-
PDF
- Split View
-
Views
-
Cite
Cite
Alvin N. Setiawan, Melanie Massaro, John T. Darby, Lloyd S. Davis, Mate and Territory Retention in Yellow-Eyed Penguins, The Condor: Ornithological Applications, Volume 107, Issue 3, 1 August 2005, Pages 703–709, https://doi.org/10.1093/condor/107.3.703
Close - Share Icon Share
Abstract
Using nest and banding data collected from 1991 to 2002, we investigated mate and territory retention rates of Yellow-eyed Penguins (Megadyptes antipodes), and the effects of reproductive success. Annual mate retention rate was 63%, and territory retention for males and females were 52% and 46% respectively. The majority of pair dissolutions were due to death of a partner, with only 6% of bonds ending in separation. Previous reproductive success was a good predictor of mate or territory retention as pairs that failed to fledge a single chick were significantly more likely to separate or move their territories than those that were successful at fledging chicks. Reproductive success of birds that changed their mates or moved territories was not higher than those that retained their mates or territories. However, birds that moved territories were less likely to have reduced fledging success relative to their previous breeding attempt. Birds that did not retain their mates, particularly males, were significantly more likely to skip breeding for at least one year. This suggests that the costs of mate or territory changes are not accrued at the end of the breeding attempt (as reflected by the number of fledged chicks), but are associated with the costs of pair formation and establishment of territories at the beginning of the breeding season.
Retención de Parejas y Territorios en Pingüinos Megadyptes antipodes
Resumen. Investigamos las tasas de retención de parejas y de territorios por parte de pingüinos Megadyptes antipodes y el efecto del éxito reproductivo sobre estas tasas con base en datos de nidificación y anillamiento recolectados entre 1991 y 2002. La tasa anual de retención de parejas fue del 63% y las de retención de territorios del 52% y 46% para machos y hembras, respectivamente. La mayoría de las disoluciones de parejas se debieron a la muerte de una de las aves y sólo el 6% de las parejas terminaron separándose. El éxito reproductivo previo predijo acertadamente la retención de compañeros y de territorios, ya que las parejas que no lograron emplumar ningún pichón tuvieron una probablilidad de disolverse o de cambiar de territorio significativamente mayor que las que criaron exitosamente. El éxito reproductivo de las aves que cambiaron de pareja o de territorio no fue mayor que el de aquellas que no lo hicieron. Sin embargo, los individuos que cambiaron de territorio fueron más propensos a presentar un éxito de emplumamiento reducido con respecto a su intento reproductivo previo. Las aves que no retuvieron sus parejas, particularmente los machos, presentaron una probabilidad mayor de no reproducirse durante al menos un año. Esto sugiere que los costos que implica cambiar de pareja o de territorio no se hacen evidentes al final del intento reproductivo (como lo indica el número de volantones producidos), sino que están asociados con los costos de la formación de parejas y el establecimiento de territorios al comienzo de la época reproductiva.
Studies have shown that mate and nest-site retention are generally high among long-lived seabirds and may be beneficial (Bried and Jouventin 2002). Mating with the same individual may result in better coordination of breeding activities due to familiarity and age or experience and is generally associated with higher reproductive success (Coulson 1966, Williams and Rodwell 1992, Bried and Jouventin 2002). Mate retention also avoids the costs associated with pair formation (Croxall and Davis 1999, Bried and Jouventin 2002). Nest-site retention is potentially beneficial due to greater familiarity with neighbors (lower aggression) and potential mates, greater knowledge of the surrounding territory (Bried and Jouventin 2002), as well as aiding reunion with a previous year's mate (Davis and Speirs 1990, Bried and Jouventin 2002). Therefore, reproductive success in the previous year is generally associated with nest site retention (Brooke 1978, Davis 1988).
