-
PDF
- Split View
-
Views
-
Cite
Cite
Rafael Villegas-Patraca, Ian Macgregor-Fors, Teresa Ortiz-Martínez, Clara E. Pérez-Sánchez, Leonel Herrera-Alsina, Carlos Muñoz-Robles, Bird-Community Shifts in Relation To Wind Farms: A Case Study Comparing a Wind Farm, Croplands, and Secondary Forests in Southern Mexico, The Condor: Ornithological Applications, Volume 114, Issue 4, 1 November 2012, Pages 711–719, https://doi.org/10.1525/cond.2012.110130
Close - Share Icon Share
Abstract.
Aside from the positive benefits of wind energy, wind farms often bring environmental problems such as noise production and wildlife collision. However, little is known about the effects of wind farms on the ecology of tropical landbirds. In this study, we evaluated changes in bird-community diversity, composition, and structure directly beneath wind turbines, 200 m away from turbines, and in nearby croplands and secondary forests. In general, our results show (1) a gradient of species richness, with values highest in croplands and secondary forests, intermediate values 200 m from turbines, and lowest values beneath turbines, (2) fairly similar bird abundance for all treatments, with values highest in secondary forests in autumn and lowest 200 m from turbines in autumn, (3) bird communities highly similar at each season, but communities at 0 and 200 m from turbines differed strongly in autumn and communities at the rest of the studied sites differed strongly during both spring and autumn, (4) evenness of the bird community greater in secondary forests and croplands and lower at both distances from wind turbines, and (5) the area covered by croplands outside the wind farm played an important role, often related to increases in species richness. Our results also suggest that wind farms have a greater effect on wintering migrants than on residents; however, further studies are required for such a comparison to be tested robustly.
Resumen.
Además de los efectos positivos que tiene Ia energía eólica, las centrales eólicas implican impactos ambientales negativos (e.g., generación de ruido, colisión de fauna voladora). Sin embargo, se sabe poco sobre los efectos que pueden tener las centrales eólicas sobre las aves tropicales. En este trabajo evaluamos los cambios que ocurren en las comunidades de aves en dos escenarios dentro de una central eólica (debajo de los aerogeneradores y a 200 m de ellos) y en campos de cultivo y bosques secundarios cercanos. Nuestros resultados muestran: (1) un gradiente de riqueza de especies, con valores máximos en campos de cultivo y bosques secundarios, valores intermedios a 200 m de los aergoeneradores y valores mínimos debajo de ellos, (2) abundancias de aves similares en los tratamientos estudiados, con valores máximos en bosques secundarios en otoño y valores mínimos a 200 m de los aergoeneradores en otoño, (3) alta similitud entre comunidades de aves relacionada con la época del año, con diferencias importantes entre las dos condiciones de la central eólica en otoño y el resto de los tratamientos estudiados en ambas temporadas, (4) alta equitatividad de las comunidad de aves en bosques secundarios y campos de cultivo, con comunidades altamente dominadas en ambas condiciones dentro de la central eólica, y (5) un papel importante del area cubierta por campos de cultivo en los tratamientos ubicados fuera de la central eólica, generalmente relacionada con aumentos en la riqueza de especies de aves. Finalmente, nuestros resultados sugieren que las centrales eólicas tienen un efecto más severe sobre las especies de aves migratorias; sin embargo, estudios posteriores son necesarios para probar dicha relación de manera robusta.
Introduction
Human population growth and development in past centuries has radically changed the scope and nature of how humans transform the land (Vitousek et al. 1997, Kennedy et al. 2010). Related to the fulfillment of basic human needs, such disturbances include agriculture, livestock production, industry, and wealth-related issues (WHO 2005) and represent a serious threat to biodiversity (Czech and Krausman 1997, Czech et al. 2000). Along with sustainable agriculture and environmentally friendly urbanization, alternative techniques to generate electric power, such as from the wind, have been developed worldwide (WWEA 2010).
