-
PDF
- Split View
-
Views
-
Cite
Cite
Luke K. Butler, Sievert Rohwer, Michelle Rogers, Prebasic Molt and Molt-Related Movements in Ash-Throated Flycatchers, The Condor: Ornithological Applications, Volume 108, Issue 3, 1 August 2006, Pages 647–660, https://doi.org/10.1093/condor/108.3.647
Close - Share Icon Share
Abstract
We describe the timing and location of the prebasic molt in the Ash-throated Flycatcher (Myiarchus cinerascens), a hawking insectivore with resident populations in Mexico and migrant populations that breed in the western United States. The timing of fall molt with respect to migration is of particular interest for Ash-throated Flycatchers from the United States because they breed in arid lowland habitats that are probably unproductive in late summer, and because time constraints on molt might force northern populations to leave their breeding grounds before molting. Adults and juveniles depart their breeding grounds to arrive and molt in the region of the Mexican monsoon shortly after the monsoon rains begin. Diminishing food on the breeding grounds and increasing food in the monsoon region seem more important than time constraints for explaining molt-related movements by northern populations, because more southerly breeding birds east and west of the monsoon region also move to the monsoon region to molt. We found age class differences in the timing and duration of molt. In adults the primary molt starts approximately 14 July and requires 76 days to complete, whereas in juveniles, primary molt starts approximately 1 August (18 days after adults) but requires only 50 days to complete. We found no evidence that juveniles molt more primaries simultaneously than adults, so we conclude that the daily growth rate of individual feathers must be higher in juveniles than adults.
Resumen
Muda Prebásica Y Movimientos Relacionados Con La Muda En Myiarchus Cinerascens
Describimos el momento y el lugar en que sucede la muda prebásica en Myiarchus cinerascens, un ave insectívora que tiene poblaciones residentes en México y poblaciones migratorias que crían en el oeste de los Estados Unidos. El momento en que sucede la muda del otoño con respecto a la migración reviste un interés particular para las poblaciones de los Estados Unidos porque éstas se reproducen en ambientes áridos de tierras bajas que probablemente son improductivos hacia el final del verano, y porque las limitaciones de tiempo sobre la muda podrían forzar a los individuos de las poblaciones del norte a abandonar sus sitios de cría antes de mudar. Los adultos y juveniles abandonan las áreas reproductivas para llegar a la región monzónica mexicana, donde mudan poco después del inicio de las lluvias monzónicas. La disminución en la disponibilidad de alimento en las zonas de cría y su aumento en la región monzónica parecen ser más importantes que las limitaciones de tiempo para explicar los movimentos de las poblaciones norteñas relacionados con la muda, pues las aves que se reproducen más al sur en áreas ubicadas al este y al oeste de la región monzónica también se desplazan hacia esta región para mudar. Encontramos diferencias entre clases de edad en cuanto al momento en que tiene lugar la muda y en cuanto a su duración. En los adultos, la muda de las primarias se incia aproximadamente el 14 de julio y requiere 76 días para completarse, mientras que en los juveniles se inicia aproximadamente el 18 de agosto (18 días después que en los adultos), pero requiere sólo 50 días para completarse. No encontramos evidencia de que los juveniles muden más primarias de forma simultánea que los adultos, por lo que concluimos que la tasa de crecimiento diario de las plumas debe ser mayor en los juveniles.
Introduction
Studies of prebasic molt and migration in North American passerines have yielded three general observations: western lowland species molt after departing the breeding grounds, western montane and eastern species molt before departing the breeding grounds, and aerial foragers molt and migrate simultaneously (Rohwer et al. 2005). Several hypotheses address these contrasts. In a recent review of east–west differences, Rohwer et al. (2005) found it conceptually helpful to consider selective forces that would favor birds leaving their breeding grounds before molting and selective forces that would favor birds moving to a particular region to molt (molt-migrating). “Pushes” are ecological or demographic forces that favor leaving a region to molt elsewhere, while “pulls” are ecological or demographic forces that favor moving to a particular region to molt. Western lowland insectivores seem to be pushed away from their breeding grounds by arid late-summer conditions, whereas eastern species and western montane species experience relatively high late-summer productivity that can support the energetic and nutritional demands of molt (Rohwer et al. 2005). Western lowland insectivores also seem to experience a complementary pull to molt in the American Southwest, resulting from a food flush (abundance of food) that follows the rains of the Mexican monsoon (Comrie and Glenn 1998, Rohwer et al. 2005). Finally, coursing aerial foragers (e.g., swallows) experience no conflict between molt and migration because they must fly to forage, so they molt slowly and overlap molt and migration (Niles 1972, Stutchbury and Rohwer 1990, Yuri and Rohwer 1997).
In addition to these ecological and foraging-related effects on the scheduling of the prebasic molt, several western species have age-related differences in molt scheduling. Among western species in which adults molt after departing the breeding grounds, there are several species in which juveniles delay departure and molt on the breeding grounds. For example, juveniles, but not adults, stay on the breeding grounds to molt in western populations of Painted Buntings (Passerina ciris, Thompson 1991), Lazuli Buntings (P. amoena, Young 1991), western Warbling Vireos (Vireo gilvus swainsonii, Voelker and Rohwer 1998), and Western Tanagers (Piranga ludoviciana, Butler et al. 2002). Two hypotheses could explain this contrast. First, in juveniles of species that stay on the breeding grounds to molt, only the body feathers are replaced before migrating, with flight feathers replaced either after partial or complete southward migration, or not until the following year. Because juveniles of these species undergo a less extensive molt on the breeding grounds, they may not experience the same “push” to migrate as adults, and in species in which juveniles retain the flight feathers until the following year, juveniles may not experience the same “pull” to molt in the productive monsoon region. Second, juveniles of species that delay migration may have juvenal body feathers that are more loosely textured, with fewer barbs per cm of rachis, than juveniles of species that, like adults, depart the breeding grounds prior to molting (Rohwer et al. 2005). Thus, there could be flight constraints on juveniles because of their lax body feather structure, which may raise the cost of migration by increasing drag (Rohwer et al. 2005).
