-
PDF
- Split View
-
Views
-
Cite
Cite
Julie A. Morse, Abby N. Powell, Michael D. Tetreau, Productivity of Black Oystercatchers: Effects of Recreational Disturbance in a National Park, The Condor: Ornithological Applications, Volume 108, Issue 3, 1 August 2006, Pages 623–633, https://doi.org/10.1093/condor/108.3.623
Close - Share Icon Share
Abstract
National parks in Alaska are generally assumed to be high-quality, undisturbed wildlife habitats. However, these parks attract recreational users, whose presence may reduce the suitability of key habitats for nesting shorebirds. In Kenai Fjords National Park, Black Oystercatchers (Haematopus bachmani) often breed on gravel beaches that are also popular campsites. In this study, we examined the effects of recreational activities in coastal Alaska on reproductive performance of Black Oystercatchers. We monitored survival of nests and chicks on 35 to 39 breeding territories annually during four breeding seasons (2001–2004). Most recreational disturbance on these territories occurred after the peak hatching date of first clutches. Annual productivity was low (average of 0.35 chicks per pair), but was not strongly affected by recreational disturbance. Daily survival of nests varied annually and declined over the season. Our results suggest that nest survival was lower during periods of extreme high tides. Daily survival rate of broods increased over the season and was higher on island than mainland territories, likely due to differences in predator communities. Territory occupancy rate and site fidelity were high; 95% of color-banded oystercatchers returned to the same breeding territory in the subsequent year. We conclude that Black Oystercatchers are resilient to low levels of recreational disturbance. However, in light of projected increases in recreation, we suggest managers move campsites away from the traditional nest sites identified in this study to minimize future disturbances.
Resumen
Productividad De Haematopus Bachmani: Efectos De Los Disturbios De Actividades De Recreación En Un Parque Nacional
Se asume con frecuencia que los parques nacionales de Alaska son ambientes no disturbados de alta calidad para la vida silvestre. Sin embargo, estos parques atraen visitantes cuya presencia puede reducir la aptitud de los ambientes clave para nidificación de las aves playeras. En el Parque Nacional Kenai Fjords, Haematopus bachmani generalmente cría en las playas de grava, lugares que son también sitios populares para acampar. En este estudio, examinamos los efectos de las actividades de recreación en la costa de Alaska sobre el rendimiento reproductivo de H. bachmani. Seguimos anualmente la supervivencia de nidos y pichones en 35 a 39 territorios de cría durante cuatro estaciones reproductivas (2001–2004). La mayoría de los disturbios ocasionados por las actividades de recreación en estos territorios se presentó luego del pico de eclosión de las primeras nidadas. La productividad anual fue baja (promedio de 0.35 pichones por pareja), pero no fue severamente afectada por los disturbios de las actividades de recreación. La supervivencia diaria de los nidos varió anualmente a lo largo de la estación. Nuestros resultados sugieren que la supervivencia de los nidos fue menor durante los períodos de mareas extremadamente altas. La tasa de supervivencia diaria de las nidadas incrementó a lo largo de la estación y fue mayor en las islas que en los territorios continentales, probablemente debido a diferencias en las comunidades de depredadores. La tasa de ocupación de los territorios y la fidelidad al sitio fue alta; el 95% de los individuos marcados con anillos de colores de H. bachmani retornó al mismo territorio de cría al año siguiente. Concluimos que H. bachmani es resiliente a bajos niveles de disturbio ocasionados por las actividades de recreación. Sin embrago, a la luz de los incrementos proyectados en las actividades de recreación, sugerimos a los responsables del manejo de estas áreas que alejen a los sitios de campamento de los sitios de nidificación tradicionales identificados en este estudio para minimizar futuros disturbios.
Introduction
Recreational activities are the fourth-leading cause of population declines of species listed as federally threatened or endangered, below only the introduction of exotic species, urbanization, and agriculture (Czech et al. 2000). Because of rapid increases in participation, recreational activities are a growing conservation concern in the U.S. (Boyle and Sampson 1985, Flather and Cordell 1995). Disturbance by recreationists can have short-term effects on wildlife behavior and physiology, and long-term effects on reproduction and survival (Boyle and Sampson 1985, Knight and Cole 1995, Carney and Sydeman 1999). However, there remains considerable debate about the effect of human disturbance on bird populations (Hill et al. 1997, Nisbet 2000).