Increasing numbers of wind farms are being established worldwide, with commercial facilities in nearly 80 countries around the globe (Redlinger et al. 2002, GWEC 2010). Compared to other techniques of producing electric power, wind energy is renewable, does not create air pollutants, and tends to reduce human dependence on fossil fuels (Kikuchi 2008, WWEA 2010). Although the economic cost of establishing and operating wind farms is often high (Royal Academy of Engineering 2004), that cost is predicted to drop in the near future (Hearps and McDonnell 2011). Wind farms also have negative effects, such as noise production, land-use transformation, modification of the view of the landscape, and increased wildlife mortality, mainly through collisions with wind turbines (Osborn et al. 2000, Johnson et al. 2002, 2004, Barrios and Rodriguez 2004, Arnett et al. 2007, Baerwald and Barclay 2009, Garvin et al. 2011). Specifically for birds, wind farms can have both direct and indirect negative effects, such as collision with wind turbines and/or displacement through barrier effects and/or habitat change or loss (Drewitt and Langston 2006). However, little is known about the effects of wind farms on landbirds, which do not typically collide with turbines (Drewitt and Langston 2006). Leddy et al. (1999) found that turbine noise and the physical movement of the turbines' blades can disturb nesting birds, but there is no information about the effect of wind farms on the diversity, structure, and composition of bird communities in tropical regions. We evaluated the bird communities directly beneath and 200 m away from wind turbines in a tropical region and contrasted these with the bird communities of croplands and secondary forests in a region of Mexico important for landbird migration.
Methods
We carried out this study on the Isthmus of Tehuantepec (17° 15′ N, 94° 31′ W), in southern Oaxaca, Mexico (Fig. 1). The climate of this region is tropical subhumid, with a summer rainfall regime (Garcia 1988). The annual average rainfall, as recorded at two meteorological stations in the region, is 1019 mm. Drought prevails from November to April; most of the year's rain falls from June to September. The annual average temperature is 25 °C (SMN 2000). Aside from urbanization, major human disturbances in the study area are related to agriculture; the establishment of wind farms represents a novel type of threat to wildlife. In this study, we surveyed bird communities in a wind farm, patches of secondary forest, and croplands within the same region. The wind farm covers an area of 982 ha, of which ∼67% are croplands and the remaining ∼33% are patches of secondary forest. Patches of tropical secondary forest have arisen mostly from the abandonment of cropland; semi-deciduous forests occur in humid areas (e.g., near intermittent streams). Thus areas of secondary forest are highly fragmented, generally embedded in a matrix of agriculture, and divided by small roads and unpaved tracks. Finally, croplands mainly contain sorghum plots that are frequently associated with lowintensity livestock grazing.
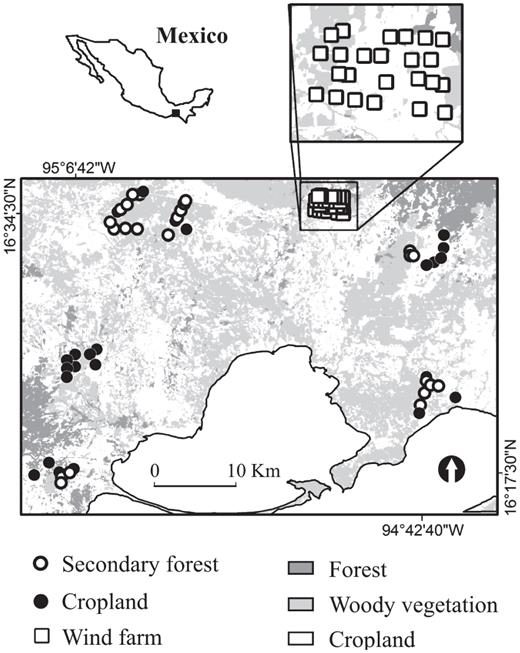
Map of study area illustrating the spatial distribution of the studied sites by treatment.
Bird Surveys
We surveyed bird communities during spring (April-May) and autumn (September—November) of 2008, using limitedradius point-counts (5 min, 25-m radius, following Ralph et al. 1995), recording all birds seen or heard from 07:00 to 10:00. Because the distance between point-counts is crucial to avoiding pseudoreplication (Ralph et al. 1995, 1996), we located our point-counts at a minimum distance of 200 m from each other. We sampled 96 independent points during spring and autumn, with 24 in each of the following treatments: (1) wind farm directly beneath wind turbines (referred to as ‘WF’), (2) wind farm 200 m away from wind turbines (‘WF200’), (3) secondary forests, and (4) croplands. We surveyed birds during major periods of bird migration because the wind farm is on the Isthmus of Tehuantepec, one of the narrowest continental corridors of migration for many species (Arizmendi et al. 2010).