The Ash-throated Flycatcher (Myiarchus cinerascens), a hawking insectivore, offers important tests of these hypotheses related to the scheduling of prebasic molt. Ash-throated Flycatchers are breeding-season migrants to western North America, occurring from Washington state south to California and southeast to central Texas (Cardiff and Dittmann 2002). There are also resident populations in the central Mexican monsoon region and in Mexico to the south and east, permitting geographical contrasts in the rate, location, and timing of molt. Furthermore, because both age classes replace all body and flight feathers during the prebasic molt (Pyle 1997), the same ecological pushes and pulls should apply to juveniles and adults from the same population.
We used museum specimens to test several hypotheses about molt in Ash-Throated Flycatchers. As low-elevation, open country insectivores, Ash-throated Flycatchers could experience the “push” of western aridity in late summer but, because they are relatively large flycatchers, they might also be able to molt before migrating by taking larger prey such as grasshoppers and beetles (Rosenberg et al. 1991, Cardiff and Dittmann 2002) that probably survive longer into the summer than the smaller, soft-bodied prey taken by foliage-gleaning western molt-migrants such as the western Warbling Vireo (Voelker and Rohwer 1998). However, even if larger prey are available to them, Ash-throated Flycatchers could nevertheless face time constraints on molting at northern latitudes if, as hawking insectivores, they are aerodynamically constrained to molt the flight feathers slowly. Ash-throated Flycatchers breed north of the monsoon region in Washington, Oregon, and Utah, west of the monsoon region in Nevada and southern California, and east of the monsoon region in Oklahoma, Texas, and eastern New Mexico. We used these contrasts to generate several predictions.
If only northern populations move to the monsoon region, then time constraints on molting in the north must be a relatively strong force in the evolution of molt-migration in this species. If all migratory populations move to the monsoon region, then both the complementary “push” of deteriorating food conditions on the breeding grounds and the “pull” of the monsoon-related food flush must be relatively strong forces in the evolution of molt-related movements, because western and eastern populations in the south gain no temporal benefit from moving longitudinally.
If Ash-throated Flycatchers use a monsoon-related food flush for molting, then monsoon-area molting should follow the arrival of the monsoon rains. Because the rains of the Mexican monsoon arrive first in the southern part of the monsoon molting area (Comrie and Glenn 1998), we also tested the prediction that molt occurred earlier in the south than the north.
Finally, we tested the prediction that juveniles have the same rate, timing, and location of molt as adults because, like adults, juveniles undergo a complete molt. To further investigate the role of juvenal body feather structure as an aerodynamic constraint on the scheduling of molt in juveniles, we quantified the structural quality of juvenal body feathers in Ash-Throated Flycatchers and compared it to published values for other molt-migrant species.
Methods
Sources of Molt Data
We examined 529 study skins from 18 North American collections including the Burke Museum. Peak incubation occurs in May in the southern part of the breeding range and in mid-June farther north (Rosenberg et al. 1991), so we scored specimens collected on or after 10 June to detect possible short-distance, molt-related movements (Butler et al. 2002). We scored specimens through 30 November to determine when molt ended and to determine whether Ash-throated Flycatchers moved to wintering areas farther south after molting. Despite our extensive loan requests, we found just 47 molting specimens among the 17 collections from other institutions, making the 26 molting specimens obtained during recent collecting trips to the Mexican monsoon region by the Burke Museum critical to this study.
In a Burke Museum expedition to Mexico in 2005, several molting Ash-throated Flycatchers were captured, and their age and primary molt status were determined before release. These molt scores added two juveniles and five adults from 5–6 August in Baja California Sur, and two juveniles from 10 August near El Fuerte, Sinaloa, to our sample. Birds from the same locality differed sufficiently in molt score that there was no possibility they were netted and counted twice. These scores were included in analyses of the sequence and rate of feather replacement, but not in the geographical and temporal analyses, which included only specimen data.
Nutting's Flycatchers (M. nuttingi) are very similar to Ash-throated Flycatchers and difficult to distinguish except by mouth color (orange in Nutting's and pink in Ash-throated). Where mouth color was not recorded on specimen labels we used characters described by Lanyon (1961) to eliminate Nutting's Flycatchers from our samples.
Aging Criteria
All birds at least one year old were classified as adults, and all birds hatched in the year they were collected were classified as juveniles. Before and during the first prebasic molt, juveniles were easily distinguished from adults by their rust-colored body feathers, orange wing bars, and tails lacking the darker brown edging of adults (Pyle 1997). Juveniles that had completed the first prebasic molt were distinguished from adults by their relatively narrow and rufous-tinged primary coverts and alula (Pyle 1997). Adults, but not juveniles, replace the primary coverts and alula at the same time as the primaries, so among birds that had completed the primary molt, the coverts and alula were new and relatively broad and dark in adults but worn and pale in juveniles (Pyle 1997).
Describing Molt in the Primaries
We used the presence of actively growing primary feathers to describe the location and timing of the prebasic molt. Replacement of most body feathers occurs during the time dedicated to flight feather molt in this species (Pyle 1997). Adults from July that had not begun primary molt had clearly worn body feathers, adults molting primaries often showed intense body molt, and adults near the end of primary molt had mostly fresh body feathers.
All molt scoring was performed under a lighted magnifying glass by gently lifting the underwing coverts with a probe to expose the base of the primaries. The ten primary feathers of each wing were scored following Rohwer (1986). Worn and faded feathers were scored as 0, and tiny pin feathers were scored as 0.01. For longer growing feathers the percentage of fully grown feather length that was replaced was estimated in increments of 0.05 and 0.1 (e.g., a growing feather at 65% of its fully grown length = 0.65). We used 0.99 to indicate growing feathers that were more than 95% grown. New, fully grown feathers were scored as 1.0, and were distinguished from old feathers by the remains of sheathing at their bases and the lack of wear. Hereafter, individual primary feathers are abbreviated with “P” followed by the position in the wing, beginning with the innermost primary (P1) and counting distally to P10.
