-
PDF
- Split View
-
Views
-
Cite
Cite
Ana S. Barreira, Dario A. Lijtmaer, Stephen C. Lougheed, Pablo L. Tubaro, Subspecific and Temporal Variation in the Structurally Based Coloration of the Ultramarine Grosbeak, The Condor: Ornithological Applications, Volume 109, Issue 1, 1 February 2007, Pages 187–192, https://doi.org/10.1093/condor/109.1.187
Close - Share Icon Share
Abstract
Ultramarine Grosbeaks (Cyanocompsa brissonii) possess a striking sexual dichromatism, with males dark blue and females brown. There are two subspecies in Argentina: the larger-bodied C. b. argentina, which is common in shrubs and semiopen areas, and the smaller C. b. sterea that inhabits forests. We measured reflectance spectra of six plumage patches from study skins to evaluate the possibility of color differences between males of each subspecies and temporal variation in plumage coloration. We found differences between subspecies in color brightness, hue, saturation, and UV chroma in the plumage patches of more conspicuous coloration, which could be related to ambient light differences between the environments that each subspecies inhabits. We also documented temporal color variation in some plumage patches, in particular a gradual decrease of UV reflectance and a gradual increase in hue after molting, possibly attributable to feather wear.
Cyanocompsa brissonii posee un marcado dicromatismo sexual, siendo los machos de color azul oscuro y las hembras marrones. Hay dos subespecies en Argentina: C. b. argentina, de mayor tamaño, que habita arbustales y áreas semi-abiertas y C. b. sterea, de menor tamaño corporal, que habita bosques. Medimos los espectros de reflectancia de seis parches de plumaje en pieles de estudio para evaluar la existencia de diferencias en la coloración de los machos entre subespecies y variación temporal en la misma. La coloración de los parches de plumaje más conspicuos difirió entre subespecies en brillo, tono, saturación y reflectancia UV, lo que podría relacionarse con diferencias en la luminosidad entre los ambientes que cada subespecie habita. También documentamos variación temporal de la coloración, en particular un decrecimiento gradual en la reflectancia UV y un aumento gradual en el tono tras la muda, posiblemente atribuible al desgaste del plumaje.
Ultramarine Grosbeaks (Cyanocompsa brissonii) exhibit a striking sexual dichromatism. Females are brown and males are mostly dark blue with lighter color patches on the cheeks, forehead, and wing coverts (Ridgely and Tudor 1989). The species has an extensive distribution that spans much of South America from Colombia and Venezuela south to Argentina. It is represented in Argentina by two subspecies, differing primarily in body size (Todd 1923), that occupy different habitats and geographic ranges (divided by the Parana River): C. b. argentina is larger, distributed through the Chaco and Yungas, and common in shrubs and semiopen areas, whereas C. b. sterea is smaller and inhabits densely vegetated forests in the Litoral region of Argentina (Olrog 1979).
Birds have a fourth type of retinal cone, lacking in humans, that extends their range of color perception into the near-ultraviolet wavelength spectrum (320–400 nm) and allows them to distinguish a larger number of colors (Toveé 1995, Ödeen and Håstad 2003). Consequently, many previous avian coloration studies, based on human visual parameters, underestimated the degree of color variation that birds actually perceive (Bennett et al. 1994, Eaton 2005).
The coloration of Ultramarine Grosbeak males has a structural origin that, unlike pigment-based coloration, is not generated by the light-selective absorption of pigments deposited in feathers, but through the interference and coherent scattering of certain wavelengths from the internal feather microstructure (Prum et al. 1998, 1999, Andersson 1999). Therefore, the internal microstructure of feathers must be finely arranged to produce high brightness, saturation, and UV reflectance (Prum et al. 2003, Shawkey et al. 2003, 2005). These components of structurally based coloration have been found to be involved in both mate selection (Bennett et al. 1997, Andersson et al. 1998, Hunt et al. 1998) and intrasexual competition Siefferman and Hill (2005) in some species. Additionally, seasonal variation in structurally based coloration has been identified in the Blue Tit (Parus caeruleus; Örnborg et al. 2002) and the Diameded Tanager (Stephanophorus diadematus; Tubaro et al. 2005), and attributed to feather wear after molting.
Although some color differences have previously been described among subspecies of Ultramarine Grosbeaks by (Todd 1923), these were based on human perception and classified according to color plates. Moreover, the study undertaken by (Todd 1923) compared subspecies other than C. b. argentina and C. b. sterea, and the subspecies classification used did not completely agree with present-day classification. In this study, we compared plumage coloration of C. b. argentina and C. b. sterea males and studied its temporal variation using reflectance spectrophotometry, a quantitative and more objective method than those previously employed by human perception-based studies.