Statistical Analyses
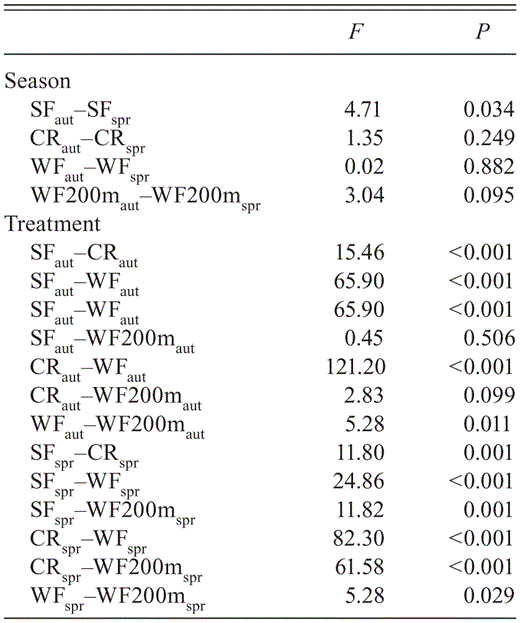
To contrast values of species richness by treatment, we compared statistical expectations (Sobs [Mao Tau]) of species richness at both seasons with Estimates (Colwell 2008). The statistical expectation of species richness is computed by the repeated resampling of all pooled samples, allowing statistical comparisons of treatments (Gotelli and Colwell 2001). To determine if species richnesses of the four treatments differed statistically, we compared their 84% confidence intervals (Payton et al. 2003). As Estimates generates 95% confidence intervals for Sobs (Mao Tau), we multiplied the SD of Sobs (Mao Tau) by the quantile (z-score of the normal curve) corresponding to two-sided intervals of 84% probabilities (1.372). Following Payton et al. (2003), we considered the data to be statistically different with an α of 0.05 if their confidence intervals did not overlap. To evaluate differences in bird abundance by treatment and season, we performed a general linear model ANOVA (GLM-ANOVA), followed by a post-hoc Newman—Keuls range test. To assess differences in species composition by treatment, we carried out a completelinkage abundance-based Bray—Curtis multivariate cluster analysis (BioDiversity Pro; McAleece 1997). This analysis generates a dendrogram with the complete linkage of similarity among the treatments compared.
To compare the evenness of the surveyed bird communities, we used rank/abundance plots (also known as Whittaker plots; Magurran 2004). Rank/abundance plots are used to depict the abundance distribution of species of a community, as they represent differences in the evenness of communities. In this sense, steep curves represent uneven communities dominated by a few species, while shallower slopes imply communities with greater evenness, in which species share similar abundances. Thus the steepness of the slope of a rank/abundance curve allows one to draw conclusions about the success of the species competing for limited resources (Magurran 2004). We compared the slopes of the regression lines in the rank/abundance plots with analysis of covariance (ANCOVA). As ranked abundances did not follow a normal distribution, we transformed the data (log10[x + 1]).
Finally, we assessed the role that some habitat traits played in determining bird species richness. For this, we quantified the area covered by additional traits (variables) in each habitat in the same 25-m radius area within which birds were surveyed (i.e., live fences, patches of secondary vegetation, small agricultural plots, bare ground) from a highquality satellite image. To assess the relative role of these additional parameters and the four treatments, we performed a general linear model multiple regression (GLM-MR), which is frequently applied to analyze multiple predictor variables, including categorical ones (StatSoft 2010) such as, in our case, treatment and season. We also carried out a classification and regression-tree analysis to evaluate which of the additional parameters were related to which treatments and under which scenarios. Classification and regression trees allow the interpretation of datasets where there are complex nonlinear relationships between the sets of response and predictor variables (Deíath and Fabricius 2000). This analysis uses binary recursive partitioning to identify threshold values of a set of predictor variables, which can be a mix of continuous and categorical variables that are related to the response variable. Thus classification and regression trees identify successive critical values of predictor variables splitting the response variable in a dichotomous and hierarchical manner (Palomino and Carrascal 2007). These types of trees are analogous to multiple-regression models, specifically those using forward selection of predictor variables (Crawley 2007).
