is indicated with asterisks (*P < 0.05, **P < 0.01, ***P < 0.001). See text for units of measurement and details of measures of vegetation and prey availability.

Covariates of Nest Density and Nnd
At the landscape level, nest density increased with the density of Sclerocarya birrea trees (F1,8 = 7.09, adjusted r2 = 0.40, P < 0.05; Fig. 2). Other measures of food availability, grass cover, cultivation, and human population size did not contribute significantly to explaining the variance in nest density at the landscape scale. Mean nest density was 3.25 nests km2 in natural habitat (±2.55; range 0.75–7.00) and 2.40 nests km-2 in transformed habitat (±1.35; range 0.50–4.25; U5,5 = 9.5, Z = -0.63, P = 0.55). NND in natural habitat (317 ± 117 m; range 81–838) differed from that in transformed habitat (449 ± 244 m; range 131–1430; U131,113 = 4496, Z = -5.36, P < 0.001). NND decreased with increasing availability of small mammals but increased with cultivation (Table 3), suggesting that depletion of prey resources and expanding cultivation negatively influenced nest density in the transformed habitat.
Characteristics measured at 40 trees with Grasshopper Buzzard nests and at paired trees without nests in a circular plot of radius 100 m centered on the nest tree, in natural and transformed habitat in northern Cameroon. Test results are of tests of the null hypothesis that the mean difference is zero.
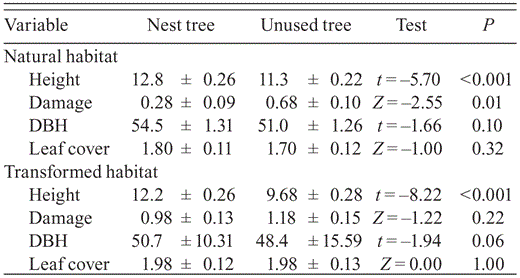
Characteristics measured at 40 trees with Grasshopper Buzzard nests and at paired trees without nests in a circular plot of radius 100 m centered on the nest tree, in natural and transformed habitat in northern Cameroon. Test results are of tests of the null hypothesis that the mean difference is zero.

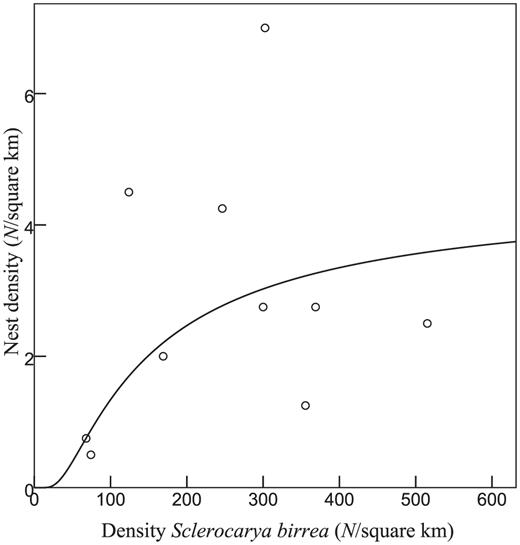
S-curve regression model for nest density and the number of Sclerocarya birrea trees ≥8 m high inside nest census areas (compare Fig. 1).
Results for a generalized linear model incorporating the effect of environmental and prey variables surrounding Grasshopper Buzzard nests in natural (n = 131) and transformed habitats (n = 113) on the distance to the nearest neighboring nest. Model fit: χ22 = 39.7, P < 0.001.

Results for a generalized linear model incorporating the effect of environmental and prey variables surrounding Grasshopper Buzzard nests in natural (n = 131) and transformed habitats (n = 113) on the distance to the nearest neighboring nest. Model fit: χ22 = 39.7, P < 0.001.

Measures of performance of Grasshopper Buzzards nesting in natural and transformed habitats in northern Cameroon, 2009–2010.
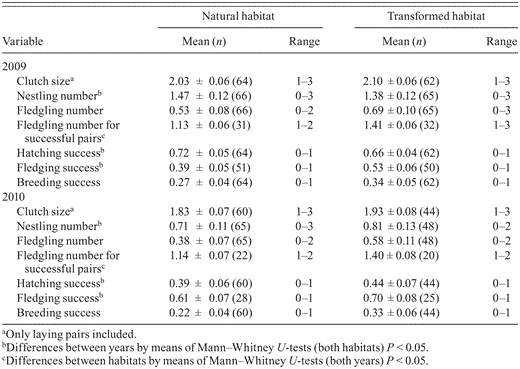
Measures of performance of Grasshopper Buzzards nesting in natural and transformed habitats in northern Cameroon, 2009–2010.