Methods
Data Collection
All measurements were made on study skins deposited in the Ornithology Collection of the Museo Argentino de Ciencias Naturales “Bernardino Rivadavia.” We only used skins of adult males in excellent condition and with complete information on subspecies and site and date of collection (n = 10 for C. b. argentina and n = 15 for C. b. sterea).
We measured reflectance spectra from six body regions: forehead, cheeks, wing coverts (hereafter referred to as coverts), back, rump, and belly. Percentage of reflectance was measured relative to a white standard of barium sulphate Osorio and Ham (2002) with an Ocean Optics 2000 spectrophotometer (Ocean Optics, Inc., Dunedin, Florida) with a PX-2 pulsed xenon light source (effective range of emission from 220 to 750 nm). Plumage was illuminated and reflected light was collected through an optic fiber probe placed on a holder that isolated ambient light from the measured surface. The probe holder was held on the plumage, with the probe illuminating and collecting light at an angle of 45° in a proximal-distal direction at 23 mm from the plumage surface. The diameter of the circular region illuminated and measured was 6 mm. Spectrophotometer resolution was 0.35 nm. Each spectral measurement was the average of three readings with an integer time of 100 ms. We recalibrated the equipment before measuring each individual to mitigate the effects of shifts on spectrophotometer performance. Boxcar smoothing was not performed. Measurements were done blind, i.e., the subspecies of each specimen was unknown when the spectral measurements were taken. For large and bilateral body regions (cheeks, coverts, back, and belly) we took two or three reflectance measurements from each specimen to obtain a more representative assessment of the complete area, and used the average of these in our analyses.
Statistical Analyses
Median reflectance values for 5 nm bins from 350 to 650 nm were included in the analyses. Readings outside this wavelength range showed considerable noise. We estimated four spectral variables for each plumage patch. Brightness was calculated as the sum of the reflectance over the complete wavelength range (ΣR350–650). We estimated hue as the wavelength of maximum reflectance (λ(Rmax)). Chroma, an index of spectral saturation, was calculated as the reflectance from 50 nm lower than to 50 nm higher than hue location, divided by total reflectance (R(Hue−50)–(Hue+50)/R350–650). Finally, due to the importance of the UV visual channel to avian communication (Bennett et al. 1997, Johnsen et al. 1998, Hausmann et al. 2003), we estimated UV chroma as the ratio of shortwave reflectance to total reflectance (R350–400/R350–650). Consistent with previous studies (McNaught and Owens 2002, Hausmann et. al 2003, Bridge and Eaton 2005), specimen age had no influence on the spectral variables. Therefore, a fading effect due to storage in the museum collection was negligible.
Because spectral variables are frequently correlated with each other, and comparing all measured variables for all body regions (24 variables in total) would require correcting the α level for multiple comparisons to a very small value, we performed a principal components analysis (PCA) on the spectral variables of all plumage regions (Montgomerie 2005). A few principal components summarized the color information of all measured patches. We performed factor varimax rotation to facilitate interpretation of the principal component loadings Tabachnick and Fidell (2001). Since our sample size was too small to fulfill the subjects-to-variables rule, we checked the component loadings by jackknifing and found them to be highly stable (Montgomerie 2005).
We performed a two-tailed independent t-test for each extracted principal component to compare subspecies, and a regression analysis with date (day and month) of capture as the independent variable to determine temporal color variation. We do not have accurate data regarding the precise time of year in which molting occurs in Ultramarine Grosbeaks, but in most Passerina species (Cyanocompsa's closest genus) an extensive molt takes place at the end of the summer Thompson and Leu (1995). Therefore, we considered March (the end of the summer in the southern hemisphere) as the time of molt in our analysis.
Results
We extracted all principal components that explained more than 10% of the total variation, which agreed with those obtained using the more subjective scree test criterion Tabachnick and Fidell (2001). This resulted in three principal components that combined accounted for 58% of the total variation (Table 1). PC1 was positively correlated with brightness and negatively correlated with chroma of the forehead, cheeks, and coverts. PC2 was negatively associated with hue of the forehead and coverts and positively associated with UV chroma of the forehead and cheeks. PC3 was positively correlated with brightness of the rump and UV chroma of the rump and belly.
Factor loadings after varimax rotation of the principal components analysis performed on four spectral variables from six different plumage patches measured on males of two subspecies of Ultramarine Grosbeak (Cyanocompsa brissonii). Significant loadings (>0.70) are denoted with an asterisk.
