-
PDF
- Split View
-
Views
-
Cite
Cite
Harry van OORT, Kenneth A Otter, Kevin T Fort, Zoe McDONELL, Habitat, Dominance, and the Phenotypic Quality of Male Black-Capped Chickadees, The Condor: Ornithological Applications, Volume 109, Issue 1, 1 February 2007, Pages 88–96, https://doi.org/10.1093/condor/109.1.88
Close - Share Icon Share
Abstract
The provisioning of offspring is limited by resource abundance and is therefore likely to vary with habitat quality and the ability of parents to obtain food. Provisioning effort may also vary because males choose different life-history strategies depending on their rank and environment. Socially dominant males have higher costs of self-maintenance compared with subordinates, yet this is likely compensated for by their priority access to resources. It is unclear, however, whether this translates into benefits for females through male provisioning effort, and how this might vary with habitat suitability. We assessed patterns of body condition, blood hematocrit levels, and provisioning effort of dominant and subordinate male Black-capped Chickadees (Poecile atricapillus) breeding in two habitats known to differ in quality. Within ranks, males were similar in size and condition across habitats. Dominant males were not structurally larger than subordinates, but they were in better condition than subordinates in both habitats. There was an additive effect of habitat and dominance rank on hematocrit level; dominant males had higher hematocrit levels than subordinates regardless of habitat, and all males breeding in poor habitat had elevated hematocrits. A habitat-rank interaction revealed a greater disparity in provisioning rates among dominant and subordinate males in poor habitats. These results suggest that dominant males may be particularly good mates when resources are scarce.
El aprovisionamiento de los pichones está limitado por la abundancia de los recursos, por lo que es probable que éste varíe con la calidad del hábitat y con la habilidad de los progenitores para obtener el alimento. El esfuerzo de aprovisionamiento también puede variar porque los machos pueden elegir diferentes estrategias de historia de vida y priorizar la inversión en diferentes componentes de las historias de vida, dependiendo de su ambiente. Los machos socialmente dominantes tienen mayores costos de auto mantenimiento que los subordinados, aunque esto probablemente se compensa con el acceso prioritario a los recursos. Sin embargo, no está claro si esto se traduce en beneficios para las hembras a través del esfuerzo de provisión de los machos, ni cómo esto podría variar con la calidad del hábitat. Evaluamos los patrones de la condición corporal, los niveles de hematocrito en la sangre y el esfuerzo de aprovisionamiento de los machos dominantes y subordinados de Poecile atricapillus que estaban criando en dos ambientes que diferían en su calidad. Dentro de los rangos, los machos fueron similares en tamaño y condición a través de los ambientes. Los machos dominantes no fueron estructuralmente más grandes que los subordinados, pero estuvieron en mejores condiciones que los subordinados en ambos ambientes. Hubo un efecto aditivo del hábitat y el rango de dominancia en los niveles de hematocrito; los machos dominantes presentaron niveles de hematocrito mayores que los subordinados, independientemente del hábitat, y todos los machos que criaron en ambientes pobres tuvieron niveles elevados de hematocrito. Una interacción entre el hábitat y el rango reveló una disparidad mayor en las tasas de aprovisionamiento entre los machos dominantes y subordinados en los ambientes pobres. Estos resultados sugieren que los machos dominantes pueden ser parejas particularmente buenas cuando los recursos son escasos.
Introduction
In monogamous mating systems, males often provide food to their social mates and young (Qvarnström and Price 2001). As resources are often unequally partitioned among territorial males, their ability to provision young is likely to correspond with their resource-holding potential. Accordingly, females are known to prefer dominant males as their social mates (Otter and Ratcliffe 1996), and dominant males appear to acquire better territories and offer greater resource provisioning to young (Qvarnström et al. 2000, Pärt 2001, Voltura et al. 2002). However, benefits associated with social dominance appear to vary among populations (Verhulst and Salomons 2004), and some studies have found reduced paternal care associated with social dominance (Qvarnström and Forsgren 1998). The circumstances that cause variability in the quality of paternal care of dominant males relative to subordinates remain poorly understood.