We described the sequence of molt in the primaries following the method of Yuri and Rohwer (1997). We compared each growing primary feather to the adjacent feathers to determine the sequence of replacement. A growing feather was considered to have initiated a molt series if it was more advanced in growth than both neighboring feathers (a nodal feather), and it was considered to have terminated a molt series if it was less advanced than both neighboring feathers (a terminal feather). A growing feather that was less advanced than the next proximal feather but more advanced than the next distal feather indicated proximal-to-distal replacement. Because P1 and P10 could only be compared to one neighboring primary feather they were considered to be either nodal or terminal, as it was clear that the primaries and outer secondaries constituted separate molt series.
Duration, Location, and Timing of Molt
If Ash-throated Flycatchers are flight-constrained to molt slowly, then molt duration should be relatively long (≥60 days) compared to other North American passerines that molt in the monsoon region (Voelker 2000). We used Pimm's (1976) regression method to estimate duration of molt, with day of year as the dependent variable and primary molt score summed across all primaries as the independent variable. This method makes our analysis directly comparable to the majority of published molt regressions in North American passerines, but it ignores variation in feather length across the wing, which is substantial in this species (Table 1). Therefore, for comparison with the standard molt score method and for age class comparisons among Ash-throated Flycatchers only, we also calculated Pimm's (1976) regression using the percent of total mm of primaries replaced as the independent variable. For this analysis, lengths of fully grown primaries were measured on five study skins of each sex and age class, and for each molting specimen in our sample, percent of total mm replaced was calculated by multiplying the molt score of each feather by the mean length of the feather when fully grown.
Sequence of replacement and lengths of primary wing feathers grown by Ash-throated Flycatchers during the prebasic molt. Shown are numbers of feathers indicating molt sequence at each position in the wing. Primary molt begins at P1 and proceeds distally to P10 in juveniles and adults. No feathers showed a distal-to-proximal replacement pattern. Within each age class, primary length (mm) is the mean of five specimens of each sex
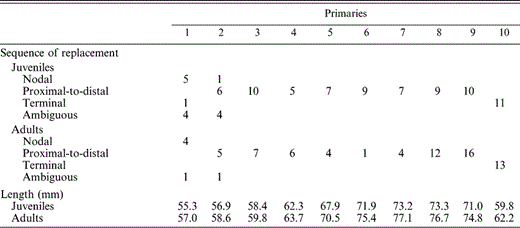
Sequence of replacement and lengths of primary wing feathers grown by Ash-throated Flycatchers during the prebasic molt. Shown are numbers of feathers indicating molt sequence at each position in the wing. Primary molt begins at P1 and proceeds distally to P10 in juveniles and adults. No feathers showed a distal-to-proximal replacement pattern. Within each age class, primary length (mm) is the mean of five specimens of each sex

To illustrate molt-related movements, we drew the geographical distribution of the peak Mexican monsoon precipitation onto a map of western North America (Comrie and Glenn 1998). Birds from the United States were then mapped to the nearest state and city where possible, or to the nearest state and county. Birds from Mexico were mapped to the nearest state and city where possible, or at the center of the state when city-level mapping was impossible based on specimen labels. We looked for evidence of molt-related movement to the Mexican monsoon region by comparing the geographical distribution of juveniles and adults that had not yet started molting (premolt) to the distribution of juveniles and adults that were molting. If Ash-throated Flycatchers depart breeding areas north and west of the monsoon region before molting, then molting specimens should be significantly less common in these areas compared to the frequency of premolt specimens in these areas. We considered specimens collected north of the northern limit of the monsoon region (37–38°N) to be from the northern population, and specimens collected west of the monsoon region but south of 37°N to be from the western population (Fig. 1). We also looked for evidence that Ash-throated Flycatchers from east of the monsoon region depart these breeding grounds before molting, as in western populations of Painted Buntings (Thompson 1991). Finally, we compared the latitudinal distributions of molting adults and juveniles.
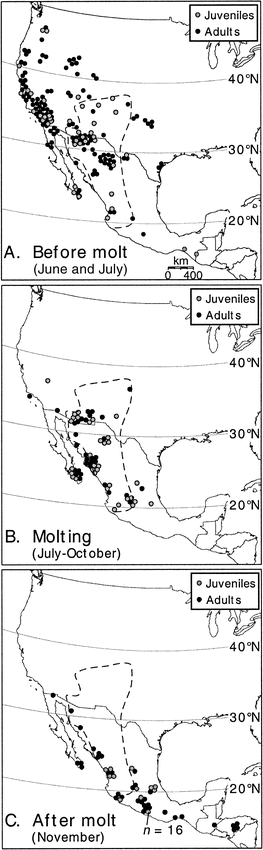
Figure 1. Geography of Ash-throated Flycatchers a) before molt in June and July, b) while molting, and c) shortly after molt. Dashed line indicates the geographical distribution of the Mexican monsoon region, including Baja California Sur (Comrie and Glenn 1998)
If adults and juveniles take advantage of a monsoon-related food flush, then they should molt in the monsoon region during or shortly after the peak monsoon rains in July and August (Comrie and Glenn 1998). We examined this possibility by mapping the localities of molting specimens, and by graphing the temporal frequency of molting specimens in semimonthly periods.
We tested the possibility that molt occurs earlier in the south than the north of the monsoon region, because southern Ash-throated Flycatcher populations breed about one month earlier than northern populations (Cardiff and Dittmann 2002), and because the monsoon rains begin about one month earlier in the southern than northern part of the monsoon region (Comrie and Glenn 1998). The residuals from Pimm's (1976) regression for estimating molt rate provided a way to compare the timing of molt among all molting individuals in the monsoon area within each age class. Among specimens at the same stage of molt, those collected earlier have low residuals and those collected later have high residuals.