Covariates of Reproductive Success
The date of laying in transformed habitat in (6 May ± 4 days) did not differ from that in natural habitat in 2009 (5 May ± 3.5 days; U31,31 = 480, Z = -0.01, P = 0.99) or in 2010 (9 May ± 8 days vs. 9 May ± 7 days; U36,28 = 484, Z = -0.27, P = 0.79). Fledglings were produced at 43% of discovered nests (yearly range 37–48%; n = 244), 41% (34–47%; n = 131) in natural habitat and 46% (42–49%; n = 113) in transformed habitat. The Mayfield estimate of overall nest success for 2009 and 2010 combined was 39%, based on a DSR of 0.986 ± 0.001 (n = 244). Measures of reproduction generally did not differ by habitat, although for successful pairs fledgling numbers were higher in transformed than in natural habitat in both 2009 and 2010 (Table 4). An ordinal regression model indicated a positive association of fledgling numbers with April rainfall, grasshopper availability, top cover, and nest visibility (Table 5). Nest-survival models showed that nests' DSR was unaffected by land use during incubation, the nest cycle, and for nests of known age (those that held nestlings) but varied with measures assumed to influence risk of predation, intraspecific competition, and food availability (Table 6). The most parsimonious model for DSR included a negative effect of nest age and brood size, suggesting that older nests and those with multiple nestlings were more likely to perish than younger broods of single chicks. Conversely, top cover was positively associated with overall nest survival and with survival of nests that held nestlings, while tree height was positively related to clutch survival. Rainfall positively influenced clutch, nestling, and nest survival, and nest survival was positively associated with NND and grass cover. Food supply around the nest (availability of grasshoppers and small mammals) was positively associated with nestling survival, but small-mammal availability was negatively associated with clutch survival.
Egg and Nestling Loss
Mammalian predation, clutch abandonment, and starvation and/or siblicide were the most common causes of failure at nests in both habitats (Table 7). Avian predation was rarely recorded (Table 7), but mammalian predation of eggs (χ21 = 24.3, P < 0.001) and nestlings (χ21 = 13.0, P < 0.001) was more common in natural than in transformed habitat. Overall, pairs in natural habitat incurred greater losses of eggs (χ21 = 7.33, P < 0.01) and nestlings (χ21 = 6.03, P < 0.05) to avian and mammalian predators than did nests in transformed habitats, while losses caused by human harvesting were relatively small.
Ordinal-regression model of nest-site and environmental variables on Grasshopper Buzzard nests with 0 fledglings (n = 136), 1 fledgling (n = 77), and 2 fledglings (n = 23) in northern Cameroon, 2009–2010 (years pooled). Nests with 3 fledglings (n = 2) omitted. Model fit: χ27 = 32.1, P < 0.001; Nagelkerke r2 = 0.22.
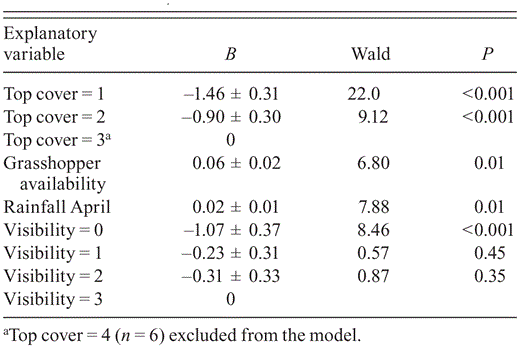
Ordinal-regression model of nest-site and environmental variables on Grasshopper Buzzard nests with 0 fledglings (n = 136), 1 fledgling (n = 77), and 2 fledglings (n = 23) in northern Cameroon, 2009–2010 (years pooled). Nests with 3 fledglings (n = 2) omitted. Model fit: χ27 = 32.1, P < 0.001; Nagelkerke r2 = 0.22.

Best-supported models (ΔAIC c = 0.00) describing survival of nests with constant daily survival rate (DSR; n = 244), nests of known age and variable DSR (n = 90), and nests in the incubation stage with constant DSR (n = 186).
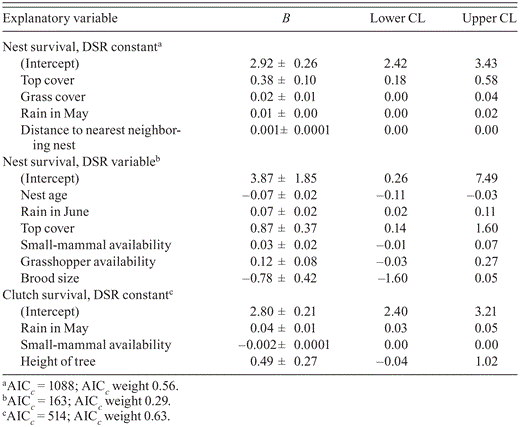
Best-supported models (ΔAIC c = 0.00) describing survival of nests with constant daily survival rate (DSR; n = 244), nests of known age and variable DSR (n = 90), and nests in the incubation stage with constant DSR (n = 186).