Results
We recorded a total of 60 bird species (Table 1). Of these, four are endemic to Mexico, 13 are nearctic—neotropical migrants that winter in the study area, one is a transient, two are considered endangered (of which one—Peucaea sumichrasti—is endemic), and two are protected by the Mexican government (SEMARNAT 2010; Table 1). For none of the four treatments did values of species richness differ by season (Table 2). Therefore, we merged the data for spring and autumn for each treatment and recalculated their statistical expectation for comparisons (Fig. 2). Species richness was lowest at WF; the higher values in croplands and secondary forests were statistically different. Interestingly, the species richness recorded at WF200 was intermediate, and not statistically different from any of the other three treatments (i.e., WF, croplands, secondary forest; Fig. 2). Bird abundance followed a different pattern (GLM-ANOVA: .F7,184 = 7.96, P < 0.001), with significantly higher values recorded at secondary forests in autumn (in comparison to the other three treatments Newman—Keuls P < 0.001). WF200 had the lowest bird abundance in autumn, and its value was statistically different from that of the secondary forests and croplands in both seasons (Newman—Keuls P values < 0.05). We found no difference in bird abundance between WF and WF200 at either season (Newman—Keuls P > 0.14).
The Bray—Curtis multivariate analysis revealed several interesting patterns of species composition in relation to the bird communities surveyed within the wind farm (i.e., WF, WF200). On the one hand, the bird communities recorded in autumn at WF and WF200 were different from the those of the other two treatments in both seasons (∼10% similarity). On the other hand, one cluster was formed with the rest of the treatments and seasons, showing two subclusters: (1) croplands in both seasons and secondary forests in autumn and (2) secondary forests, WF, and WF200 m in spring (Fig. 3).
Bird-community evenness also differed by treatment and season (Table 3, Fig. 4). When we compared the rank/ abundance slope of each treatment in both seasons, we found statistical differences only between secondary forests, with a nonsignificant tendency at WF200 (in both cases the steepest curve was for spring). In the paired comparisons, we also found significant differences in the slopes for most treatments at the same season, except in two comparisons: WF200—secondary forests and WF200—croplands, both during autumn (Table 3).
Species richness in the four studied treatments in both seasons of 2008 expected on the basis of a comparable abundance cut-off point of 38 accumulated individuals (lesser total abundance recorded at the wind farm [WF200] in spring).
![Species richness in the four studied treatments in both seasons of 2008 expected on the basis of a comparable abundance cut-off point of 38 accumulated individuals (lesser total abundance recorded at the wind farm [WF200] in spring).](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/condor/114/4/10.1525_cond.2012.110130/1/m_t02_711.gif?Expires=1750188893&Signature=u3hy6ld3ikF6-w12vGFWdmmgNQYWwDmoOO9-KIGqShksoDP0dwq0qullTn7KsNyYxY~hpB7XNIxqT4bPJdW5lULGInJFXx~QbQkVC5V0YEZeC2op82YPQ0m8YrlfSVPdWptXdGJu8VEbqVxosGpHE6om4XxdCV4AJjB7im4V2Gv6WvbFxvXC87g0rmsqDTZVHIn6lCaB6VVrRmiI5FMGXpdKCD8PKSTrD6S9fy5lOfNIp-PIGqCS3Z-BD4IU7tiGW-sLuhqy-2wyZl55SUsv7JQKSYM4Sn9HTIsHEGQjWuVS~Yb2L5ihq3NsigK96Pc8f4EuHGKX40-E9SbmWNOKHA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Species richness in the four studied treatments in both seasons of 2008 expected on the basis of a comparable abundance cut-off point of 38 accumulated individuals (lesser total abundance recorded at the wind farm [WF200] in spring).
