-
PDF
- Split View
-
Views
-
Cite
Cite
Neil Losin, Chris H. Floyd, Todd E. Schweitzer, Sarah J. Keller, Relationship Between Aspen Heartwood Rot and the Location of Cavity Excavation by a Primary Cavity-Nester, the Red-Naped Sapsucker, The Condor: Ornithological Applications, Volume 108, Issue 3, 1 August 2006, Pages 706–710, https://doi.org/10.1093/condor/108.3.706
Close - Share Icon Share
Abstract
We investigated nest-hole excavation by the Red-naped Sapsucker (Syphrapicus nuchalis) in aspen (Populus tremuloides) woodlands in western Colorado. Sapsuckers excavate nest cavities primarily in aspens infected with a heartwood rot fungus (Phellinus tremulae), which softens the heartwood of infected trees. We assessed the interior condition of fungus-infected aspen trunks by extracting wood samples with an increment corer to determine whether sapsuckers chose nest-hole locations based on the extent of healthy sapwood remaining. Comparing fungus-infected trees with and without cavities, cavity-bearing trees had thinner healthy sapwood. The depth of healthy sapwood also varied with compass direction, being thinnest on the south sides of fungus-infected aspens. Cavity entrance orientations were significantly biased to the south-southeast, corresponding with the directional bias in heartwood rot. These results suggest that the depth of healthy sapwood, and hence excavation effort, may be important in determining nest hole location for the Red-naped Sapsucker.
Resumen
Relación entre un Hongo de Populus Tremuloides y la Ubicación de Excavaciones de Syphrapicus nuchalis, un Ave que Nidifica en Cavidades Primarias
Investigamos la excavación de cavidades de nidificación por parte de Syphrapicus nuchalis en bosques de Populus tremuloides en el oeste de Colorado. Esta especie excava las cavidades principalmente en árboles infectados con el hongo Phellinus tremulae, el cual ablanda la madera. Evaluamos la condición interna de los troncos de los árboles infectados mediante la extracción de muestras de madera con un barreno de incremento para determinar si las aves eligen la localización de las cavidades basadas en la magnitud de restos de madera saludable. Comparando los árboles infectados con hongos con y sin cavidades, los árboles que presentaron cavidades tuvieron una madera saludable más delgada. La profundidad de la madera saludable también varió con la orientación cardinal, siendo más delgada en las caras sur de los árboles infectados. La orientación de la entrada de las cavidades estuvo significativamente sesgada hacia el sur-sureste, correspondiendo con el sesgo direccional del hongo. Estos resultados sugieren que la profundidad de la madera saludable, y por ende el esfuerzo de excavación, pueden ser importantes para determinar la localización de las cavidades de nidificación en S. nuchalis.
Primary cavity-nesting birds use a variety of criteria to select nest trees and nest-hole locations within trees. Excavation effort may be one important factor in the selection process. Many woodpeckers prefer to excavate nest cavities in trees with soft wood (Schepps et al. 1999). Heartwood fungus infection can soften the wood of many tree species (Jackson and Jackson 2004), and many woodpeckers are known to prefer fungus-infected trees for nesting (Kilham 1971, Hooper et al. 1991, Daily 1993).
Compass orientation also influences the placement of nest cavities by cavity-excavating birds (Inouye 1976). Cavities may be oriented for thermoregulation (Butcher et al. 2002) or to allow easy access to foraging areas (Dobkin et al. 1995). Red-naped Sapsucker nests, for example, have been found to be oriented predominantly toward the south-southeast in some areas, perhaps to maximize exposure to direct sun (Inouye 1976). Multiple factors, including nest microclimate, access, and defensibility may together determine optimal cavity orientation (Zwartjes and Nordell 1998). Heartwood rot could affect the radial placement of nest cavities, because cavity excavation may be easier in a rotted section of trunk than in a healthy one; to our knowledge, however, no such association has been documented (Conner 1977, Jackson and Jackson 2004).
To investigate the role heartwood rot plays in woodpecker nest-hole location, we studied the nest cavity characteristics of the Red-naped Sapsucker (Syphrapicus nuchalis, hereafter sapsucker) in aspen (Populus tremuloides) woodlands in the Rocky Mountains of western Colorado. Sapsuckers are the most abundant primary cavity-nesters in this region, and their nest holes are used by at least eight secondary cavity-nesting species (Daily et al. 1993; NL, pers. obs.). Sapsucker nests are commonly found in aspens infected with a heartwood rot fungus (Phellinus tremulae), which rots the trunk from the inside out and produces distinctive reproductive structures, called conks, on the exterior of the trunk (Crockett and Hadow 1975). Daily (1993) found that all sapsucker nest trees studied were infected by P. tremulae, compared with a low infection rate in trees without cavities. These findings suggest that sapsuckers may choose their nest trees based on the presence or absence of fungus, selecting trees with external signs of heartwood fungus infection, such as wounds, which provide a route for infection, or fungal conks. Whether the extent of heartwood rot influences sapsucker nest tree choice, however, is unknown.