Habitat quality may influence the relative phenotypic quality of dominant males and their ability to provide direct benefits (Ellis 1995, Qvarnström and Forsgren 1998). There are several mechanisms that could cause such habitat-rank interactions on the phenotypic quality of males. For example, the challenge of living in less suitable habitats may be disproportionately greater for subordinates because they are inefficient at competing for resources relative to dominant individuals (Sutherland 1996). Such interference competition may result in a greater advantage to being dominant when living conditions are difficult (Ellis 1995, Carrascal et al. 1998). Alternatively, it is possible that the net nutritional benefit of being dominant could diminish in poor habitats. Although dominant males have priority access to preferred foraging sites, they also have high metabolic rates (Røskaft et al. 1986, Hogstad 1987), causing their nutritional requirements to be greater compared with subordinate males. Consequently, there is a positive relationship between social dominance and sensitivity to nutritional stress (Swaddle and Witter 1994). If resources are generally scarce, the energy gained from priority access to resources may not offset the high metabolic costs of dominance, and dominant males may suffer disproportionately in poor environments compared to subordinates (Qvarnström and Forsgren 1998). Hence, the relative advantage of being mated to a dominant male could potentially decrease in a poor environment.
Despite these and other potential mechanisms that could lead to habitat-rank interactions in free-living populations, we have very little understanding of how habitat quality affects the relative phenotypic quality of dominant males. To date, empirical evidence offers conflicting information about environmental impacts on the nutritional condition of dominant versus subordinate males (Carrascal et al. 1998, Hay et al. 2004). The interactive effects of social dominance and habitat quality on parental care remain entirely unexplored. In this study, we investigated patterns of reproductive effort, body condition, and hematocrit levels among breeding dominant and subordinate male Black-capped Chickadees (Poecile atricapillus) nesting in two habitats that differed in suitability. We used provisioning rates as an indicator of reproductive effort and size-corrected body mass as a measure of body condition. We measured hematocrit levels to gain information about the metabolic or physical workload of breeding males (Carpenter 1975, Saino et al. 1997a, 1997b).
Methods
Study Species
Black-capped Chickadees are small, nonmigratory songbirds in the titmouse family (Paridae), associated with deciduous or mixed forests (Smith 1991). During winter, they forage in flocks that can be readily observed to determine within-flock social dominance hierarchies (Ficken et al. 1990). These hierarchies are linear and stable—once the relative relationship is established between two birds, it does not change among sites or years (Smith 1991, Otter et al. 1998). Typically, older and more experienced individuals are dominant over younger birds, and males are dominant over females.
Black-capped Chickadees are socially monogamous, and males make large contributions to within-pair reproduction (Smith 1991). Males feed their mates extensively during the laying period and throughout the incubation period (early to late May in the study site). When nestlings hatch (late May to early June), males are initially the primary providers for the nestlings, but females contribute greater amounts of provisioning as the nestlings grow and brooding becomes less necessary. Females are known to prefer dominant males as social partners (Otter and Ratcliffe 1996) and female reproductive success is closely tied to the relative rank of the male she is partnered with (Otter et al. 1999).
Study Site
Our study population was located adjacent to the Prince George campus of the University of Northern British Columbia. The study site consisted of two strikingly different habitats, both extensively used by Black-capped Chickadees. Approximately half of the 200 ha study site was comprised of mature mixed forest dominated by stands of trembling aspen (Populus tremuloides), paper birch (Betula papyrifera), hybrid spruce (Picea glauca × P. engelmannii), lodgepole pine (Pinus contorta var. latifolia), subalpine fir (Abies lasiocarpa), Douglas-fir (Pseudotsuga menziesii), and black cottonwood (Populus balsamifera var. latifolia). The trees in this undisturbed forest were approximately 80 to several hundred years old (canopy height ~25 m). In the remaining half of the study site, the forest was much younger and dominated by conifers. This disturbed habitat was a patchwork of various young regenerating forests, including several lodgepole pine plantations and areas of natural regeneration of alder (Alnus crispa) and willow (Salix spp.), ranging in age from 25–40 years (canopy height of 5–15 m). There were also several small fragments (0.5–4.0 ha stands) of mature forest that together comprised less than 10% of the total disturbed area. Further details can be found in Fort and Otter (2004a, 2004b).