![Species richness in the four studied treatments in both seasons of 2008 expected on the basis of a comparable abundance cut-off point of 38 accumulated individuals (lesser total abundance recorded at the wind farm [WF200] in spring).](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/condor/114/4/10.1525_cond.2012.110130/1/m_t02_711.gif?Expires=1750188893&Signature=u3hy6ld3ikF6-w12vGFWdmmgNQYWwDmoOO9-KIGqShksoDP0dwq0qullTn7KsNyYxY~hpB7XNIxqT4bPJdW5lULGInJFXx~QbQkVC5V0YEZeC2op82YPQ0m8YrlfSVPdWptXdGJu8VEbqVxosGpHE6om4XxdCV4AJjB7im4V2Gv6WvbFxvXC87g0rmsqDTZVHIn6lCaB6VVrRmiI5FMGXpdKCD8PKSTrD6S9fy5lOfNIp-PIGqCS3Z-BD4IU7tiGW-sLuhqy-2wyZl55SUsv7JQKSYM4Sn9HTIsHEGQjWuVS~Yb2L5ihq3NsigK96Pc8f4EuHGKX40-E9SbmWNOKHA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
The GLM-MR was robust and significant (R = 0.49, F5,186 = 11.92, P < 0.001), revealing significant relationships only between bird species richness and (1) treatment: significantly higher in croplands and secondary forests (F = 17.93, P < 0.001), (2) season: significantly higher in autumn (F = 5.34, P = 0.02), and (3) live fences: positively related (F= 9.78, P = 0.002). The classification and regression tree yielded results similar to those of the GLM-MR, showing that treatment was the most important factor determining bird species richness, making a first important dichotomy between two groups: (1) WF and WF200 and (2) croplands and secondary forests (Fig. 5). Bird species richness for both wind farm treatments (i.e., WF, WF200) was lowest than for the other two treatments. On the other hand, the area covered by croplands (including small plots at sites of secondary forest) was, in general, positively related to bird species richness, while the area covered by bare ground did not show any clear pattern.
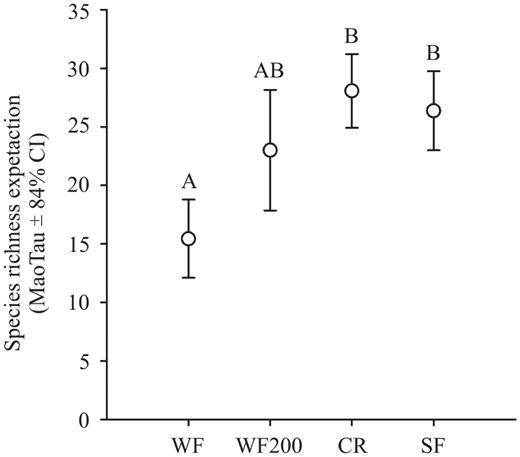
Statistical expectation of bird species richness for both studied wind farm treatments (i.e., beneath the wind turbine, 200 m away), croplands, and secondary forests. Values were calculated on the basis of a cut-off point of 92 individuals (lesser total abundance for WF200). WF, wind farm; WF200, wind farm 200 m; CR, croplands; SF, secondary forests. Letters above the whiskers for the CI represent statistically significant differences.
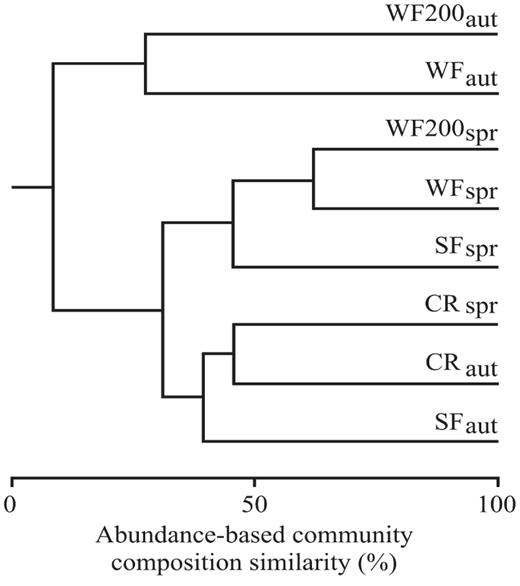
Bray—Curtis abundance-based multivariate cluster analysis representing similarity in composition of the bird community. SF, secondary forests; CR, croplands; WF, wind farm; WF200, wind farm 200 m; spr, spring; aut, autumn.