Feather Structure
We examined the texture of the first (juvenal) body feathers of Ash-throated Flycatchers by comparing the number of barbs per distal cm of rachis (barb count) to published values for species in which the juvenal plumage is replaced before or after migration (Rohwer et al. 2005). Our results showed that young Ash-throated Flycatchers migrate south in juvenal plumage, so we predicted the quality of their body feathers would be high relative to adults, similar to other species in which immatures migrate in juvenal plumage (Rohwer et al. 2005). We measured the barb count of feathers from both sides of the lateral lower breast and upper belly. Our sample included three feathers from each of six adults and six juveniles.
Statistical Analyses
We used Fisher's exact test to compare the frequency of molting and nonmolting specimens in and out of the region of the Mexican monsoon. We used a G-test with Williams' correction (Sokal and Rolf 1995) for contingency analysis of age, molt status, and semimonthly time period. We tested for latitudinal differences in molt timing within the monsoon region by regressing the residuals from Pimm's (1976) regression (percent mm replaced method) against latitude. We used ANCOVA to test for age differences in the slope of Pimm's (1976) regression, treating day of year as the dependent variable, with the independent variables age class and percent mm replaced. In the ANCOVA, the interaction term age class * percent mm replaced was used to test for any dependence of molt rate on age class. Because we found significant age class differences in the duration of molt, we performed post-hoc Mann-Whitney U-tests for possible age class differences in the number of simultaneously growing primaries. Data are presented as means ± SD. We concluded statistical tests were significant when P < 0.05.
Results
Sequence and Rate of Primary Molt
The primaries constitute a single molt series in both adults and juveniles (Langston and Rohwer 1995) that starts with P1 (and often P2 simultaneously), proceeds distally, and ends at P10 (Table 1). Among the 14 specimens and four field-scored birds molting P1 and P2, P1 was 10% longer than P2 in nine (P1 nodal), P1 = P2 in seven, and P2 was 10% longer than P1 in one (P2 nodal). P1 was 40% longer than P2 in one field-scored adult sampled on 5 August in Baja California Sur.
We found an unexpected age class difference in the amount of time required to replace the primaries. As predicted for a hawking insectivore, adults appeared to molt slowly, requiring 76 days to complete primary feather replacement (Fig. 2). In contrast, juveniles required only 50 days to complete this molt (Fig. 2). The age difference in molt duration, indicated by differences in the slope of the regression of day of year on molt score, was statistically significant (ANCOVA, interaction between age and molt score: F1,67 = 6.4, P = 0.01). Essentially the same results were obtained regressing day of year on percent mm replaced. In this latter analysis, adults started molt two days later and required 75 days, and juveniles started molt one day later and required 49 days, to molt (ANCOVA, interaction between age and percent mm replaced: F1,67 = 6.4, P = 0.01).
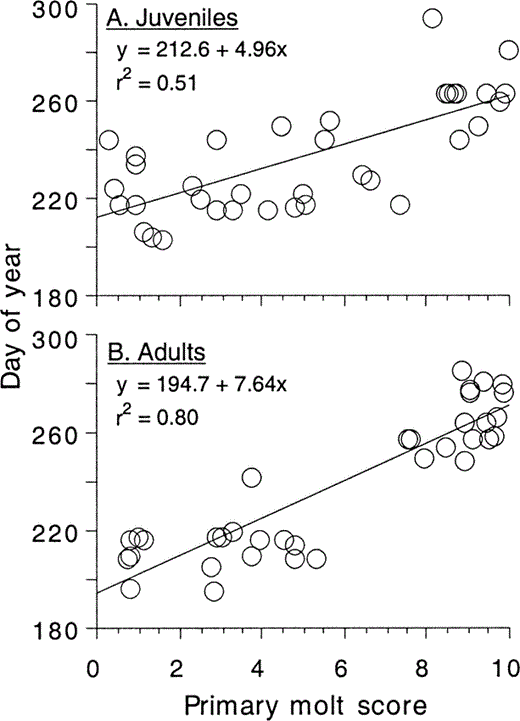
Figure 2. Regression of day of year on primary molt score for a) molting juvenile and b) molting adult Ash-throated Flycatchers (Pimm 1976). Replacement of the 10 primaries requires 50 days in juveniles and 76 days in adults. Day 180 = 29 June and day 300 = 27 October. A molt score of 0 indicates no new feather has been produced and a molt score of 10 indicates that all 10 primaries have been replaced
A potentially confounding problem with our molt rate analysis is that apparent molt rate tends to slow down near the end of molt when the more massive outer primaries are grown. This nonlinearity in the daily increase in feather length near the end of molt (Dawson 2003) could account for the apparently faster molt in juveniles than adults because only 31% of our 36 molting juveniles were growing P9 or P10, but 49% of our 35 molting adults were growing P9 or P10. This greater percentage of adults molting P9–P10, combined with the nonlinearity of length increase near the end of molt, would tend to increase the estimate of time required for molt in adults more than in juveniles. To address this problem, we compared molt rate among specimens in which molt was not complete past P8. By calculating and comparing Pimm's (1976) regressions among the first eight primaries only, and treating a new set of P1–P8 as 100% mm replaced, we removed any effect of nonlinearity in outer primary growth, which is limited to the outermost one or two feathers among the small but diverse set of species for which the pattern has been described (Dawson and Newton 2004). As in the ten-primary analysis, juveniles molted the first eight primaries at a significantly faster rate than adults (ANCOVA, interaction between age and percent mm replaced: F1,58 = 4.5, P = 0.04). The eight-primary estimate seems to be a realistic measure of molt duration over the inner wing because the slope of the eight-primary regression was not significantly different from the ten-primary regression within each age class (separate ANCOVAs, each P > 0.25). Furthermore, the estimates of eight-primary molt duration were 11 days shorter than ten-primary molt for both juveniles and adults, and the estimate of when molt started (the y-intercept of the regression) did not change for juveniles (day of year = 214) and decreased by only one day for adults (to day of year = 196).