Previous research in our study site determined that the young forest habitat is less suitable for chickadees, as seen by a difference in nesting success (Fort and Otter 2004b). Population density tends to be slightly lower in the young forest than the mature forest (Hansen et al. 2005), although this effect is slight and not always detected (Fort and Otter 2004b). Other studies have shown behavioral differences between chickadees in these habitats that are consistent with the hypothesis that food is less available in young forests (Fort and Otter 2004a, van Oort and Otter 2005, van Oort et al. 2006, Otter et al. 2007).
Winter Dominance Observations
Over 90% of the individuals in our study site were captured and color-marked with leg bands from December to mid-February each year using potter traps at feeding stations. Nine feeders were distributed throughout the study site (approximately one feeder per 20 ha), each used by several flocks. Feeders were stocked with black oil sunflower seeds. We began observing dominance interactions within flocks after we had marked most of the population. Dyadic interactions between marked individuals, such as chases, supplants, submissive posturing, and waiting for access to the feeders, were recorded. In most cases, males could be easily classified as either dominant or subordinate within their flocks because the flocks tended to consist of two pairs: one experienced pair and one yearling pair. Bird feeders were equally available across habitats during winter, so any effect of providing additional food would only make our habitat comparisons more conservative. However, the winter feeders were taken down following banding (late February), well before flock breakup (late April). Winter conditions, with freezing temperatures and snow cover, persist into April in the study site, thus it is unlikely that supplemental food could have greatly influenced our habitat comparisons that took place late in the breeding season (mid-June).
Male Provisioning Effort
We observed nests in the breeding seasons of 2001 and 2003 to assess male provisioning effort. Nests were typically located during the period of nest excavation and the progression through the nesting cycle was monitored. Pairs are highly conspicuous when females are laying because females solicit the male for food throughout the day using begging vocalizations (Smith 1991). As the laying period progressed, territories were monitored every second day to estimate when females initiated incubation. Chickadee incubation lasts approximately 12–13 days (Smith 1991) and nests were not monitored closely during this stage. Nest monitoring commenced two days prior to the predicted date of hatching. Nests were monitored every second day until evidence of hatching was documented, occasionally through looking into the nest cavity, but primarily by noting behavioral changes in the parents. Prior to hatching, males typically deliver food items to females at rates of one to three trips per hour. Behavioral evidence of hatching was determined by a combination of: (1) male delivery rates increasing to more than five food deliveries per hour, (2) males entering the cavity with food, or (3) either parent removing fecal sacs. Such estimates of hatching date appeared to be reliable (Otter et al. 1999), as judged by nestling development during banding six to ten days later.
To investigate male provisioning rates, nests were observed for two 1-hr observation periods. Observations were limited to 2 hr to maximize the number of territories that could be visited. Chickadees were relatively synchronous in their breeding activities, so it was logistically difficult to conduct more extensive observations throughout the nesting period without sacrificing sample size. All observers were experienced at reading leg bands. During nest watches, the observer was positioned far enough from the cavity to avoid disrupting chickadee behavior (>10 m). The first 1-hr observation took place when nestlings were about three days old, and the second 1-hr observation was carried out three days later (estimated at six days posthatching). During nest observations, all trips made by the male and female were counted separately. Chickadees often build nest cavities well above the ground in the upper canopy, which can occasionally make nest observations difficult. However, parents could readily be identified and in most cases it was possible to see whether food items were delivered to the nest. Males were never observed entering the cavity without food, thus we assumed that all male visits to the cavity were feeding events. Nest trips were also noted for females, however these data are less reliable as females were often seen returning to the cavity without food items. We did not directly analyze female provisioning rates in this study; however, we did compare the number of feeding trips made by the male with the total number of visits made by the female (regardless of whether or not she had food) as a conservative estimate of the relative contribution of male provisioning during this study period. For the main analysis of provisioning rates, we summed day 3 and day 6 data for a total of 2 hr of observation per territory. Although we could not control for the amount of food delivered by males during each visit to the nest, food delivery rates should act as an adequate index of relative provisioning effort; food delivery rates have been found to be predictive of total prey biomass delivered in other species (Nolan et al. 2001).