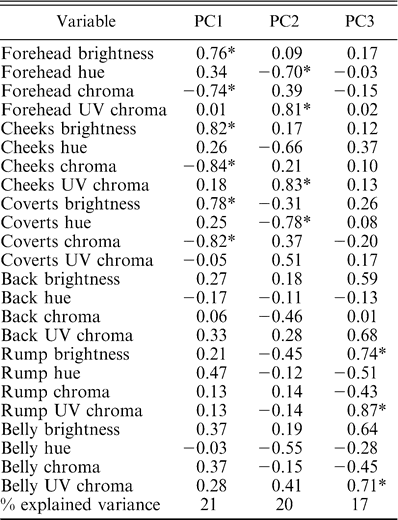
Factor loadings after varimax rotation of the principal components analysis performed on four spectral variables from six different plumage patches measured on males of two subspecies of Ultramarine Grosbeak (Cyanocompsa brissonii). Significant loadings (>0.70) are denoted with an asterisk.

PC1 was significantly higher for C. b. argentina than for C. b. sterea (t23 = −3.3, P < 0.01), meaning that the coloration of the forehead, cheeks, and coverts was brighter and less saturated in the males of C. b. argentina than in the males of C. b. sterea (Table 1, Fig. 1). PC2 also differed between subspecies (t23 = 3.0, P < 0.01), with a significantly higher value for C. b. sterea; thus, males of C. b. argentina had higher hue in the forehead and coverts and lower UV chroma in the forehead and cheeks (Table 1, Fig. 1). PC3 did not differ between subspecies (t23 = 0.1, P = 0.93).
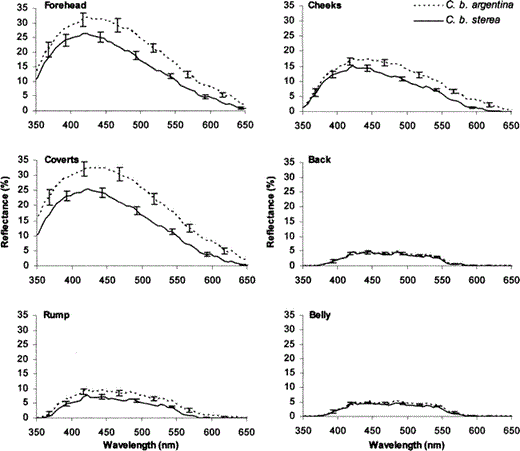
Reflectance spectra of males of the two examined Ultramarine Grosbeak (Cyanocompsa brissonii) subspecies.
Each spectrum represents mean reflectance (± SE) of each body region for C. b. argentina (n = 10) and C. b. sterea (n = 15, except for the forehead where n = 14). Significant differences were found between species. Brightness was higher in C. b. argentina in the forehead, cheeks, and coverts, and chroma was higher in C. b. sterea in the same body regions. UV chroma was higher in C. b. sterea in the forehead and cheeks and C. b. Argentina had a higher hue in the forehead and coverts.
Only PC2 showed a significant decreasing temporal tendency (Fig. 2). This indicates a gradual temporal reduction of UV chroma in the forehead and cheeks as well as a gradual increase in hue in the forehead and coverts after the end of the summer.
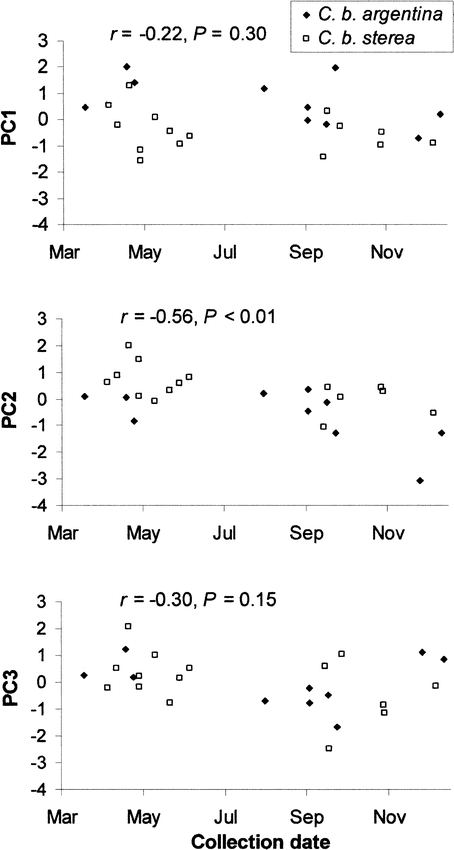
Regressions between the three principal components extracted from a principal components analysis performed on the spectral variables of six different body regions and date of collection of the specimens of Ultramarine Grosbeak (Cyanocompsa brissonii).