The long-term resilience of a species to disturbance is a function of its ability to survive and reproduce. Understanding the processes that adversely affect reproductive success is crucial for developing effective management or conservation plans. Ground-nesting birds, including shorebirds, generally have low rates of reproductive success, with predation the predominant cause of nest failure (Evans and Pienkowski 1984). Additionally, ground-nesting birds in coastal habitats are particularly vulnerable to human disturbance, given the propensity for humans to live and concentrate recreational activities in these regions (Burke et al. 2001). These combined threats have resulted in population declines and the listing of several beach-nesting shorebirds as endangered species (e.g., Western Snowy Plover [Charadrius alexandrinus nivosus]; Page et al. 1995, Piping Plover [C. melodus]; Dyer et al. 1988, New Zealand Dotterel [C. obscurus]; Dowding 1993).
Black Oystercatchers (Haematopus bachmani) have been negatively affected by human disturbance throughout their range along the Pacific coast of North America. Local extirpations of breeding populations have occurred on islands off the coast of Baja California, Mexico (Kenyon 1949, Jehl 1985) and Alaska (Andres and Falxa 1995). Furthermore, human disturbance has caused changes in abundance and distribution of breeding oystercatchers from California to Alaska (Andres and Falxa 1995). More than half the world's Black Oystercatcher population breeds in Alaska, where recreational activities are increasing (Bowker 2001, Colt et al. 2002). In addition to being vulnerable to human disturbance, the Black Oystercatcher has a small population size, limited distribution, and low rate of reproductive success; consequently, it has been designated a Species of High Conservation Concern (Alaska Shorebird Working Group 2000, Brown et al. 2001).
Productivity is a highly variable component of avian life history that significantly influences population dynamics, and is therefore an important measure for assessing the impacts of human disturbance. Black Oystercatchers are ground-nesters with semiprecocial young, thus their reproductive success may be lower in areas with human disturbance. Additionally, long-lived iteroparous species such as the Black Oystercatcher (average lifespan is greater than 10 years; Andres and Falxa 1995) have many opportunities to breed in a lifetime; therefore, individuals may reduce reproductive effort when breeding conditions are unfavorable (Stearns 1992). Black Oystercatchers may lower reproductive effort in areas with human disturbance by foregoing nesting or renesting. Alternatively, human disturbance could adversely affect productivity by displacing or lowering site fidelity of breeding pairs in areas with recreational disturbance.
Over the past decade, recreational use of backcountry coastal habitats in Kenai Fjords National Park, Alaska has increased (MDT, unpubl. data), causing concern for oystercatchers and providing the impetus for this study. Our primary goal was to acquire baseline data on the breeding ecology of Black Oystercatchers while levels of recreational use in the park were still relatively low compared to national parks elsewhere in the United States. An additional objective of this research was to determine whether daily survival rates and site fidelity differed at sites with recreational disturbance. The park included areas differentially affected by recreational disturbance, allowing comparisons between areas with and without human use. We also examined other sources of variation in daily survival rates of nests and broods. In our analyses, we considered the effects of both ecological factors and recreational disturbance on survival rates. Based on our results, we suggest preventative management recommendations in light of projected increases in recreational activities in the park.
Methods
Study Area
Our study was conducted in Aialik Bay (59°52′N, 149°39′W) and Northwestern Fjord (59°45′N, 149°53′W), the two most popular coastal destinations of tourists in Kenai Fjords National Park, Alaska (Fig. 1). Mountains, tidewater glaciers, deep bays, and steep, rocky, and convoluted shorelines characterize the study area. The area is remote, approximately 60 km across the Gulf of Alaska from the nearest town of Seward, and accessible only by boat. Most tourists visit the coastal areas of Kenai Fjords National Park on small tour boats and never set foot on the shoreline; only 1% of park visitors stay overnight in the backcountry (Colt et al. 2002).