Because we pooled the two hours of observation, we controlled for time of day primarily by conducting all observations in a narrow window of time, between 07:30 and 11:30. The start times for day 3 and day 6 observations were alternated between early (07:30–08:30) and late (09:30–10:30) for each nest, so that individual cases would not be biased by time of day. In addition, when possible, start times were balanced between social ranks and habitats within a day. Brood size was known for a subsample of nests accessed after the provisioning trials were completed (n = 22).
Male Captures
During late May to mid-June of 2002 and 2003, we captured males at their nest cavities using nets mounted on 5 m extension poles in conjunction with tree-climbing apparatus. Males were captured when their nestlings were 4–8 days old. Upon capture, males were weighed (to 0.1 g) using a Pesola spring balance, and their tarsus length was measured (to 0.1 mm) with vernier calipers. We scored their furcular fat stores from 0 to 5 (after Gosler 1996). To measure hematocrit levels, we drew ~50 µl of blood with a heparinized capillary tube from a puncture in the brachial vein. Blood samples were refrigerated immediately for up to 2 hr and then centrifuged for 11 min at 11 500 rpm. Hematocrits were measured as the total packed cell volume (not including the buffy coat layer) divided by the total volume of blood. Handling time was typically less than 15 min. For territories where we sampled both male body condition and provisioning rates (in 2003), male captures always took place after male provisioning observations were completed. Males that were captured in 2002 were not captured in 2003. The same person performed all captures and measurements.
Statistical Analyses
We used parametric tests to compare the effects of habitat and rank on dependent variables. Residuals were checked for normality and homogeneity of variance and these assumptions were met in all comparisons. All statistical procedures were performed with Statistica 6.1 (StatSoft 2003) with significance levels (α) set at 0.05. For the provisioning analysis, six out of 37 males were observed in both field seasons. Two of these males changed rank between these sampling periods and, given that the observations were taken from different nesting attempts two years apart, we treated these observations as independent samples. We report mean values ± SE.
Results
We found no relationship between tarsus size and either dominance rank (dominants: 19.1 ± 0.2 mm, subordinates: 18.9 ± 0.1 mm; F1,23 = 1.1, P = 0.30) or habitat type (mature forest: 19.1 ± 0.1 mm, young forest: 19.0 ± 0.2 mm; F1,23 = 0.1, P = 0.74), and no evidence of an interaction between these factors (F1,23 = 0.0, P = 0.94). Body mass did not differ among years (one-factor ANOVA: F1,30 = 0.02, P = 0.88) so the data were pooled. Using an ANCOVA model that controlled for body size (tarsus length), dominant males had significantly greater mass than subordinates (F1,22 = 4.8, P = 0.04; Fig. 1a), but habitat did not contribute to variation in mass (F1,22 = 0.2, P = 0.69). The habitat-rank interaction term was not a significant predictor of body mass in this model (F1,22 = 1.4, P = 0.25).