High retention of mates and nest sites are also evident among penguins. With the exception of the Emperor (Aptenodytes forsteri) and King Penguins (A. patagonicus), mate and site retention are generally high (60%–90%, Williams and Rodwell 1992, Williams 1996, Bried and Jouventin 2002), and breeding failure is likely to lead to separation in penguins (Davis 1988, Williams and Rodwell 1992, St. Clair et al. 1999). Nest site retention is also related to reproductive success in Adelie (Pygoscelis adeliae, Davis 1988), Little Blue (Eudyptula minor, Johannesen et al. 2002) and in Fiordland Penguins (Eudyptes pachyrhynchus, but only in one out of two years, St. Clair et al. 1999). Interestingly, however, retention of the previous year's mate does not necessarily result in high reproductive success (Reilly and Cullen 1981, Williams and Rodwell 1992, St. Clair et al. 1999).
The Yellow-eyed Penguin (Megadyptes antipodes) is an endangered and endemic species found only on the south east coast of the South Island and Subantarctic islands of New Zealand (Marchant and Higgins 1990). Richdale (1957) provided detailed descriptions of mate retention rates, the lengths of pair bonds, reasons for pair dissolutions, ages of the breeding birds, and reproductive success. However, the data were presented in the form of tables and anecdotes, and were not analyzed statistically. As a result, the relationship between the different factors and their importance relative to each other in determining mate retention has not been fully investigated.
Even less is known about nest-site retention in Yellow-eyed Penguins, with the only published data being that of Darby and Seddon (1990), who reported that less than 30% of pairs returned to their previous nest sites. Excluding the non-nesting, ice-dwelling Emperor Penguins, this is the lowest known level of nest fidelity rate among penguins (Williams 1996). Yellow-eyed Penguins are considered semicolonial or solitary nesters, having nests that are far apart and visually isolated from each other (Seddon 1988, Darby and Seddon 1990, Williams 1995). They are known to defend a territory of up to 20 m around the nest (Marchant and Higgins 1990), and collect nesting materials from up to 40 m away (Darby and Seddon 1990). Therefore, unlike other penguins, nest-site changes need not amount to territory changes in Yellow-eyed Penguins and nest-site retention of 30% (Darby and Seddon 1990) is likely an underestimate. Yellow-eyed Penguins are the only penguins that establish territories beyond the nest. Apart from them, only the King Penguins establish observable territories at the beginning of the breeding season, albeit they do not build nests (Davis and Renner 2003). Given the unusual nest patterns of Yellow-eyed Penguins (Seddon 1988, Seddon and Davis 1989), a detailed analysis of the factors that determine territory retention in this species is overdue.
Therefore, in this study we aimed to: 1) determine the current rates of territory and mate retention, 2) investigate the factors that determine site and mate retention, and 3) assess the effect of mate or nest retention on reproductive success in Yellow-eyed Penguins.
Methods
Study Area and Data Collection
Data were collected between 1991 and 1998 at three breeding areas, and at four breeding areas between 1999 and 2002 at Boulder Beach, Otago Peninsula (45°89′S, 170°61′E), New Zealand, from a population of banded birds of known age and sex. Sexes were determined through morphometric measurements, DNA analyses (Setiawan et al. 2004), and behavioral observations (Richdale 1951). As part of routine population monitoring, detailed and systematic nest searches were conducted each year during the pre-egg (September) and incubation periods (October–November). Nests were visited during the guard period (a period when chicks are brooded or attended by at least one adult at the nest at all times; November–January) and when chicks were banded near fledging (February). Any unbanded adults or juveniles encountered during these visits were also banded. Penguins were banded at the base of the right flipper using stainless-steel bands. As such, we obtained data annually on the identities of breeding adults and their reproductive success (number of chicks fledged).
In 2000, exact nest locations were determined using GPS in three out of the four breeding areas and from all nests at all four breeding areas in 2001 and 2002. As part of concurrent projects, during the 2000–2002 breeding seasons, nests were visited daily during the pre-egg period through to hatching, and every two days during the guard period. Some birds were also handled for blood sampling once every 14 days from egg laying through to the middle of the guard period.
A bird was considered to have retained its mate if it bred with the partner that was its last known mate. A bird was considered to have lost a mate if its mate was not known to breed again in the area. Birds were considered to have separated if at least one partner was recorded as breeding with a bird other than its previous mate, while its mate was recorded as still being alive. A bird was considered to have moved its breeding territory when GPS data showed that it had moved at least 20 m from its previous nest-site. Given that our GPS data did not take the slope of the terrain into account, the distances of nest movements were underestimated.