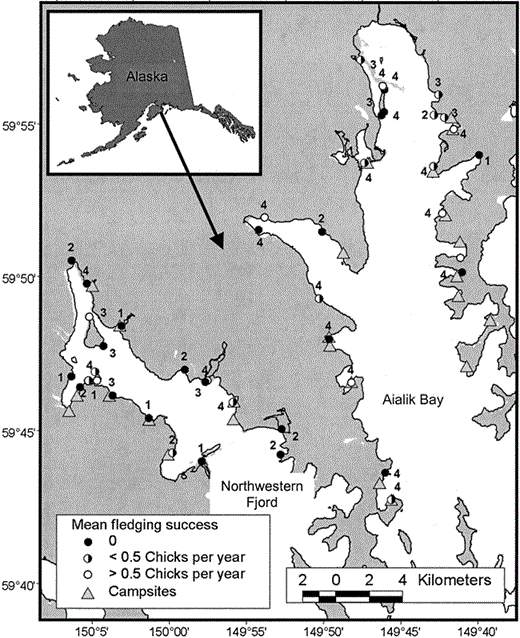
Figure 1. Kenai Fjords National Park location and study area, and fledging success of all Black Oystercatcher breeding territories monitored from 2001 to 2004. The number beside each territory indicates the number of years (max. = 4) the site was occupied
Recreational activities on the shoreline are primarily limited to camping by sea kayakers. Much of the shoreline is too steep for landing a small boat and camping, therefore recreational disturbance is isolated to gravel beaches (slope <10°). With over 150 km of shoreline in the study area, there are only 25 camping beaches, 12 of which are established campsites (with permanent bear lockers for food storage) and used regularly. It was difficult to quantify the amount of human use over such a large area; the best available data came from Park Ranger patrols and were limited to Aialik Bay (MDT, unpubl. data). These data show that the number of campers in the study area in general was low and varied annually and seasonally, with peak use on weekends in July (Fig. 2).
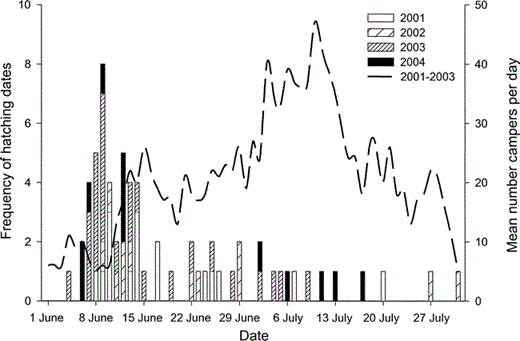
Figure 2. Comparison of Black Oystercatcher breeding chronology and level of recreational disturbance at Kenai Fjords National Park, Alaska. Solid bars show estimated and observed hatching dates from 2001 to 2004, and dashed line shows the mean number of campers in Aialik Bay each day from 2001 to 2003
Field Methods
Crews of two to four people conducted fieldwork from mid-May to mid-August, 2001–2004. We completed one annual systematic survey of 150 km of shoreline during peak nest initiation (late May) to locate territorial pairs; additional pairs were located opportunistically throughout the rest of the season. When a territorial pair of oystercatchers was observed, the area was searched on foot for a nest. Once a nest was found, it was checked on average every six days (2001–2002), or every four days (2003–2004), until chicks fledged or the nest or brood failed. Similar research on European Oystercatchers (H. ostralegus) showed that frequent nest visits did not have deleterious effects on breeding success (Verboven et al. 2001). Regardless, on subsequent visits we often could observe a bird incubating from the boat without flushing it off the nest; thus, we minimized researcher disturbance at all nest sites. We considered a nest successful if we observed at least one chick, and a brood successful if at least one chick was observed flying. When a nest failed, the immediate area was searched for shell fragments, predator tracks, or evidence of recent flooding (Mabee 1997). We were only able to attribute predation events to specific predators if whole eggshells remained (avian) or recent tracks or scat were immediately adjacent to the nest site. In 2003 and 2004, we floated two eggs from each clutch to estimate date of hatching (Dinsmore et al. 2002).
We began capturing and banding birds with unique combinations of color bands for individual identification in 2003. Adults were captured at the nest site during early incubation with either a dipnet or noose mats. Most oystercatchers returned to incubating eggs before we departed the territory, therefore we do not think our research activities affected nesting success. All chicks were banded between 10 and 20 days after hatching.