We examined the relationship between sapsucker nest-hole location and aspen heartwood fungus. Using nest-site selection at two scales, nest tree choice and cavity orientation, we tested the hypothesis that sapsuckers excavate cavities where healthy sapwood is thinnest, thereby minimizing excavation effort. We predicted that trees with sapsucker nests would have thinner healthy sapwood than trees without cavities. We measured healthy sapwood in each cardinal direction to determine whether there was a directional bias in heartwood rot. If such a bias existed, we expected sapsucker cavities to be oriented in the direction of the thinnest healthy sapwood.
Methods
We conducted this study at the Rocky Mountain Biological Laboratory in Gunnison County, Colorado during summer 2004 and 2005. In 2004, we located 95 aspens with sapsucker cavities, containing 19 active sapsucker nests and 195 unused holes. An additional 79 fungus-infected trees, both with (n = 30) and without (n = 49) cavities, were located in August 2005, after the conclusion of breeding activity. We measured a total of 272 sapsucker holes in 2004 and 2005. Cavity-bearing trees were located by visual search; trees without cavities but with heartwood rot were located by looking for fungal conks in the same aspen groves. From visual inspection, we could not determine whether a cavity had been used by sapsuckers in past years. We identified sapsucker nest cavities based on their entrance hole size and habitat characteristics. Only one other cavity-excavating species, the Northern Flicker (Colaptes auratus), was common in our study area, and its nest cavities were much larger than sapsucker cavities. We recorded the compass orientation of each cavity entrance to the nearest half-degree using a handheld sighting compass and corrected orientations to true north before analysis.
To assess the interior condition of a tree, we used an increment corer to extract a wood sample, or core, approximately 5 mm in diameter, extending from the outer bark to the center of the tree (hereafter “coring”). In 2003 we collected pilot data, coring infected trees with (n = 12) and without (n = 8) cavities at three heights: 1 m, 3 m, and 5 m. We sampled wood from the north and south side of each tree. These data suggested that the patterns of sapwood depth did not vary substantially with coring height up to 5 m, so for simplicity we cored trees at breast height (about 1.5 m) only in 2004 and 2005. The latter strategy did not require climbing equipment, and allowed us to core many more trees than climbing would have permitted. In 2004 and 2005, each live tree was cored to its center four times, once in each cardinal direction. Sometimes a core could not be taken because another tree was obstructing the path of coring; we cored only when it was possible to take a core within a few degrees of the appropriate cardinal direction. We determined the extent of rot in each core by visual inspection—rotted wood appeared discolored and crumbly, contrasting sharply with healthy wood. Dead trees were used by sapsuckers but were not cored, because we could not distinguish reliably between rotted and unrotted wood. We measured the depth of healthy sapwood in each core and the diameter at breast height (dbh) of each tree cored.
Statistical Analyses
Depth of healthy sapwood, a linear variable, was analyzed using mixed-model ANOVAs. To test for pairwise differences in sapwood depth between cardinal directions, we used Tukey's test (familywise α = 0.05). We tested the circular uniformity of distribution for cavity orientations using Rayleigh's test, and used circular ANOVA to test for differences in orientation between active and unused cavities. We performed linear analyses using SAS v. 9.1 (SAS Institute 2003) and circular analyses using R v. 2.1.1 (R Development Core Team 2005) with the package “circular” v. 0.3–2 (Lund and Agostinelli 2005) for circular variables. Core measurements were log-transformed to meet ANOVA assumptions. Means are reported ± SD.
Results
Sapsucker cavity orientation was significantly biased toward the south-southeast, with a mean orientation of 166° (Rayleigh's test, n = 272, ρ = 0.41, P < 0.001; Fig. 1). Because cavity orientations within a tree may not be independent, we analyzed the data using mean cavity orientations by tree and obtained a similar result, with a mean bearing of 157° (Rayleigh's test, n = 124, ρ = 0.45, P < 0.001). In 2004, cavities containing active sapsucker nests did not differ in their orientations from unused holes (circular ANOVA, F1,212 = 0.1, P = 0.70).