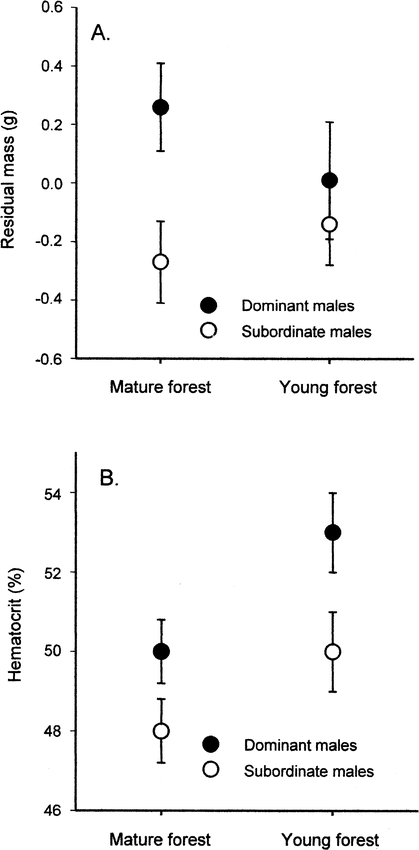
The effects of habitat quality and social dominance on (a) body mass and (b) blood hematocrit levels of male Black-capped Chickadees that were actively provisioning nestlings in central British Columbia.
These data suggest that dominant males are in better condition and have elevated metabolic rates. All males breeding in the young forest have elevated hematocrits, suggesting a physiological impact of habitat quality. In both graphs, means (± SE) are shown; however, the means in graph ‘a’ are standardized after controlling for tarsus size in an ANCOVA model.
Hematocrit levels did not differ across years (F1,32 = 0.1, P = 0.79) so the data were pooled. In a factorial ANOVA, there was no evidence of a habitat-rank interaction (P = 0.71), which was removed from the model. The two-factor ANOVA showed that dominant males had higher hematocrit levels than subordinates (dominants: 0.51 ± 0.01, subordinates: 0.49 ± 0.01; F1,23 = 6.2, P = 0.02) and males living in the young forest had higher hematocrit levels than their counterparts living in the mature forest (mature forest: 0.49 ± 0.01, young forest: 0.51 ± 0.01; F1,23 = 6.7, P = 0.02; Fig. 1b). Furcular fat stores were low in all breeding males (ranging from 0 to 1) and were not analyzed.
During the observation periods, females spent a large portion of their time brooding (35 ± 2 min per hr on day 3 and 25 ± 2 min per hr on day 6). Among all trials, males made the majority (65%) of the total trips to the nest. Because many of the trips made by females were not food delivery trips, the average male contribution was undoubtedly greater than 65% during the first six days of nestling provisioning.
After combining the data from day 3 and day 6 observations, the total number of male provisioning trips varied from six to 23 trips in 2 hr of observation. The number of provisioning trips was not significantly different between years (F1,38 = 3.0, P = 0.09) and was not related to brood size (linear regression: F1,20 = 2.1, r2 = 0.05, P = 0.17), but there was a linear decrease in provisioning associated with date (linear regression: F1,38 = 4.6, r2 = 0.12, P = 0.04). Brood size was not predicted by habitat, rank, or by a habitat-rank interaction term (factorial ANOVA; all P > 0.19). We therefore included date as a covariate in an ANCOVA model (n = 37). First, we checked for homogeneity of regression (Tabachnick and Fidell 2001) by including covariate-factor interaction terms. Interaction terms between the covariate and the factors were not significant (all P > 0.7) and were removed from the model. In the final model, date was a significant predictor (F1,32 = 8.9, P = 0.005) of the total number of provisioning trips made by males. The main effects (habitat and rank) were not significant terms in this model (both P > 0.25), however there was a significant habitat-rank interaction (F1,32 = 9.1, P = 0.005). The interaction showed a large effect of rank in the young forest, where dominant males made more trips than subordinate males, whereas there was no indication of a rank effect in the mature forest habitat (Fig. 2).