Statistical Analyses
We employed logistic regression models to investigate the probability of separation or territory movement due to variables such as number of chicks fledged, sex, age, individual breeding experience, and common-pair breeding experience. A pilot analysis showed that birds were more likely to skip one or more years of breeding following pair dissolution (due to death of partner or separation). Therefore, we conducted logistic regression to determine the role of mate retention status (mate retained, new mate due to mate loss or separation) and the aforementioned variables on the probability of skipping breeding. Logistic regression was also employed to measure the effect of age, common-pair and individual breeding experience on reproductive success following territory movement. To avoid pseudoreplication, we randomly selected from only one year's breeding data for each individual in each model. To optimize the sample size for the analysis of territory retention, we only included data collected in 2000–2002, with no individuals contributing more than one data point of the same type (moved or retained territories). For models that included breeding experience (individual and common-pair), only data collected at the three breeding areas for which we had complete datasets from 1993 onwards were used, as breeding experience data prior to 1991 were not readily available. Pearson's Chi-squared analyses were used to determine the effects of pair dissolution (through death of partner or separation) or territory movement on the number of fledglings produced in a breeding season and relative to those produced in the previous breeding attempt.
All statistical analyses were conducted using SYSTAT 10.2 (Systat Software Inc. 2002). Values are reported as means ± SE, and in all cases, statistical significance was accepted when P ≤ 0.05.
Estimate of the Cost of Separation on Lifetime Reproductive Success
Our analyses found a greater probability of skipping at least one year of breeding following separation. We estimated the reproductive cost of separation associated with a missed breeding attempt (skipping) by multiplying the probability of skipping due to separation for males and females with the average number of chicks fledged per breeding attempt. This cost needs to be seen in relation to the potential lifetime reproductive success. Potential lifetime reproductive success was estimated as the adult life expectancy multiplied by the average number of chicks fledged per breeding attempt. Adult life expectancy was derived from adult survival (S) according to Seber (1973): Adult life expectancy = 0.5 + (1 − S). Survival was derived from 1991–1999 data, and measured as the average proportion of adults surviving to breed in subsequent years over the number of adults breeding in one year. Therefore, adult life expectancy denotes life expectancy of adults from the first year of breeding.
Results
Mate retention between 1991 and 2001 varied between 39% and 83% with an overall average of 63% ± 5% (Table 1). The majority of pair dissolutions were due to the death of a partner (31% ± 4%), while only 6% ± 1% of bonds ended in separation.
The number of chicks per nest in relation to the fate of Yellow-eyed Penguin pair bonds at the end of the season, 1991–2001. For each pair-bond fate, we present the number of nests (n) with the percent of all nests that fall within this fate category given in parentheses
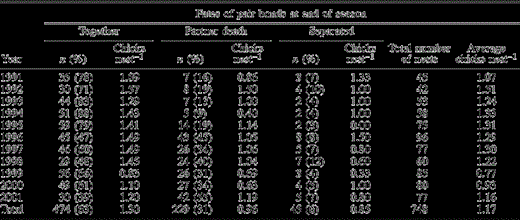
The number of chicks per nest in relation to the fate of Yellow-eyed Penguin pair bonds at the end of the season, 1991–2001. For each pair-bond fate, we present the number of nests (n) with the percent of all nests that fall within this fate category given in parentheses

Pairs that failed to fledge any chicks were more likely to separate than those that fledged at least one chick. A logistic regression model with number of chicks fledged, sex, age, individual breeding experience and common pair breeding experience fitted as independent variables against the probability of mate retention and separation, was constructed using data collected from 1993 onwards. Failure to fledge any chick was found to have a significant effect on the probability of separation (n = 173; no chicks fledged: P < 0.01; one chick fledged: P = 0.43; sex: P = 0.15; age: P = 0.58; individual breeding experience: P = 0.35; common breeding experience: P = 0.08). The model was shown to provide a slightly better fit than the intercept-only model (log-likelihood χ26 = 14.5, P = 0.03) and correctly predicted 85% of all cases. To calculate the probability of separation, only the number of chicks fledged was fitted into the model (n = 202; no chick fledged: P < 0.01; one chick fledged: P = 0.85; log-likelihood χ22 = 10.6, P < 0.01; total correct = 0.83). The probabilities of separation among birds that failed to fledge any chicks and those that fledged at least one chick were 0.23 and 0.06, respectively.