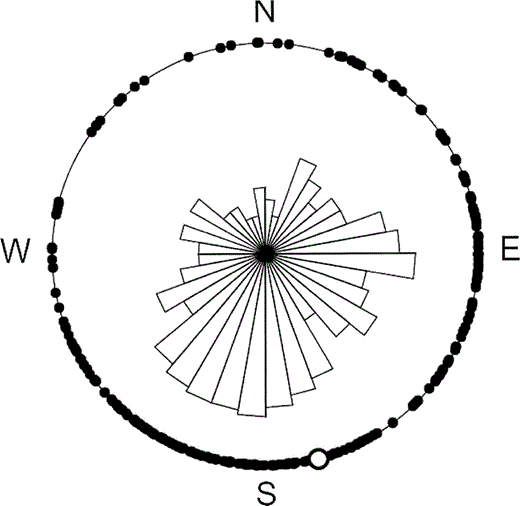
Figure 1. Distribution of the orientations of Red-naped Sapsucker cavities in aspen trees in western Colorado. Each point on the perimeter of the circle represents the orientation of an individual cavity (n = 272). The unfilled circle represents the mean bearing of all cavities (166°). In the center, a rose diagram divided into 10-degree bins shows the relative distribution of cavity orientations about the compass
Analysis of samples collected in 2003 showed that fungus-infected aspens had thinner healthy sapwood on their south sides than on their north sides (F1,83 = 11.9, P < 0.001), and infected trees with sapsucker cavities tended to have thinner healthy sapwood than infected trees without sapsucker cavities (F1,18 = 4.1, P < 0.06). The thickness of healthy sapwood decreased somewhat with increasing core height (F2,83 = 4.0, P = 0.02), but we observed no significant interactions between core height and direction (F2,83 = 0.5, P = 0.62), or between core height and cavity presence (F2,84 = 1.5, P = 0.24). Because no interactions with core height were observed, we conclude that cores taken at breast height allow us to infer patterns of sapwood depth higher in infected trees.
In 2004 and 2005, all sapsucker cavity-bearing trees had evidence of heartwood rot. In 2005, we cored fungus-infected trees both with and without cavities. The depth of healthy sapwood differed significantly between cavity-bearing trees and infected trees without cavities, with dbh as a covariate (F1,77 = 12.6, P < 0.001). Trees with sapsucker cavities had thinner healthy sapwood overall (4.5 ± 2.5 cm, n = 30) than trees without cavities (7.0 ± 4.2 cm, n = 49). Trees with and without cavities did not differ significantly in dbh (F1,77 = 0.69, P = 0.41). Trees with active sapsucker nests and those with unused holes in 2004 did not differ in the depth of healthy sapwood (F1,65 = 1.0, P = 0.32).
Healthy sapwood depth differed significantly among cardinal directions (F3,410 = 53.9, P < 0.001). Southern cores had the thinnest healthy sapwood (4.2 ± 3.2 cm, n = 144), followed by eastern (5.2 ± 3.0 cm, n = 140), western (5.8 ± 3.1 cm, n = 136), and northern (6.2 ± 3.3 cm, n = 142) cores (Fig. 2). Pairwise comparisons between directions showed that southern cores had significantly thinner healthy sapwood than cores from any other direction (all P < 0.001, Tukey's test). We analyzed trees without cavities alone to test whether a directional bias existed independent of sapsucker excavation, and the same pattern was observed (F3,138 = 21.4, P < 0.001): southern cores had the thinnest healthy sapwood, followed by eastern, western, and northern cores, and southern cores had significantly thinner sapwood than any other direction (all P < 0.001, Tukey's test).
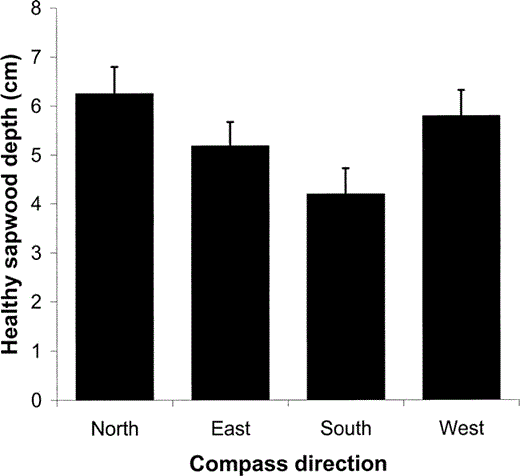
Figure 2. Depth of healthy sapwood in each cardinal direction for all aspen trees sampled at the Rocky Mountain Biological Laboratory in Gothic, Colorado in 2004 and 2005 (n = 146). Wood samples were collected using an increment corer to extract a piece of wood approximately 5 mm in diameter, extending from the outer bark to the center of the tree. Error bars represent 95% confidence limits
Discussion
We observed two significant trends in the extent of heartwood rot in aspen trees. First, trees with sapsucker cavities had thinner healthy sapwood than trees without cavities. Second, fungus-infected trees had a nonuniform radial distribution of heartwood rot, with the thinnest healthy sapwood on the south sides of their trunks. Sapsucker nests were correspondingly biased in their compass orientations toward the south-southeast.