Discussion
Values of species richness were lowest at WF, intermediate at WF200, and highest in croplands and secondary forests. The high values in croplands may be associated with the presence of irrigation channels, offering a scarce vital resource for birds. This is most likely to apply in spring, a time of less rainfall, although October and November represent the gradual end of the rainy season. Intermediate values of species richness recorded at WF200 suggest that the direct effect of wind turbines gradually subsides, as reported in previous studies (e.g., Leddy et al. 1999, Pearce-Higgins et al. 2009). Our habitat analysis considering additional variables showed that the relative effect of the four treatments (i.e., WF, WF200, croplands, secondary forest) overrode that of additional variables (i.e., live fences, patches of secondary vegetation, small agricultural plots, bare ground), although complementary analyses underline the importance of croplands, including small plots within secondary forests, as secondary drivers of bird species richness.
Slight differences in species richness between WF and WF200 suggest two possible explanations: (1) landbirds are being affected directly by the wind farm through collisions with wind turbines, or (2) landbirds are avoiding turbines (as recorded for other human-made structures; Wallander et al. 2006, Pruett et al. 2009, Torres et al. 2011). As our field observations provided no evidence for a substantial rate of collision, the second scenario is more likely, as previously suggested by Fox et al. (2006). Interestingly, in southeastern England (East Anglia) Devereux et al. (2008) found that turbine location, controlled for other potential effects, did not affect the distribution of four functional groups of wintering farmland birds (i.e., seedeaters, corvids, gamebirds, and the Sky Lark [Alauda arvensis]) at various distances from the turbines.
Another interesting result regarding species richness was that values for secondary forests and croplands were not different, which differs widely from results of previous studies comparing bird communities of forested areas and croplands (e.g., Estrada and Coates-Estrada 1997, MacGregor-Fors and Schondube 2011). This difference might be due to (1) the degree of disturbance and human management of the secondary forests in our study area and/or (2) the high density of irrigation channels distributed among croplands. Hence further studies are needed to test these hypotheses.
Although bird abundances under the four treatments were quite similar at both seasons, we found statistical differences for two of them: (1) bird abundance was significantly higher in croplands during autumn and (2) was significantly lower at WF200 during autumn. Such differences are related to the presence and abundance of generalist landbirds that are often associated with disturbed habitats. On the one hand, highest values of abundance in croplands during autumn were due primarily to the high abundance (>10 individuals per species) of species such as the Stripe-headed Sparrow (Peucaea ruficauda), Groove-billed Ani (Crotophaga sulcirostris), Wilson's Warbler (Cardellina pusilla), Inca Dove (Columbina inca), and Greattailed Grackle (Quiscalus mexicanus), which were also recorded in croplands during spring but in lesser numbers. On the other hand, the low bird abundances recorded at 200 m from the wind farm during autumn might be caused by the flocking of some generalists (e.g., Q. mexicanus, C. inca), so their absence during point counts does not necessarily mean their absence from the area (Ralph et al. 1995).
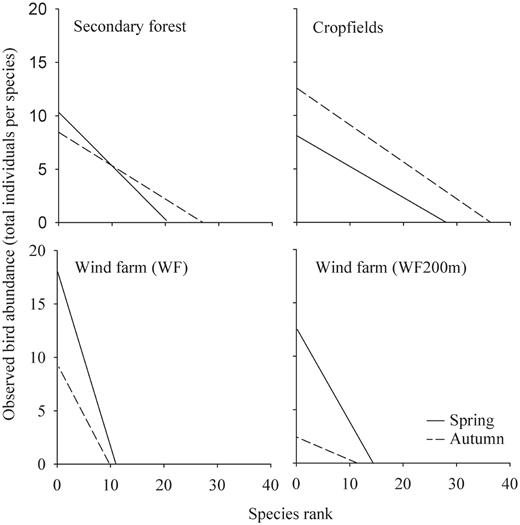
Rank/abundance plot slopes for the treatments and seasons surveyed. See Table 3 for results of ANCOVA.