Age differences in the total length of primary feathers produced cannot account for the estimated 26-day difference in molt duration between adults and juveniles. Total length of the ten primaries produced in the prebasic molt is 650 mm in juveniles and 676 mm in adults (sexes combined; feather length measurements n = 5 of each sex within each age class). This 25 mm difference should only account for about three more days of molt in adults, assuming daily production of 3.2 mm day−1 follicle−1 (Rohwer 1999) and an average of 2.65 feathers growing simultaneously (see below).
We also found no evidence that juveniles completed the primary molt faster than adults because they skipped replacement of the inner primaries, as suggested by Pyle (1997). No molting juvenile specimens (n = 32) appeared to have retained P1–P2 based on examination of color and wear patterns of these primaries. It is possible that we did not detect retained juvenal P1–P2 because they were not sufficiently different in appearance from the other inner primaries, but juveniles molting P1 and P2 were common (25%) in our combined sample of specimens and field-scored birds (n = 36). Three of the nine molting juveniles were molting slightly late in the season (23 and 26 August and 2 September), so it is unlikely that skipping replacement of P1–P2 is a strategy to reduce the time allocated to the primary molt.
Finally, we found no evidence that juveniles molted more intensely than adults. First, we found no significant difference in the number of simultaneously growing primaries between age classes. Among molting specimens in which molt had not yet progressed to P10, juveniles had 2.46 ± 0.81 simultaneously growing primaries (n = 26) and adults had 2.65 ± 0.75 simultaneously growing primaries (n = 20; U = 299.5, P = 0.34). Second, we found no significant difference in the relative lengths of neighboring growing feathers. Rapid molt should be characterized by small differences in length between adjacent growing feathers because little time should pass between initiation of molt in adjacent follicles. To test this possibility, we determined the difference in length between neighboring growing feathers, excluding feathers >90% grown because growth rate of individual feathers may decrease toward the end of growth. To account for the quadratic relationship between adjacent feather length differences and primary position (large differences between neighbors in the middle primaries and small differences in the inner and outer primaries), we combined age classes and used a quadratic fit of the difference in mm between each pair of neighbors to the number of the proximal feather pair (r2 = 0.38, df = 2, 96, P < 0.01). We then used the residuals from that quadratic regression to compare age classes, but found no evidence that juveniles had lower residuals (i.e., smaller differences between neighboring primaries) than adults (U = 1282.0, juvenile n = 34 and adult n = 32, one-tailed P = 0.33).
Given the lack of any other evidence for how juveniles completed molt more rapidly than adults, we estimated the daily per-feather growth rate for each age class. Adults replaced 675 mm of feather in 75 days, giving an average daily production rate of 9 mm of new feather per wing. Adults averaged 2.65 feathers growing simultaneously, so the per-feather production rate was 3.4 mm day−1 (9 × 2.65−1), very similar to the rate of 3.2 mm per day calculated by Rohwer (1999) for passerines. In contrast, juveniles replaced 650 mm of feathers in 50 days, for an average daily production rate of 13 mm of new primary feather per wing, and an average daily per-feather growth rate of 5.3 mm day−1 (13 × 2.46−1). Unfortunately, growth bands in the primaries were too difficult to see to independently evaluate these surprising rate differences (Grubb 1989, Rohwer 1999).
Premolt Movements
Molt in juveniles started about 1 August (Fig. 2a). Therefore, to illustrate molt-related movements in juveniles, we compared the geographic distribution of premolt juveniles collected in June and July to the distribution of molting juveniles (date range: 22 July–21 October; Fig. 1a–b). These comparisons showed that juveniles departed breeding areas north and west of the monsoon region before molting. Four premolt juveniles were collected north of the monsoon region, but no molting juveniles were collected there. This difference was marginally nonsignificant (Fisher's exact test, one-tailed P = 0.06), probably because so few premolt juveniles were collected in the north. Similarly, 14 premolt juveniles were collected west of the monsoon region, but just one molting juvenile was collected there (Fisher's exact test, one-tailed P < 0.01), illustrating movement east or south by juveniles hatched west of the monsoon region.
We found the same molt-related movements in adults. Adults started molting in mid-July (Fig. 2b), so we used the geographic distribution of adults collected in June to estimate their premolt distribution. Although 25 premolt adults were collected north of the monsoon region, no molting adults (date range: 1 July–13 October) were collected there (Fisher's exact test, one-tailed P < 0.01). Similarly, there were 45 premolt adults west of the monsoon region but only two molting adults there (Fisher's exact test, one-tailed P < 0.01).
Finally, ten premolt adults and one premolt juvenile were collected in the United States east of the monsoon region, but no molting birds of either age class were collected there. Thus, it appears that nearly all adults and juveniles of all migrant populations of Ash-throated Flycatchers depart their breeding grounds before molting.
Adults appeared to depart northern breeding areas before juveniles. Adults comprised 100% of specimens collected in the north in June, but adult frequency declined to 80% by late July and to 30% by late August (Table 2). Thus, juveniles probably departed the north after adults, corresponding to the later start of molt in juveniles in the monsoon region. We could not make this comparison with eastern populations because of small samples, and samples of western populations in August probably included migrants from the north, making interpretation of age ratios difficult.