These findings suggest that sapsucker nest-site choice may be strongly influenced by the depth of healthy sapwood through which they must excavate to reach the fungus-softened heartwood. We propose that sapsuckers minimize their excavation effort by excavating nest holes in trees with extensive heartwood rot, and by nesting on the south sides of fungus-infected trees, where healthy sapwood is thinnest.
Our data cannot address causation. One alternative to the above explanation is that sapsucker cavity excavation helps to transmit the fungus, or creates a favorable fungal growth environment, leading to the biases we observed. Indeed, a similar effect has been documented in pine forests: woodpeckers carry fungal spores on their bills, and their foraging activity may contribute to fungus-mediated sapwood decay (Farris et al. 2004). Cavity excavation, however, is much more localized than foraging activity, and it is difficult to imagine that the large difference we observed in heartwood rot between trees with and without cavities is due to sapsucker-mediated fungus transmission alone. We also find it improbable that cavity excavation encourages fungal growth, for two reasons. First, nest cavities expose the interior of the trunk to the drying effects of wind and sunlight, effects that are unlikely to favor fungus growth (Jackson and Jackson 2004). Second, the directional differences in heartwood rot exist even in trees without sapsucker cavities, so sapsuckers cannot be responsible for this directional effect. In both tree choice and cavity orientation, the pattern of sapsucker cavity locations is consistent with nest-site selection for minimal excavation effort.
In each of the above alternatives, cavities and heartwood rot are causally linked, though the direction of causation differs. Each directional bias, however, may be caused independently by the same or different environmental factors. Many birds are known to select nest cavities based on microclimate characteristics (Wachob 1996), and woodpeckers and secondary cavity-nesters may prefer south-facing cavities in cool environments (Inouye 1976, Dobkin et al. 1995, Rendell and Robertson 2004) or north-facing cavities in hot environments (Inouye et al. 1981, Hardy and Morrison 2001). In the high-altitude aspen woodlands near the Rocky Mountain Biological Laboratory, sapsuckers may orient their cavities to the south to maximize their exposure to direct sun, thereby creating a warmer nest microclimate. Warm nest temperatures increased survival and growth rates of Tree Swallow chicks (Dawson et al. 2005), and were associated with increased clutch size in Northern Flickers (Weibe 2001). Wind has also been implicated in the orientation of nest entrances (Facemire et al. 1990), though we have no evidence of such an effect in the placement of sapsucker nest holes.
Wind, however, is one important dispersal mechanism for fungal spores, so prevailing winds could create a directional bias in heartwood rot (Jackson and Jackson 2004). If more spores arrived on the south side of an aspen trunk, this could lead to more extensive rot on the south side of the tree. Similarly, the fungus likely invades healthy trees from branch stubs (Jackson and Jackson 2004), and if aspens grow more branches on the south side of the tree to capture available solar radiation, then more branch stubs will be formed on that side, creating additional sites for fungal infection. Finally, the greater sunlight on the south side of an aspen may create warmer conditions inside the trunk, which could facilitate fungus growth. Thus, greater solar radiation may favor sapsucker excavation on the south sides of aspens due to a warmer nest environment for rearing chicks, easier excavation of the nest cavity due to more extensive heartwood rot, or a combination of these factors.
Other authors have suggested that sapsuckers use fungal conks and signs of external damage as indicators of the suitability of a tree for nesting (Kilham 1971, Daily 1993). If sapsuckers select nest sites based on the depth of healthy sapwood, however, rather than simply the presence or absence of rot, they may excavate test holes or use nonvisual cues to select nest trees. It has been suggested that a woodpecker may use its bill to “sound” a tree trunk to assess the interior condition of the tree (Conner et al. 1976). Perhaps Red-naped Sapsuckers can assess the depth of healthy sapwood in aspens in such a manner.
Nest-site selection in primary cavity-nesters like the Red-naped Sapsucker is a complex process involving factors at multiple spatial scales (Lawler and Edwards 2006). Our results strongly suggest that the extent of heartwood rot caused by aspen heartwood fungus (Phellinus tremulae) may influence nest tree choice and nest cavity placement within a tree. Further research is needed to confirm the causal relationship between fungus-mediated heartwood rot and nest orientation, and to elucidate the proximate mechanisms of nest-site selection.
Acknowledgments
This research was supported by a National Science Foundation grant (Research Experiences for Undergraduates) to the Rocky Mountain Biological Laboratory, an Explorers Club Youth Activity Fund Grant to SJK, and a Garden Club of America Scholarship in Environmental Studies grant to SJK. We thank the Rocky Mountain Biological Laboratory for providing research facilities and living quarters during the study, N. Waser for assistance with pilot study design, D. Carr for statistical comments, and D. Miller and E. Reynolds for field assistance. Two anonymous reviewers provided valuable comments on a previous version of the manuscript.
Literature Cited