Discussion
Contrary to expectations, we recorded no significant effect of land use on the Grasshopper Buzzard's productivity and nest success, suggesting that the agricultural landscape in northern Cameroon provided adequate habitat. This was related primarily to the opposing effects of generally more favorable resource conditions in natural habitat, whereas pairs in transformed habitat profited from fewer losses due to natural predation and longer distances between nests, which perhaps reduced intraspecific interference and competition (Newton 1979). The importance of natural predation to breeding Grasshopper Buzzards was supported by our observation that nest-tree selection and reproductive output were strongly influenced by the nest's vulnerability to predation. First, the greater odds of failure of nests with increasing age and brood size suggests that increased conspicuousness of larger, older broods, perhaps through excrement, heightened activity, and the smell from prey remains, increased the odds of detection and predation (Di Giacomo et al. 2011). The positive effect of tree height on clutch survival further implied that selection for relatively high trees was adaptive, possibly because it reduced access or detectability by common mammalian predators, such as the African Wild Cat (Felis silvestris) or viverrids. Several adults were killed at the nest by these or other mammalian carnivores, which were responsible for most losses of eggs and nestlings in natural habitat, underlining their importance as nest predators.
Although we recorded little direct evidence of predation by avian predators, the canopy cover shielding the nest from the sky, and thus avian predators (Tome 2003, Rodriguez et al. 2006), was positively associated with nest success and fledgling number. This suggests that Grasshopper Buzzards could escape detection and predation by avian predators by improving nest concealment. Raptors frequently pose the greatest risk of predation to small and medium-sized raptors (Sergio and Hiraldo 2008), and this also seems true in African savannas (Thiollay 1976), where seasonal migrants may be among the most important predators of Grasshopper Buzzard nestlings. Indeed, Wahlberg's Eagle (Aquila wahlbergi) and the Red-necked Buzzard (Buteo auguralis), intra-African migrants, were common in our study area and frequently mobbed by Grasshopper Buzzards, suggesting they were perceived as an important threat (Coulson et al. 2008). Additionally, canopy shielding might have protected broods from rainstorms or excessive heat gain from solar radiation, which may harm eggs and nestlings (Collias and Collias 1984). Although this factor might have affected nestlings' survival, top cover was not significantly associated with clutch survival. Finally, the somewhat counterintuitive positive association of productivity with increasing visibility of the nest from the ground might be explained partly by the low rate of human harvesting and mammalian predators detecting prey primarily by olfactory and auditory cues (Selås 1997, Kleindorfer et al. 2005). Greater visibility from the nest might have also improved the Grasshopper Buzzards' ability to detect predators (Götmark et al. 1995) or defend the nest (Selås 1997), benefiting productivity rather than depressing it.
Causes of loss of eggs or nestlings (%) at Grasshopper Buzzard nests in natural (n = 131) and transformed habitats (n = 125) in northern Cameroon, 2009–2010.
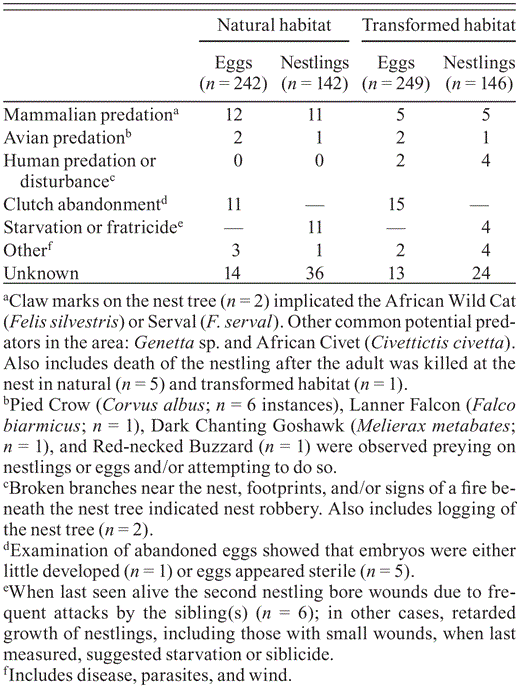
Causes of loss of eggs or nestlings (%) at Grasshopper Buzzard nests in natural (n = 131) and transformed habitats (n = 125) in northern Cameroon, 2009–2010.