Age ratios of Ash-throated Flycatchers collected north of the Mexican monsoon region. Adults become relatively uncommon in August, and both age classes are rare in September
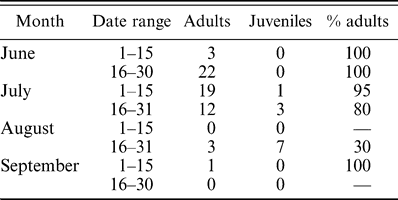
Age ratios of Ash-throated Flycatchers collected north of the Mexican monsoon region. Adults become relatively uncommon in August, and both age classes are rare in September

Location and Timing of Molt
After departing their breeding grounds, migrant Ash-throated Flycatchers moved to the Mexican monsoon region to molt: 30 of 33 molting juveniles (91%), and 29 of 32 molting adults (91%) were collected in the monsoon region (Fig. 1b). The percentage of molting adult and juvenile specimens, a rate that is unaffected by collecting effort, was highest at 26–30°N (Fig. 3), near the latitudinal center of the monsoon region (Fig. 1).
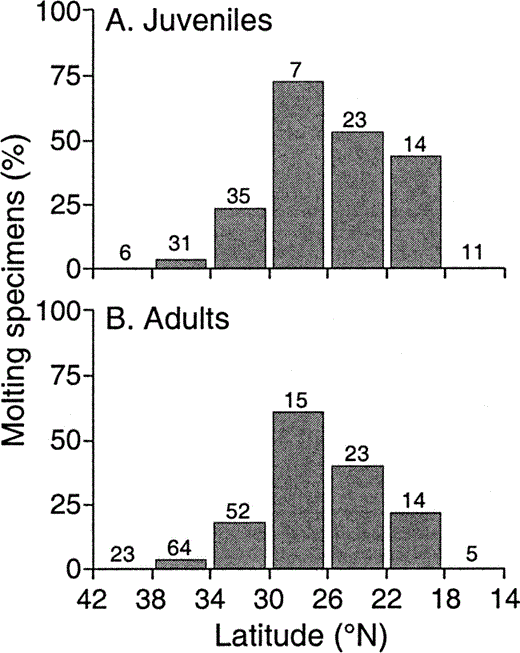
Figure 3. Latitudinal distribution of primary feather molt in a) juvenile and b) adult Ash-throated Flycatchers. Breeding populations occur as far north as 42°N. The Mexican monsoon region occurs from about 20–37°N. Sample sizes are shown above bars
The timing of molt also corresponded to the monsoon rains. The Mexican monsoon is characterized by sharp increases in precipitation in southwestern Mexico in June, progressing to northwestern Mexico, eastern Arizona, and western New Mexico starting in July (Comrie and Glenn 1998). The highest frequency of molting adults and juveniles occurred about six weeks after the start of these rains (Fig. 4). There was no age class difference in the temporal distribution of molting specimens (Gadj = 6.9, df = 9, P = 0.65; Fig. 4).
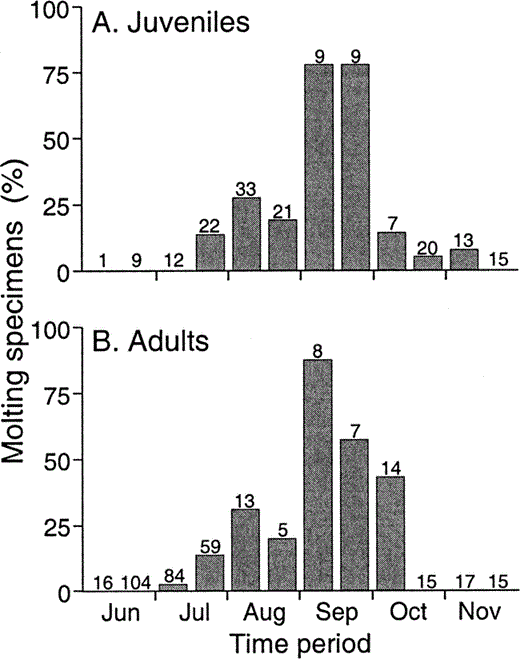
Figure 4. Temporal distribution of flight feather molt in a) juvenile and b) adult Ash-throated Flycatchers. Each bar represents a semimonthly period (e.g., 1–15 July). Sample sizes are shown above bars
We predicted that molt in the monsoon region would start earlier in the south than the north following the progression of the monsoon rains. However, among birds molting in the monsoon region we found no relationship between our measure of relative molt timing (the residuals from Pimm's [1976] regression using percent mm replaced) and latitude (juveniles: r2 = 0.01, df = 1, 32, one-tailed P = 0.30; adults: r2 = 0.03, df = 1, 30, one-tailed P = 0.19).
Postmolt Movements
After molting, most juveniles and adults moved to wintering areas south of peak molting latitudes (Fig. 1c). The midpoint latitude of all molting and freshly plumaged birds in November was 27°06′N among juveniles (range = 17°30′–36°36′N) and 25°54′N among adults (range = 15°0′–36°42′N). Whereas 39% of 33 molting juveniles were collected north of the juvenile midpoint, none of 27 freshly plumaged juveniles in November were collected there (Fisher's exact P < 0.01). Likewise, whereas 69% of 32 molting adults were collected north of the adult midpoint, only 20% of 35 freshly plumaged adults in November were collected there (Fisher's exact P < 0.01).
Juvenal Feather Structure
As predicted for species in which juveniles migrate in juvenal plumage, juvenile Ash-throated Flycatchers had relatively tightly textured, adult-like contour feathers (juvenile barb count = 17.7 ± 2.3, adult barb count = 20.4 ± 1.7). Barb count was only 15% higher in adults than juveniles, similar to the 19% ± 11% higher adult barb count for 15 other species in which juveniles migrate in juvenal plumage, and well below the 30% ± 12% higher adult barb count among six species in which juveniles, but not adults, replace their body feathers before migrating (Rohwer et al. 2005).