There was no difference between the number of chicks fledged by birds that retained or changed mates due to death of mate or separation (males: n = 115; χ22 = 1.6, P = 0.45; females: n = 106; χ22 = 0.1, P = 0.96, Fig. 1).
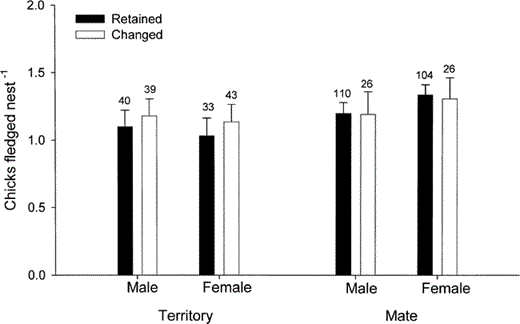
The number of chicks fledged per nest following retention or change in territory or mate in male and female Yellow-eyed Penguins. Data are presented as means ± SE with sample sizes indicated above each error bar
We found that the probabilities of skipping at least one year of breeding were highest among males and those that separated (Table 2). Sex, age, individual and common breeding experience, number of chicks fledged, and mate-retention status were fitted as independent variables against the probability of skipping at least one year of breeding in a logistic regression using data collected from 1993 onwards. Sex and mate-retention status, but not age, individual or common breeding experience, or number of chicks fledged had significant effects on the probability of skipping breeding (n = 212; sex: P = 0.02; new mate due to death of previous mate: P < 0.01; new mate due to separation: P < 0.001; age: P = 0.88; individual breeding experience: P = 0.95; common breeding experience: P = 0.76; no chicks fledged: P = 0.69; one chick fledged: P = 0.84; log-likelihood χ28 = 34.4, P < 0.001; total correct = 0.84). The average probabilities for skipping breeding are higher among males (0.31) than females (0.13), and among birds that separated from their previous mate (0.42) than those whose mates died (0.20) or those that retained their mates (0.04).
The numbers of male and female Yellow-eyed Penguins that bred or skipped one or more years of breeding in relation to mate retention. One sample from each bird was randomly selected from data collected in 1991–2002. Birds either retained their previous mate or bred with a new mate due to death of the previous mate or separation. Values in parentheses denote the probability of skipping one or more years of breeding in relation to mate retention and sex as calculated by logistic regression
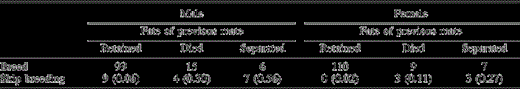
The numbers of male and female Yellow-eyed Penguins that bred or skipped one or more years of breeding in relation to mate retention. One sample from each bird was randomly selected from data collected in 1991–2002. Birds either retained their previous mate or bred with a new mate due to death of the previous mate or separation. Values in parentheses denote the probability of skipping one or more years of breeding in relation to mate retention and sex as calculated by logistic regression
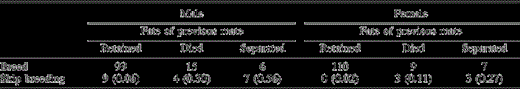
On average, male and female potential reproductive success decreased 9% and 5%, respectively, if they separated. Males and females fledged 1.05 and 1.11 chicks per breeding attempt, respectively. Therefore, on average, the estimated cost of separation for males and females were 0.6 and 0.3 fledged chicks per separation, respectively. However, these figures are underestimates as they assume that only one year of breeding was skipped for each separation. Adult life expectancy since the first year of breeding was 6.8 years for males and 5.7 years for females. Thus, potential lifetime numbers of chicks fledged for an average male or female are 7.1 and 6.3, respectively.