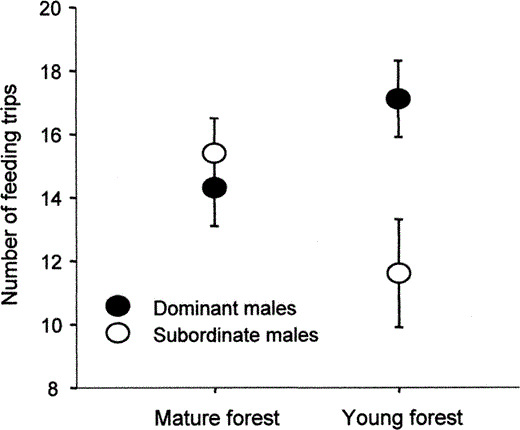
The interactive effect of habitat quality and social dominance on the number of provisioning trips made by male Black-capped Chickadees in 2 hr of nest observation in central British Columbia.
The provisioning advantage of being mated to a dominant male is only realized in the relatively poor-quality young forest. Standardized means (± SE) are plotted after controlling for date.
Discussion
Forests that differ in age, structure, and species composition are also likely to differ in suitability for forest songbirds. Habitat quality may affect foraging efficiency (Stauss et al. 2005), provisioning rates (Nour et al. 1998), body condition (Lambrechts et al. 2004), physiological stress levels (Suorsa et al. 2004), and nesting success (Fort and Otter 2004b). In this study, we found additive effects of habitat and dominance on hematocrit levels and interactive effects of habitat and dominance on provisioning levels of male Black-capped Chickadees. Social dominance was associated with body condition, but habitat quality was not. We discuss these findings in greater detail below.
An association between habitat and hematocrit levels has been reported before (Ots et al. 1998); however, some studies have found little evidence of habitat associations (Dawson and Bortolotti 1997 and references therein). It may be difficult to detect habitat effects in field studies because hematocrit measures show considerable within-individual variability (Ots et al. 1998). Because of this, habitat associations with hematocrit levels may not have been detected in previous studies when other important variables were not statistically or experimentally controlled for. In this study, we would not have found a significant habitat effect had we not controlled for the effect of social dominance. Furthermore, the habitat effect size was considerable in our data: males breeding in the young forest habitat had hematocrits that were generally elevated by 20% of the total variability. Thus, a combination of appropriate controls and large effect size may have allowed us to detect this association.
The hematocrit data demonstrate that habitat quality in our study site is associated with a physiological difference in breeding males. We can think of two potential hypotheses for this finding. First, daytime temperatures could be greater in young forest habitat due to the lack of an upper strata tree canopy, leading to dehydration and a reduction in blood plasma volume. We do not have data to test this hypothesis, but it may be an unlikely explanation, given that open water is freely available in the young forest (a large lake and numerous streams and ephemeral ponds). Alternatively, the low foraging efficiency experienced by birds breeding in poor habitats may lead to an increase in daily energy expenditure (Godfrey 2003); if males breeding in young forest are working harder than males breeding in mature habitat (Stauss et al. 2005), they may synthesize more red blood cells to compensate for their heightened oxygen consumption (Carpenter 1975, Saino et al. 1997a, 1997b). Although we can conclude that there is a physiological difference between males in each habitat, more detailed work is required to understand exactly what mechanisms cause the habitat differences in hematocrit levels found in our study.
If reproduction is more physiologically demanding in the young forest habitat, life history theory predicts that provisioning rates, body condition, or both should be reduced in this environment. Although we detected an effect of rank on body mass, we found that body condition was relatively unaffected by habitat quality. Rather, provisioning effort appeared to be more sensitive to habitat type. That health is prioritized over reproduction in poor conditions agrees with experimental findings in other passerines (Spencer and Bryant 2002). However, reduced provisioning rates were seen only in subordinate pairs, as shown by a strong habitat-rank interaction on the number of provisioning trips made by males to the nest. This finding suggests that: (1) socially dominant males are somehow able to mitigate habitat shortcomings to maintain their investments in provisioning and self-maintenance, and (2) the elevated metabolic and nutritional requirements of dominant males (Røskaft et al. 1986, Hogstad 1987, Swaddle and Witter 1994), which were reflected in the hematocrit data from this study, are more than compensated for by their superior resource acquisition, regardless of habitat quality. As such, our study fails to support the idea that the metabolic costs of dominance will cause dominant males to be less desirable mates in poor quality habitats (Qvarnström and Forsgren 1998); instead, our results suggest that dominant males potentially offer heightened reproductive benefits to females breeding in poor habitats (Ellis 1995).