Statistical Analyses
We calculated the following components of reproductive success: mean clutch size, percent of females renesting, apparent nesting success (number of nests that hatched at least one chick per the total number of nests), fledging success (number of nests that fledged at least one chick per number of nests hatched), and productivity (chicks fledged per pair). Annual fledgling productivity (number of chicks fledged per number of years the territory was occupied) was also calculated for territories occupied in multiple years of the study. Percentage of color-banded oystercatchers that returned to breed was calculated based on resightings throughout the 2004 breeding season. These descriptive statistics are presented for comparison with other studies.
We used program MARK to model the daily survival of Black Oystercatcher nests and broods with respect to recreational disturbance and other ecological variables (Dinsmore et al. 2002, Cooch and White 2005). We examined year- and time-specific variation in survival rates, and simultaneously evaluated the importance of individual covariates on daily survival rates. We used an information-theoretic approach for model selection and evaluated models using Akiake's information criterion corrected for small sample size (AICc; Burnham and Anderson 2002). To directly assess the effects of individual covariates we compared AICc scores of models with and without the covariate of interest. The penalty for adding a single parameter to a model is 2 AICc, therefore a change in AICc score (ΔAICc) close to 2 indicates the covariate does not explain any additional variation in survival rates. We also used beta parameter estimates to indicate effect size. Currently, there is no goodness-of-fit test for nest survival data in program MARK (Dinsmore et al. 2002); therefore, we estimated variance inflation factor values (ĉ = deviance of the global model/degrees of freedom). This estimate of overdispersion is known to be positively biased (Dinsmore et al. 2002), thus although we report these values we did not adjust model selection statistics.
We limited our analyses to a small set of a priori models to address our questions of interest. First, we considered a model in which daily survival was constant, comparable to traditional Mayfield (1961, 1975) techniques of estimating nest survival. We then modeled daily survival rates of nests and broods relative to year and temporal trends within a year; we examined models with a linear and quadratic trend in survival over the nesting season. Temporal trends within a season may be confounded with nest age (Klett and Johnson 1982, Dinsmore et al. 2002); however, nests were initiated throughout the season due to renesting. We did not treat nest age as a separate covariate because we lacked accurate estimates of nest age in all years. Seasonal trends in survival may also be confounded with renesting attempts, therefore we also considered one model with a categorical renesting covariate.
We next examined variation in nest and brood survival rates relative to two categorical covariates by adding them to the best temporal model. We hypothesized that: (1) predation rates, and therefore survival, would vary between territories on the mainland and on islands (landform effect), and (2) survival rates would be lower during periods of extreme high tides due to a higher potential for nests to be flooded. We treated tide height as a categorical covariate because we expected a threshold effect; extreme tide periods were defined as days when the maximum observed tide height (data obtained from the National Oceanic and Atmospheric Administration station in Seward, Alaska) was greater than monthly mean high high water. We modeled additive effects of landform and tide, and their interaction. Once we had determined the most parsimonious model from this set, we compared this model to a model with the same structure and a campsite covariate added. We hypothesized that survival rates on established campsites would be lower than on territories with no camping disturbance. This model-building strategy provided the best means to evaluate a campsite effect, our primary objective (Lebreton et al. 1992).
Nest and brood survival were likely independent with respect to temporal variation and covariate effects, therefore these were treated as separate analyses. However, survival rates derived from these analyses are not independent, as the same pairs and locations were used in both analyses. Estimated date of hatching (derived from either direct observation or egg flotation data) was used to divide exposure days between the nest and brood analyses. In cases (n = 14) where hatching date was unknown, we used the midpoint between the last nest visit and the first chick visit as the estimated date of hatching. Lack of precise hatching date estimates for all nests may bias our estimates of daily survival probability (Stanley 2000); however, we expect this bias to be small due to the small sample of nests with unknown hatching dates. Hatching and fledging survival probabilities were calculated as the product of daily survival rates, and variances for these estimates were calculated with the delta method (Seber 1982).