Overall territory fidelity was 52% ± 6% for males and 46% ± 2% for females (Table 3). Seventy-five percent of males and 63% of females that are known to have changed territories were found to breed with the same partner.
Territory movement (defined as birds whose nest sites moved more than 20 meters from last year's position), and the number of chicks per nest for male and female Yellow-eyed Penguins in 2001 and 2002

Territory movement (defined as birds whose nest sites moved more than 20 meters from last year's position), and the number of chicks per nest for male and female Yellow-eyed Penguins in 2001 and 2002

We found that females that bred with a new mate in the following season had the highest probabilities of moving territories (Table 4). Sex, age, individual and common breeding experience, number of chicks fledged, and mate retention status were fitted as independent variables against the probability of moving territories in a logistic regression model. Only the number of chicks fledged was found to have a significant effect on the probability of moving territories by this model (n = 113; no chick fledged: P < 0.001; one chick fledged: P < 0.001; sex: P = 0.38; age: P = 0.25; individual breeding experience: P = 0.17; common breeding experience: P = 0.27; mate retained: P = 0.38; new mate due to death of previous mate: P = 0.74; log-likelihood χ28 = 42.5, P < 0.001, total correct = 0.66). We then fit only sex, number of chicks fledged, and mate-retention status to the model as these variables were found to have significant effects in the previous analyses. This model showed significant effects of sex, number of chicks fledged, and an interaction between mate-retention status and sex on the probability of moving territories (Table 4; n = 158; sex: P < 0.01; no chick fledged: P < 0.001; one chick fledged: P < 0.01; sex*mate retained: P = 0.02; log-likelihood χ25 = 43.9, P < 0.001, total correct = 0.63).
The probability of Yellow-eyed Penguins moving territories in relation to sex, mate retention, and the number of chicks fledged in as calculated from logistic regression for data collected in 2000–2002. Significant effects of sex, number of chicks fledged, and an interaction between sex and mate retention (fe male and retained mate) were found
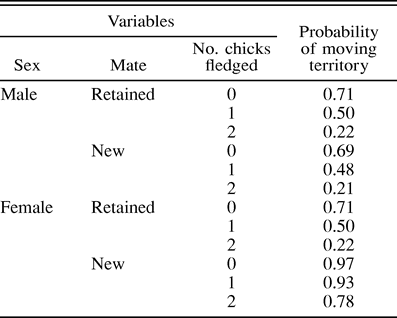
The probability of Yellow-eyed Penguins moving territories in relation to sex, mate retention, and the number of chicks fledged in as calculated from logistic regression for data collected in 2000–2002. Significant effects of sex, number of chicks fledged, and an interaction between sex and mate retention (fe male and retained mate) were found

There was no difference between the number of chicks fledged by birds that retained or moved territories (Fig. 1; males: n = 78; χ22 = 1.2, P = 0.54; females: n = 84; χ22 = 0.7, P = 0.69). However, birds that moved territories maintained or increased fledging success (males: n = 36; χ21 = 18.8, P < 0.001; females: n = 46; χ21 = 17.0, P < 0.001), even when compared to birds that retained territories (males: n = 77; χ21 = 8.2, P < 0.01; females: n = 83; χ21 = 6.6, P = 0.01). Among birds that moved territories, we found that common and individual breeding experience and age, were not correlated with the probability of reduced fledging success (n = 59; common breeding experience: P = 0.09; individual breeding experience: P = 0.47; age: P = 0.73; log-likelihood χ23 = 6.1, P = 0.01, total correct = 0.81).
Discussion
Our results are generally in agreement with those of Richdale (1957). We found that 63% of Yellow-eyed Penguin pairs stayed together for two or more years while 31% of pair-bonds were broken due to death of one partner; similar to the 60% and 27% values reported by Richdale (1957). However, only 6% of pair-bonds in our study ended in separation which is lower than the 14% reported by Richdale (1957).