Total sample size = 25 (10 C. b. argentina and 15 C. b. sterea). PC1 is positively associated with brightness and negatively associated with chroma of the forehead, coverts, and cheeks. PC2 is negatively correlated with hue of the forehead and coverts, and positively correlated with UV chroma of the forehead and cheeks. PC3 is positively correlated with brightness of the rump, and UV chroma of the rump and belly. Only PC2 was significantly associated with date of collection, which means that hue in the forehead and coverts significantly increased and UV chroma of the forehead and cheeks significantly decreased after molting (in March).
Discussion
We found plumage color differences between males of the two studied subspecies of Ultramarine Grosbeak, particularly in those patches with the most conspicuous coloration (forehead, cheeks, and coverts). Males of C. b. argentina had brighter and less saturated coloration, with higher hue and lower UV reflectance, than males of C. b. sterea. Although two subspecies are not enough to draw strong conclusions about the causes promoting color divergence in this species, the differences between the environments that each subspecies inhabits could be a driving factor. Because ambient light varies among environments (Endler 1993), and color perception changes as a consequence, different signal properties may be favored in different habitats (Endler 1992, Marchetti 1993, McNaught and Owens 2002). In open spaces, such as those inhabited by C. b. argentina, ambient light derives directly from the sun and is mainly white with no particular prevailing wavelength (Endler 1993). In contrast, the ambient light in forests, like those inhabited by C. b. sterea, results from dispersion that occurs in the sky, which gives it a bluish aspect (“woodland shade”; Endler 1993). Highly saturated colors vary in intensity, while unsaturated colors vary in hue, in response to changes in the light wavelengths striking surfaces (Endler 1992). In open areas with white illumination, the brighter and less saturated plumage coloration of C. b. argentina would be more conspicuous because it reflects a wider range of wavelengths and has a higher intensity. In contrast, a shift toward shorter wavelengths and higher chroma could be favored in the plumage of C. b. sterea, since this subspecies inhabits an environment of lower luminosity where blue wavelengths prevail.
Differences in other sexual characters might also arise in response to environmental differences, as a result of maintaining a balance between their conspicuousness and their capacity to function as an honest signal of quality (Schluter and Price 1993, Price 1998). Indeed, a negative association between song complexity and conspicuousness of plumage coloration has been identified in species with carotenoid-based coloration (Badyaev et al. 2002). This may hold true for C. b. sterea males, where nuptial songs are both louder and more complex than in C. b. argentina (Ridgely and Tudor 1989; R. Straneck, Museo Argentino de Ciencias Naturales “Bernardino Rivadavia,” pers. comm.). Louder and more complex songs may compensate for the lower detectability of plumage color due to low ambient light intensity and higher vegetation density in the environment where this subspecies occurs.
In addition to color differences between subspecies, we found significant temporal variation in plumage color, probably as a result of feather wear. Plumage UV chroma gradually decreased and hue gradually increased after the end of the breeding season. These results are consistent with those of (Örnborg et al. 2002), who found the same temporal pattern in the Blue Tit and associated it with plumage wear after molting (in particular the deposition of dirt and fat on feathers, because these substances often absorb short wavelength light).
In summary, our study is the first to objectively and quantitatively document significant differences in plumage coloration between subspecies of Ultramarine Grosbeaks, as well as temporal variation in plumage attributes in this species. Approaches that take into account characteristics specific to the avian visual system for reflectance spectra analyses, such as those suggested by Endler and Mielke (2005) or employed by (Eaton 2005), might help to clarify whether the differences we found are actually perceived by the birds. Regardless, our results show a higher level of divergence than previously recognized between the two studied subspecies, establishing Ultramarine Grosbeaks as an interesting model species to study the ecological and behavioral factors that influence the evolution of structural coloration patterns.
Acknowledgments
We thank Gabriela Ibarguchi for bringing the spectrophotometer from Canada to Argentina and two anonymous reviewers who made valuable suggestions on a previous version of the manuscript. This work was supported by the Consejo Nacional de Investigaciones Científicas y Técnicas, Argentina (PEI 6001 to PLT), a Natural Sciences and Engineering Research Council of Canada Discovery Grant (to SCL), and a Canada Foundation for Innovation New Opportunities grant (to SCL).
Literature Cited