Results
Breeding Ecology
We monitored the reproductive success of 35 to 39 breeding pairs annually (Table 1). Eight to twelve pairs nested each year on steep rocky islands; the remaining pairs nested on mainland gravel beaches. Eight of these beaches were also established campsites regularly used by sea kayakers. Recreational disturbance at these campsites was more frequent starting in mid-June, after the peak date of hatching of first-laid nests (Fig. 2). Most breeding territories were occupied in multiple years, and 21 territories were occupied in all four years of the study (Fig. 1). We banded 45 breeding adults in 2003 and, with the exception of two pairs, at least one individual from every known breeding pair in the study area was banded. Almost all (96%, n = 43) birds banded in 2003 returned and bred again in 2004; except for two individuals, all returned to the same breeding territory.
Annual measures of reproductive success of Black Oystercatchers in Kenai Fjords National Park, Alaska, from 2001–2004. Values are means ± SE
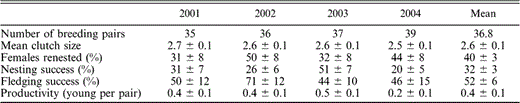
Annual measures of reproductive success of Black Oystercatchers in Kenai Fjords National Park, Alaska, from 2001–2004. Values are means ± SE

In 2003 and 2004 we estimated that at least 65% of our nest failures were likely due to predation. Evidence of specific predators was rarely apparent; however, we were able to attribute 8% of these depredated nests to black bears (Ursus americanus), and 6% to avian predators. Over the four years of this study we observed various predators taking oystercatcher eggs and chicks, including black bears, wolverines (Gulo gulo), river otters (Lutra canadensis), Bald Eagles (Haliaeetus leucocephalus), and Common Ravens (Corvus corax). Flooding accounted for roughly 6% of nest failures, and approximately 6% of nests were abandoned; cause of failure for the remaining 23% was unknown.
Nest Survival
Daily nest survival rates varied annually and declined as a quadratic function of date (Table 2, Fig. 3). There was no evidence that survival rates of subsequent nests differed from first-laid clutches. The campsite covariate explained very little variation in survival rates; adding this covariate to the most parsimonious model did not improve model fit and only slightly decreased the model deviance (βcampsite = −0.02, 95% CI: −0.19, 0.15; Table 2). Evidence was strong that tide influenced nest survival; there was a trend toward lower survival during periods of extreme high tides (βtide = −0.37, 95% CI: −0.86, 0.13). There was little support for an effect of landform on nest survival, and this parameter was not precisely estimated (βlandform = −0.13, 95% CI: −0.53, 0.27). There was no support for the null model in which daily survival was constant. The model-averaged estimates of annual nest survival for a nest hatching on 13 June (mean hatch date) were 0.32 (95% CI: 0.17–0.46), 0.25 (95% CI: 0.12–0.38), 0.48 (95% CI: 0.32–0.64), and 0.22 (95% CI: 0.10–0.33) in 2001–2004, respectively (Fig. 4). The estimated variance inflation factor (ĉ) for this analysis was 3.1.
Ranking of all a priori models estimating daily survival rates (DSR) of Black Oystercatcher nests and broods at Kenai Fjords National Park, Alaska, 2001–2004. Models were ranked based on the difference in Akaike's information criterion adjusted for small sample size (ΔAICc). The model deviance (Dev), number of parameters (K), difference in AICc from the best model (ΔAICc) and model weights (wi) are presented for all models