Discussion
The duration of molt is an important factor for understanding variation in the scheduling of fall molt with respect to migration. Species and age classes that require a long time to molt should molt after southward migration due to time constraints at northern latitudes (Hemborg et al. 2001, Rohwer et al. 2005), whereas species and age classes that molt rapidly should face weak breeding-ground time constraints. Rapid molt could also be the reason that birds migrate to the monsoon region to molt (Voelker 2000). Replacement of the primary feathers by Ash-throated Flycatchers requires about 75 days in adults, but only about 50 days in juveniles. Because of this large difference between age classes, we discuss molt scheduling in each age class separately.
Molt and Migration in Adults
Adult Ash-throated Flycatchers take more than a month longer to molt than eastern migrants that molt on their breeding grounds (e.g., 38 days in eastern Warbling Vireos [Vireo gilvus gilvus]; Voelker and Rohwer 1998, 44 days in Gray Catbirds [Dumetella carol inensis]; Dwyer-Heise and Rimmer 2000, and 40–44 days in Yellow Warblers [Dendroica petechia]; Ryder and Rimmer 2003), and about three weeks longer than other western passerines that molt in the monsoon region (49-day average among four species: Lazuli Bunting [57 days]; Young 1991, western Warbling Vireo [55 days]; Voelker and Rohwer 1998, Western Tanager [43 days]; Butler et al. 2002, and Baird's Sparrow [Ammodramus bairdii], 39 days; Voelker 2004). In contrast to these terrestrial foragers and foliage-gleaners, the Northern Rough-winged Swallow (Stelgidopteryx serripennis), which molts and migrates simultaneously, takes about 100 days to molt (Yuri and Rohwer 1997). Our estimate of long molt duration in adult Ash-throated Flycatchers (75 days) suggests that hawking as a foraging method might be an aerodynamic constraint necessitating slow molt, and thus that time constraints might favor adults from northern populations moving south before molting. However, time constraints alone cannot explain premolt movements of adults from all Ash-Throated Flycatcher populations because adults of eastern and western populations also departed the breeding grounds before molting.
About 90% of adult Ash-throated Flycatchers concentrated in the monsoon region to molt, and most birds that molted in the northern part of the monsoon region appeared to move farther south after they molted. This suggests that both the push of food shortages and the pull of food abundance in the monsoon region drive this molt-migration. However, as noted by Butler et al. (2002), we currently lack direct evidence of declining foraging conditions for lowland western insectivores and, because Ash-throated Flycatchers include in their diets large proportions of hard-bodied insects such as beetles (Coleoptera) and grasshoppers (Orthoptera; Cardiff and Dittmann 2002), they may not experience the same decline in food resources that has been hypothesized to explain premolt movements in other western insectivores (e.g., western Warbling Vireos; Voelker and Rohwer 1998).
In contrast to other molt-migrants that move to the monsoon region, rapid molt (Voelker 2000) cannot explain why adult Ash-throated Flycatchers molt in the monsoon region, because they have the longest molt duration of any species molting in the monsoon area, and because they could probably molt much faster (i.e., in ≤50 days like juveniles). What benefit pulls adults to the monsoon region? One possibility is that abundant insects simply allow for more efficient foraging, thereby reducing the time required for foraging and the resultant costs of energy use, thermoregulation, feather wear, risk of damage to growing feathers, and predation risk. This hypothesis could be tested in Ash-throated Flycatchers and other flycatchers with year-round monsoon distributions (e.g., Cassin's Kingbird [Tyrannus vociferans]; Tweit and Tweit 2000) by comparing foraging behavior during and outside the monsoon season. We predict that time spent foraging will decrease and foraging efficiency will increase shortly after the rains arrive.
Molt and Migration in Juveniles
Like adults, juveniles from populations north, east, and west of the monsoon region move to the monsoon region to molt before migrating to wintering grounds farther south. Unlike adults, juveniles molt rapidly. Does the rapid molt hypothesis (Voelker 2000) explain why juveniles molt in the monsoon region? Rapid molt could be important for juveniles, because they start molting about 1 August, 18 days later than adults, but they finish molting around 20 September, approximately one week before adults. Juveniles might be forced to molt rapidly if replacing flight feathers in the monsoon region is difficult after September. Other species molting in the monsoon area with comparable data complete molt in late September or early October: 17 September in Western Tanager (Butler et al. 2002), 19 September in western Warbling Vireo (Voelker and Rohwer 1998), and early October in Lazuli Bunting (Young 1991). The few available data suggest that Bullock's Orioles also complete molt in late September (Rohwer and Manning 1990). Time constraints on molt in juvenile Ash-throated Flycatchers could explain why one juvenile in our sample (UCMVZ 111033), collected on 27 October in Guerrero, Mexico, had failed to replace juvenal P9 and P10.
Despite the much shorter time taken by juveniles than adults to replace the primaries, juveniles did not grow more primary feathers simultaneously than adults. Instead, juveniles appeared to increase molt rate by increasing the rate of production of individual feathers. Based on the mm of feathers produced and the time used for molt, juveniles and adults produced individual primaries at 5.3 mm per day and 3.4 mm per day, respectively. Assuming our estimate of daily growth rate in juveniles is accurate, this finding differs from other passerines, in which rapid molt is achieved by molting a relatively high number of primaries simultaneously (Hall and Fransson 2000, Ryder and Rimmer 2003, Dawson 2004).
Why do juveniles postpone molt by more than three weeks compared to adults, especially if they may face declining foraging conditions in October? The late start of molt in juveniles probably reflects late arrival in the monsoon region. Juveniles depart northern breeding areas later than adults, and therefore probably arrive in the monsoon region later. In many passerines, juveniles are less skilled at foraging than adults (Burger 1988, Wunderle 1991), and remain so for several weeks after fledging in some species (Grubb et al. 1991, Woodrey and Moore 1997). As hawking insectivores, juvenile Ash-throated Flycatchers probably require several weeks to become as proficient at foraging as adults. Latent acquisition of foraging skills could delay their migration to the monsoon region, and also delay their molt after arrival until their foraging skills develop sufficiently to overcome the loss of flight ability caused by flight feather molt (Swaddle and Witter 1997). This hypothesis would be refuted if there were no age differences in foraging ability at the time adults depart northern breeding areas.