Similar to other seabirds (Johnston and Ryder 1987, Bradley et al. 1990, Williams and Rodwell 1992, Dubois and Cezilly 2002, Johannesen et al. 2002), breeding success seems to be a good predictor of whether a pair bond will be maintained in the next breeding attempt. Pairs are almost four times as likely to separate or change territories following a breeding failure (no chicks fledged) than those that produced at least one fledgling. In fact, fledging success seems to be the only factor that affects separation, with no effects of sex, age, individual breeding experience or length of common breeding experience. Due to lack of sample size and the low incidence of separation, we were unable to analyze why among birds that failed to fledge any chicks, certain birds separate while some do not.
As has been observed in some other penguin species (Little Blue, Reilly and Cullen 1981; Macaroni and Gentoo, Williams and Rodwell 1992; Fiordland Penguins, St. Clair et al. 1999), we found no difference in the reproductive success (number of fledged chicks) of newly formed or reunited pairs in any given year. We agree with the assertion of Croxall and Davis (1999) that the evidence for the reproductive benefits of mate retention in penguins is equivocal and that the costs of mate changes are not accrued at the end of the breeding, but in the beginning, those associated with pair formation and loss of breeding status (Williams 1996, Croxall and Davis 1999). Indeed, pair formation seems to be relatively costly or difficult in Yellow-eyed Penguins as birds that separate or have lost their mates are more likely to miss one or more years of breeding than those that retain their mates. This cost seems to be even greater among males. Therefore, although mate changes may not diminish reproductive success in any given year, they potentially may have considerable effects on lifetime reproductive success through missed breeding opportunities, roughly estimated at 9% and 5% per separation for males and females, respectively.
Despite its cost, separation can be adaptive when the cost of retaining an incompatible or inferior mate is high (Bried and Jouventin 2002). According to the “Incompatibility Hypothesis”, pairs suffer low reproductive success not because one or both partners are of low quality (e.g., low body condition, inefficient foraging, etc), but simply because the combination of their individual traits (behavioral or physiological) are incompatible for successful breeding (e.g., erratic incubation patterns; Coulson 1966, 1972, Johnston and Ryder 1987, Ens et al. 1993). From this hypothesis, both members of the pair would benefit from separation as it would enable them to mate with more compatible partners and increase their reproductive success. This is unlikely to be the case for Yellow-eyed Penguins, as birds did not improve (nor decrease) their fledging success upon separation. However, the “Better Option Hypothesis”, where separation is initiated by one partner in order to breed with a bird of a higher quality than its previous partner (Ens et al. 1993), may apply to Yellow-eyed Penguins. Only initiators are expected to benefit from separation, thus, on a population level which includes victims, this benefit may not be detectable (Ens et al. 1993). Lack of detectable improvement in breeding success may be due to the fact that we were unable to differentiate which individual initiated the separation.
When breeding site is expressed in terms of territory rather than nest location, site fidelity in males and females were 52% and 46%, respectively, higher than the 30% reported by Darby and Seddon (1990), but still low relative to other penguins (Williams 1996, Bried and Jouventin 2002). Similar to Little (Johannesen et al. 2002), Gentoo, and Macaroni Penguins (Williams and Rodwell 1992), high reproductive success was correlated with site retention. In Yellow-eyed Penguins, territory movement was significantly less likely among birds that fledged two chicks and among males, and most likely among females with new mates, but there was no difference in reproductive success between birds that retained or moved territories. However, birds were less likely to have reduced fledging success following territory movement, even when compared to those that stayed on their previous territory. This suggests that territory movement is not costly and may even be adaptive. Given its high incidence, territory movement may play a greater role in determining lifetime reproductive success in Yellow-eyed Penguins than previously thought. Given their unique nesting behavior, greater emphasis should be placed on aspects of nest-site selection behavior in the future.