We found that rainfall was positively associated with the Grasshopper Buzzard's nest success and productivity, adding to existing evidence of the positive role of rainfall in raptors' productivity in arid African savannas (Hustler and Howells 1990, Wichmann et al. 2003). Rainfall triggers a flux of prey (Thiollay 1978), which is unpredictable at the start of breeding, limiting possibilities for opportunistic movements to other, more productive areas during the breeding season. We found clutch and brood survival positively associated with rainfall at corresponding periods during the breeding season, which may point to the potential for adjustment of breeding investment through continuous resource tracking (Sergio et al. 2011). Despite the Grasshopper Buzzard's apparent behavioral flexibility in response to fluctuating conditions, the decline of rainfall in the region since the late 1960s (Hulme et al. 2001) might have depressed its reproductive output consistently, potentially contributing to its population decline from 1969 to 2004 (Thiollay 2006). According to predictions of some climatic models (e.g. Held et al. 2005), the drying of the Sahel is likely to continue, which would aggravate conditions for breeding.
Conservation Implications
Although the majority of afrotropical raptors have probably been affected significantly by land-use changes in the West African savanna (Thiollay 2006, 2007), in northern Cameroon Grasshopper Buzzards showed a high degree of flexibility in the face of the transformation of their breeding habitat. Although human land use reduced the availability of grasshoppers and small mammals, sparse grass cover in the transformed habitat improved the accessibility of important reptile prey. In our study area, Grasshopper Buzzards were dependent on Sclerocarya birrea trees for nesting and selected relatively large trees, but such trees were as widely available in transformed as in natural habitat. Pairs even nested in stunted, isolated trees in croplands, and in five instances continued using nests in trees that were heavily pruned during the breeding season. Importantly, in transformed habitat pairs incurred lower losses to natural predators than in natural habitat in our study area, and this pattern may be widespread, as agroecosystems generally support fewer mammalian and avian predators than do natural habitats (Blaum et al. 2007, Thiollay 2006, 2007). Finally, we detected little evidence of nest harvesting by humans in our study area, which Thiollay (2007) and Anadón et al. (2010) mentioned as a significant factor contributing to declines of raptors in West African savannas. Although we suspect that our presence in the study area might have contributed to the low incidence of human harvesting, it appeared to be largely related to the unsuitability of raptors as food for the predominantly Muslim population in the area, the majority of whom consider consumption of raptor products and their derivatives unlawful (R. Buij, unpubl. data).
Despite conditions apparently suitable for breeding in transformed habitat, we found that the distance between Grasshopper Buzzard nests increased with increasing cultivation around nests. This may have been related to human activities in cultivated fields; although breeding pairs seemed fairly tolerant of farmers working near the nest, the coincidence of the breeding season with the start of the crop-growing season might have increased the odds of disturbance and early desertion of nests in our study area. Furthermore, grass cover and the availability of small mammals and grasshoppers, which positively influenced nest density, productivity, or nest success, were more favorable in natural than in transformed habitat. This suggests that human land use, in the form of livestock grazing and expanding cultivation, has the potential to depress Grasshopper Buzzard populations. Such changes, which have characterized the Sudano-Sahelian breeding range of the Grasshopper Buzzard over the past four decades (Wardell et al. 2003, Brink and Eva 2009), are likely to continue unabated as the region experiences some of the world's highest rates of human population growth (∼3% per year; UN World Population Prospects 2010) and agricultural yields generally show little sign of improvement (Njomaha 2004). Education about the potential value of raptors in pest control (SánchezZapata et al. 2007, Sergio et al. 2008) might prove valuable to local initiatives for conservation of breeding raptors in agroecosystems, which should focus on sustainable intensification of agriculture and retaining native trees in croplands.
Acknowledgments
This study was financially and logistically supported by the Institute of Environmental Sciences (CML) of the University of Leiden, the Netherlands, through its collaborative program with the University of Dschang, Cameroon, at the Centre for Environment and Development Studies in Cameroon (CEDC). We gratefully acknowledge A. Boukar, A. Ali, I. Folkertsma, and B. Croes of CML for their important contribution to data collection. We thank B. Croes, P. Scholte, and two anonymous reviewers for comments that improved an earlier draft. We thank J.-P. Mvondo and H. Hamadou of CEDC for their assistance with logistics.
Literature Cited