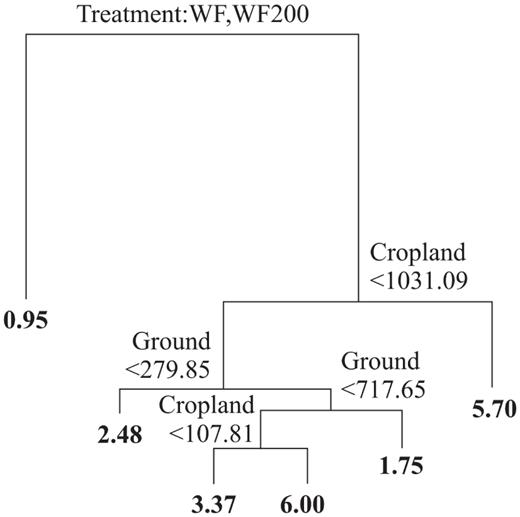
Classification and regression tree showing the relationship between bird species richness and the studied treatments (i.e., WF, WF200, cropland, secondary vegetation), seasons (i.e., spring, autumn), and additional variables (i.e., live fences, patches of secondary vegetation, small agricultural plots, bare ground).
The composition of the bird community varied in three main patterns: (1) major differences between bird communities in and near the wind farm (i.e., WF, WF200) during autumn and those of the other treatments and seasons, (2) high similarity between the two wind-farm treatments and the secondary forest during spring, and (3) high similarity between the secondary forest in autumn and croplands in both seasons. Differences between the two wind-farm treatments in autumn and spring indicate that seasonal variations occur. The high similarity between the two wind-farm treatments and the secondary forest during spring shows that the types and numbers of resident birds able to use the wind farms are similar to those recorded in highly disturbed forests during the dry season. However, no seasonal effect was related to bird community composition in croplands, as both seasons were grouped with secondary forests in autumn. This supports the idea that irrigation channels might mitigate differences caused by water scarcity during spring and part of autumn.
In general, bird communities at the wind farm we studied were depauperate even in comparison to those of croplands and secondary forests. Thus only a few generalist species are capable of using the available resources, in spite of the hazard, while many other species simply avoid the turbines (as recorded for other human-modified systems; Blair 1996). However, the species richness and abundance recorded at WF200 during autumn was also extremely low. This result might be due to two non-exclusive explanations: (1) wind farms may represent an important hazard for both residents and wintering species, and (2) the gregarious behavior of some generalists that manage to dwell within the wind farm might have compromised our capability to record them by the method we used.
Unfortunately, information about the effects of wind farms on migrant landbirds is limited (e.g., Farfán et al. 2009, Tellería 2009). In a previous study, Hötker (2006) stated that wind farms have, in general, small effects on breeding birds but larger effects on migrants. Our results agree with Hötker's findings, as the bird communities we recorded at the wind farm consisted mostly of residents; we recorded only two individuals of one wintering species, Setophaga petechia. Although our results suggest that winter visitors can avoid the wind farm we studied, this observation might apply only to this one isolated wind farm. If more wind farms are established over a greater area of the Isthmus of Tehuantepec, which is expected because of the isthmus's unique conditions for generating wind energy, the effect of wind farms on wintering landbirds could potentially alter their stay and/or phenology. However, further studies are needed to document the effects of wind farms on birds as more wind farms are established.
Acknowledgments
We are grateful to the anonymous reviewers for their valuable comments. We also thank M. Mora for providing GIS data, O. Muñoz, T. Hernández, A. Bombela, A. González, A. Aguilar, E. Almanza, E. Senties, S. Cabrera, and L. Contreras for assistance in the field, and the many farmers who allowed us to work in their lands. We are grateful to the Federal Electricity Commission (Comisión Federal de Electricidad) for authorizing the publication of this paper, which information is based on the ‘Plan de vigilancia de la fauna (aves y murciélagos) en la central eólica La Venta II, municipio Juchitlán, Oax. (Periodo 2008–2011)’ and completed under four agreements (9400039484, 9400047488, 9400053437, 9400060686) between the Instituto de Ecología, A.C., and the Federal Electricity Commission.
Literature Cited