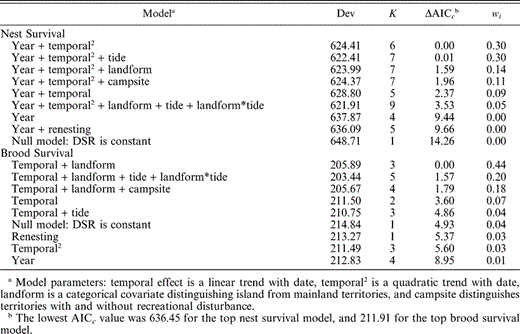
Ranking of all a priori models estimating daily survival rates (DSR) of Black Oystercatcher nests and broods at Kenai Fjords National Park, Alaska, 2001–2004. Models were ranked based on the difference in Akaike's information criterion adjusted for small sample size (ΔAICc). The model deviance (Dev), number of parameters (K), difference in AICc from the best model (ΔAICc) and model weights (wi) are presented for all models

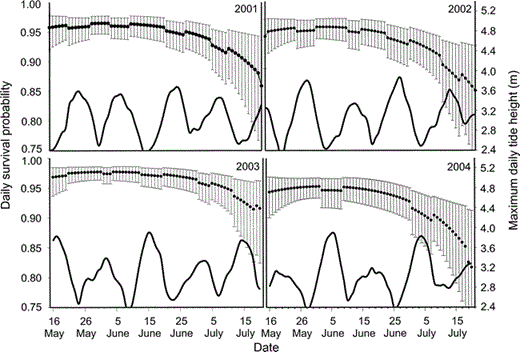
Figure 3. Comparison of daily survival rates of Black Oystercatcher nests and maximum daily tide height at Kenai Fjords National Park, Alaska, 2001–2004. Model-averaged estimates ± 95% CI of daily nest survival generated for each year. Solid line represents the highest tide height observed for each day in each year
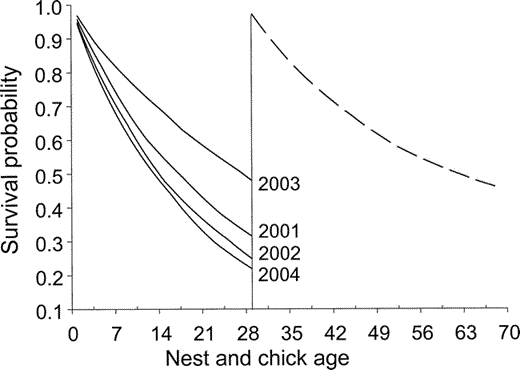
Figure 4. Cumulative survival curves for Black Oystercatcher nests (solid lines) and broods (dashed line) in Kenai Fjords National Park, Alaska, 2001–2004. The vertical line represents hatching; estimates are based on a mean hatching date of 13 June for all years (including renests). Values are the product of model-averaged daily survival estimates for nests, and daily survival estimates derived from the best model for broods
Brood Survival
Daily survival rates of broods increased as a linear function of date and did not vary annually or with renesting (Table 2). As for nest survival, there was little support for a campsite effect on brood survival; adding the campsite covariate to the most parsimonious model did not improve model fit and only slightly decreased model deviance (βcampsite = −0.09, 95% CI: −0.48, 0.29). Survival of chicks was higher on island territories than mainland territories (βlandform = −0.98, 95% CI: −1.87, −0.09). There was some support for an effect of tide on brood survival, and an interaction between tide and landform, but these parameters were not precisely estimated (βtide = −1.57, 95% CI: −3.73, 0.59, βtide*landform = 1.51, 95% CI: −0.93, 3.95). The model-averaged estimate of brood survival to 40 days for a nest hatched on 13 June was 0.46 (95% CI: 0.30–0.63; Fig. 4). Estimated variance inflation factor (ĉ) for this analysis was 3.2.
Discussion
During the four years of this study, we monitored the survival of 500 eggs, from which only 51 chicks fledged. With the exception of Middleton Island, a relatively predator-free island in the Gulf of Alaska where estimates of nest success were considerably higher (Gill et al. 2004), our low estimates of reproductive success are similar to those reported for Black Oystercatchers in other areas (Andres and Falxa 1995, Murphy and Mabee 2000, Hazlitt 2001). Similar to these studies, we found substantial annual variation in nest survival rates, with most nest failures due to predation. Evidence of specific predators was rarely apparent in our study area, but the suite of potential predators was extensive. Due to the low density of breeding oystercatchers, we considered all predators to be opportunistic. We suggest that the high temporal (i.e., year and season effects) and spatial variability (i.e., island versus mainland effects) in breeding success of Black Oystercatchers was primarily due to local variation in predator abundance.