In many penguins, males arrive at the breeding area before females and establish territories or nest-site (Warham 1974, Davis and Speirs 1990, Williams 1995, Davis 2001). Adelie Penguins use the previous year's nest site as a meeting place at the start of breeding (Davis and Speirs 1990). Males consequently adopt a strategy of returning to this site as soon as possible to aid reunion, and by doing this as early as possible, avoid cuckoldry while increasing the likelihood of other matings (Davis and Speirs 1990). Williams and Rodwell (1992) indicated that male Macaroni Penguins adopt a similar strategy, whereby their primary aim is nest-site retention and, only secondarily, mate retention. This strategy of males being primarily responsible for territory establishment also applies in Yellow-eyed Penguins. Although both sexes are present year-round, male Yellow-eyed Penguins establish territories through occupation and vocal displays during the pre-egg phase (Seddon and Darby 1990). This strategy would also explain why failure to retain one's mate more often results in missed breeding opportunity in males, as territory establishment would require them to be tied to one area, thus reducing the opportunities for mate prospecting. On the other hand, females are less attached to territories than males, making them more likely to change territories, and moving with each new mate.
So why do Yellow-eyed Penguins have low site fidelity? Territory change may be promoted under certain conditions, such as: 1) low reproductive cost of territory change, 2) low cost of obtaining of new territories due to an abundance of available territories or low levels of competition, and 3) high likelihood of reuniting with the previous year's partner. All three conditions may occur in our Yellow-eyed Penguin study population. We found that moving territories entails little or no reproductive cost and cost of obtaining new territories may also be low, as our study area offered an abundance of suitable nesting habitat relative to the number of pairs present. This would have likely reduced competition. In fact, territorial disputes are rare among Yellow-eyed Penguins (Seddon and Darby 1990). The resident nature of Yellow-eyed Penguins would allow many opportunities for partners to locate each other in the event of a territory change, increasing the likelihood of remating. A considerable proportion of birds that have changed territories managed to retain the previous year's partner.
However, all studies of Yellow-eyed Penguin nest-site selection have been conducted on the mainland while populations on predator-free offshore islands (Enderby, Campbell, Codfish Islands) with little human modification have never been monitored in sufficient detail to allow for even a cursory analysis of mate or territory fidelity. The destruction of the native coastal podocarp and hardwood-forest habitat over the past 50 years on the mainland, along with mammalian predation have been implicated in reducing breeding numbers by up to 75% in some areas, and forcing Yellow-eyed Penguins to use alternative habitats which may be suboptimal (Darby and Seddon 1990).
Birds on Campbell Island in the 1987 season had a breeding success of 1.4 chicks per nest that is comparable to populations on the mainland only for the most successful breeding years or in areas with low chick predation (Moore 1992). As our findings indicate, differences in reproductive success may also produce differences in territory and mate retention. Yellow-eyed Penguin nest characteristics on the mainland and Campbell Island were similar. In both localities, nests normally have a solid back and are sheltered laterally on three sides and overhead, presumably to provide shelter from heat stress (Seddon and Davis 1989, Moore 1992). Scrub habitats are thought to be best suited to provide this level of cover. On the Campbell Island study area, only 8% of nests are situated in a grassland habitat with the rest being in scrub or fern habitats. However, in one grazed farmland habitat on the Otago Peninsula with an effective predator trapping regime, the number of penguins nesting in grassland almost doubled in 1994–1996 accompanied by a decrease in breeding success (McKay et al. 1999). It is unclear why these penguins would choose to breed in what is thought to be an inferior habitat despite the consistent presence of habitable scrub.
Mate and site retention may differ between populations and be influenced by external factors (Bried and Jouventin 2002). Differences in reproductive success and nest-site selection between the mainland and Campbell Island habitats may have repercussions on mate and territory retention behavior. Studies on mate and territory retention of populations on offshore islands or ancient data are necessary to allow for comparison between modified and unmodified habitats. The current study also shows that greater emphasis needs to be given to the cost of pair formation in future studies on separation in seabirds.
Acknowledgments
We are grateful for the help of Mike Hazel, Brad Robson and Danilo Hegg, and the other workers who collected breeding data throughout the years. We also thank and acknowledge the help of Dave Houston of the Department of Conservation for maintenance of the database, R. Scott Davidson, and Brian Niven for statistical advice, and two anonymous reviewers for their constructive comments and suggestions. Institutional financial support was provided by the University of Otago through the Otago Research Grant, Antarctic and Southern Ocean Research Group, and The Yellow-eyed Penguin Trust.
Literature Cited