This is the first study to report a habitat-dominance interaction effect on provisioning rates, however the general pattern—that subordinates are more sensitive to habitat quality—is supported by other resource-dependent attributes. In our study population, there is a tendency for the begging rates of females to be higher in subordinate pairs than in dominant pairs breeding in young forest; this rank effect is less pronounced in mature forests, where begging rates are reduced (Otter et al. 2007). Likewise, nesting success is rank-dependent for pairs breeding in the young forest habitat, yet subordinate pairs maintain similar reproductive success to dominant pairs breeding in the mature forest (Fort and Otter 2004b). In other tit species, dominant males also appear to show better abilities to compete for resources in harsher environments (Carrascal et al. 1998). Hence, there is a considerable body of empirical data suggesting that subordinate individuals are more sensitive to habitat quality across an array of life history traits or investments (but see van Oort et al. 2006).
It is not clear why habitat quality appears to have less effect on dominant males. In our study population, it is unlikely that dominant male success is driven entirely by their enhanced resource-holding potential, because territory defense appears to be reduced in the young forest habitat (Fort and Otter 2004a). It is possible that dominant males are better at using adaptive strategies in each habitat, perhaps because they are more experienced breeders. For example, only dominant males show a large decrease in dawn chorus singing across habitats in our study population (van Oort et al. 2006). Reducing investment in costly advertisement may allow dominant males to maintain adequate reproductive potential for the remainder of the breeding season. Because dominant males tend to be older, such reduced advertisement could be a learned strategy, or a function of different natural selection regimes (advertisement-dependent survivorship) across habitat types (van Oort et al. 2006).
It is also possible that males of differing social rank breeding in each habitat type are using different life history trade-off strategies with regard to maintenance of body condition. Although nonsignificant, there was a very weak trend in the body-mass data for a larger rank effect to be seen among males breeding in the mature forest; in the young forest, dominant males appeared to have a small drop in body mass, while subordinates appeared to have a small increase in body mass. Increased sample sizes are needed to verify this pattern, but this trend could suggest that dominant males in young forests sacrifice body condition to some degree to maintain their reproductive effort. Conversely, subordinate males breeding in young forests show a slight increase in body condition compared to counterparts in mature forest, which, coupled with evidence of increased likelihood of nest abandonment (Fort and Otter 2004b) and lower provisioning rates (this study), may suggest that low-ranked males in poor habitats prioritize self-maintenance over reproduction. It is worth noting, however, that the current analysis had sufficient power to detect the effect of rank on body mass, suggesting that the biological importance of such an interaction term may not be large, should it exist. Future work should endeavor to explain what mechanisms are involved in habitat-rank interactions on male provisioning levels and other life history traits.
Acknowledgments
We thank Inge-Jean Hansen, Mandy Kellner, and Dave Gummeson for field assistance, and David Lank, Russ Dawson, Mike Gillingham, Staffan Lindgren, and two anonymous reviewers for their comments on an earlier draft of this paper. Mark Shrimpton kindly provided a centrifuge for this research. Funding and support for this work came from the National Science and Engineering Research Council (Canada), Canada Foundation for Innovation, British Columbia Knowledge Development Fund, and the University of Northern British Columbia. All work was conducted under permits from the University of Northern British Columbia's Animal Care Committee, and met the guidelines outlined by the Canadian Animal Care and Use Committee.
Literature Cited
Author notes
Present address: Kingbird Biological Consultants, Box 8617, Revelstoke, British Columbia, V0E 2S2, Canada. E-mail: [email protected]