Despite low estimates of productivity, we found little evidence that survival rates of nests or broods were adversely affected by recreational disturbance in Kenai Fjords National Park. This result contrasts with other recent studies on beach-nesting shorebirds exposed to recreational disturbance (Ruhlen et al. 2003 and references therein). However, the level of recreational disturbance in our study area was considerably lower than other areas studied, was not consistent throughout the breeding season, and affected only a small number of territories. Additionally, some predators in our study area, namely black bears, may be deterred from areas with human disturbance. Levels of recreational disturbance in Kenai Fjords National Park began increasing in mid-June, after the peak date of hatching of oystercatcher nests. Thus, brood-rearing was potentially the most vulnerable breeding stage, yet we found very little evidence that recreational disturbance affected brood survival. Our ability to detect differences in daily survival rates of broods reared on popular campsites may have been precluded by low hatching success and the resultant small sample size. We suggest that a threshold level for recreational disturbance may exist, below which productivity is not adversely affected or effects are not detectable.
Ecological factors did explain variation in daily survival rates of nests and broods. Our results provide evidence that daily nest survival rates were lower during periods of extreme high tides. Black Oystercatchers typically nest close to the high tide line and are therefore highly vulnerable to flooding events (Andres and Falxa 1995). Yet we did observe, as have others, that eggs could survive mild flooding events (Lentfer and Maier 1995). Daily survival of chicks was higher on island territories than on mainland beaches. This contradicts the finding of higher Black Oystercatcher breeding success on shallow-sloped territories (Hazlitt et al. 2002). Slope of the breeding territory has been used as an indicator of forage availability; presumably, lower-sloped territories have more forage available and are more directly accessible to chicks. However, predation rates may override any advantage incurred from better forage on shallow-sloped shorelines. The suite of potential predators was certainly more limited on island territories; usually only avian predators were observed at these sites. Common Ravens are typically a primary predator in other areas (Andres 1999), but we observed few ravens in our study area. Thus, higher brood survival on island territories was likely a result of lower predation rates by mammals at these sites, and may have been a very localized effect.
We also found that Black Oystercatchers exhibited strong site fidelity to their breeding territories, consistent with other studies (Hazlitt and Butler 2001 and references therein). High site fidelity in 2004 may have been correlated with high breeding success in 2003 (Hazlitt and Butler 2001). Breeding site fidelity can provide ecological benefits through familiarity with foraging sites and local predator communities (Oring and Lank 1984). These ecological advantages may contribute to increased nest and adult survival, and improved feeding efficiency. We could not directly assess the effect of disturbance on site fidelity because very little recreational disturbance occurred during the critical period in early May when pairs were establishing territories. However, most of the campsite territories were occupied by oystercatchers in all four years of our study, thus we suspect that Black Oystercatchers are unlikely to be displaced from breeding territories by recreational disturbance. Ecological advantages of site fidelity, combined with the potential lack of alternative nest sites on the rocky coastline (Hockey 1996, Andres 1998), would likely override avoidance of areas with human activity, except in cases of extreme disturbance (e.g., development).
Our study found little evidence that recreational disturbance adversely affected the productivity of Black Oystercatchers in Kenai Fjords National Park. Nevertheless, the projected increase in recreational disturbance in the park is a cause for concern, as responses of oystercatchers to higher levels of disturbance are currently unknown. Thus, we encourage resource managers to take a precautionary approach to managing recreational disturbance. While managers can do little to control ecological variables, we suggest that preventative management actions be taken to minimize disturbance during critical breeding stages. For example, given the strong site fidelity observed in Black Oystercatchers, established campsites could be moved away from the nest sites identified in this study. Managers of Kenai Fjords National Park have a unique opportunity to largely control where people camp because most recreational users stay at established campsites with food storage lockers for bear protection. Because of the high levels of predation observed, we further suggest more research be done on the effects of recreational disturbance on predator communities.
Acknowledgments
This study was funded through the U.S. Geological Survey Natural Resources Preservation Program with additional support provided by Kenai Fjords National Park. Capture and banding activities were conducted under appropriate permits from the University of Alaska Fairbanks Institutional Animal Care and Use Committee, and the U.S. Geological Survey Bird Banding Laboratory. We thank the staff of Kenai Fjords National Park for logistical support with fieldwork, especially the crew of the M/V Serac. We are indebted to several technicians who spent many hours in the cold and rain visiting nests; we especially thank K. Charleton, M. Grey, L. Garding, G. Keddie, and K. Rozell. Earlier drafts of this manuscript were greatly improved with comments and help from B. Andres, P. Flint, R. Lanctot, E. Rexstad, and J. Schmutz.
Literature Cited