In passerines in which juveniles migrate in juvenal plumage, the quality of the juvenal feathers (as measured by barb counts) approaches that of adults (Rohwer et al. 2005). Ash-throated Flycatchers followed this pattern, but the functional significance of this pattern remains debatable. Further, we do not know if this similarity merely permits juveniles to migrate before molting or if it has evolved to facilitate migrating before molting.
Molt, Time, and Feather Quality
Recent studies suggest that rapid, late-season molt produces low-quality feathers. Dawson et al. (2000) found that late-molting European Starlings produced lighter and less wear-resistant primary feathers than birds that molted early. In Gray Plovers (Pluvialis squatarola), molt duration explained almost 60% of variation in primary feather wear among seven populations, with slow-molting populations producing higher quality feathers (Serra 2001). Costs to feather quality probably explain why late, rapid-molting Blue Tits (Parus caerulescens) had higher thermoregulatory costs and lower survival in the following winter, and lower reproductive success in the following breeding season, compared to birds that molted early (Nilsson and Svensson 1996). In this study, juvenile Ash-throated Flycatchers appeared to grow flight feathers rapidly so as to complete molt about the same time as adults. Thus, we predict that juveniles have lower quality primaries than adults. This prediction could be tested using museum specimens by comparing primary feather wear in definitive and first basic primary feathers.
The precise functional relationships between molt rate, food intake, and feather quality are unknown, but it seems plausible that a monsoon-related food flush facilitates the production of high quality flight and body feathers in adults. Feathers are produced with protein that is ingested on a daily basis (Murphy and King 1992), and the production of body feathers alone requires a considerable increase in daily food consumption. For example, under laboratory conditions, White-crowned Sparrows (Zonotrichia leucophrys) replacing about 25% of their body feathers consumed around 20% more food daily than nonmolting birds (F. Bonier, University of Washington, unpubl. data). Thus, benefits to feather quality might provide another “pull” to the monsoon region for some molt-migrants.
Considerations for Future Studies
Ash-throated Flycatchers are the sixth species shown to migrate to the region of the Mexican monsoon to molt (Rohwer et al. 2005), suggesting this region is of special conservation importance for migrants (Krueper 2000, Butler et al. 2002, Leu and Thompson 2004). Thus, additional descriptions of molt in western birds are needed, and we suggest that future studies should consider the following points.
First is the need to recognize the possibility of geographic, age, and sex differences in the rate or timing of molt. For example, Thompson (1991) combined data from monsoon-molting Painted Buntings with molting Painted Buntings from the southeastern United States in calculating molt duration. Because passerines in eastern North America molt rapidly, combining eastern and western molting birds is likely to bias estimates of the time required for molt. In Ash-throated Flycatchers, the statistical assumption that all specimens came from the same population of molting birds (Pimm 1976) was probably met because molting specimens were collected in such a limited geographical area.
Second, because collecting effort has historically been biased away from the location and season of the Mexican monsoon, the relative frequency of molting specimens, not counts of specimens, should be used to assess geographic and temporal patterns in molt. For example, 14 Ash-throated Flycatchers collected on a recent trip to the monsoon region by the Burke Museum accounted for 56% of all specimens (among the 18 museums in our sample) collected mid-July to mid-August between 22°N and 30°N. Based on counts of molting specimens, Voelker (2000) concluded that Gray Vireos did not move to monsoon regions to molt, contrary to prediction. However, figure 2 in Voelker (2000) shows that about 70% of molting Gray Vireos were collected in or at the edge of the monsoon region and three of these specimens were taken between the breeding and wintering range. A study of Lucy's (Vermivora luciae) and Virginia's Warblers (V. virginiae) similarly relied on raw counts rather than rates of occurrence of molting birds to conclude that these species were not using the monsoon region for molt (Voelker and McFarland 2002), and this is now known to be an erroneous conclusion for Lucy's Warblers (SR et al., unpubl. data).
Finally, declining foraging conditions are thought to be the selective force favoring migration before molting in western passerines, but reduced food availability in late summer on their breeding grounds has not yet been demonstrated (Butler et al. 2002). Thus, the importance of the hypothesized breeding grounds “push” to the scheduling of molt and migration remains to be demonstrated.
Acknowledgments
In addition to the specimens at the Burke Museum, specimens were examined from the following museums in 2004: American Museum of Natural History, Carnegie Museum of Natural History, Field Museum of Natural History, Moore Laboratory of Zoology at Occidental College, Museum of Comparative Zoology at Harvard University, Museum of Vertebrate Zoology at University of California Berkeley (UCMVZ), Natural History Museum of Los Angeles County, Sam Noble Oklahoma Museum of Natural History, Slater Museum of Natural History at University of Puget Sound, Texas A&M University, University of California Los Angeles Dickey Collection, University of Kansas Natural History Museum, University of Michigan Museum of Zoology, University of Utah Museum of Natural History, Washington State University Charles R. Conner Museum, and the Western Foundation of Vertebrate Zoology. Specimens collected in Sinaloa and Baja California Sur in 2005 that were included in this study will be deposited at the Burke Museum and at the Museum of Zoology at Universidad Nacional Autónoma de México. D. Froehlich, T. Heady, P. Loesche, and two anonymous reviewers made valuable comments that improved the manuscript. LKB was supported during this work by a Burke Museum Eddy Fellowship. MR received a Collection Study Grant from the American Museum of Natural History. We discovered that Ash-throated Flycatchers were molt-migrants during expeditions to the monsoon region supported by a grant from the Nuttal Ornithological Club's Blake Fund, and by the Burke Museum's Endowment for Ornithology. We are sincerely grateful to these individuals and institutions.
Literature Cited
Author notes
Present address: Department of Biology, Tufts University, Medford, MA 02155. [email protected]



