-
PDF
- Split View
-
Views
-
Cite
Cite
Jonas Lehmacher, Betül Toprak, Nils Arne Sörensen, Ramona Bei der Kellen, Alina Goßling, Tau Sarra Hartikainen, Paul Michael Haller, Alina Schock, Raphael Twerenbold, Tanja Zeller, Stefan Blankenberg, Dirk Westermann, Johannes Tobias Neumann, Validation of a 0/1 h Algorithm for Rapid Diagnosis of Myocardial Infarction Using a High-Sensitivity Troponin I Assay, Clinical Chemistry, Volume 69, Issue 5, May 2023, Pages 482–491, https://doi.org/10.1093/clinchem/hvad019
Close - Share Icon Share
Abstract
Current guidelines recommend 0/1 h algorithms using high-sensitivity cardiac troponin (hs-cTn) for fast diagnosis of myocardial infarction (MI). Yet, for some assays, existing data is limited. We aimed to evaluate the diagnostic performance and the prognostic value of a rapid 0/1 h algorithm for the Access hs-cTnI assay.
In consecutive patients presenting with suspected MI, we measured concentrations of Access hs-cTnI at presentation and after 1 hour. Final diagnosis was adjudicated independently by 2 cardiologists. Parameters for diagnostic performance were calculated, applying the recently derived European Society of Cardiology (ESC) 0/1 h algorithm for Access hs-cTnI. Additionally, we assessed the prognostic utility of Access hs-cTnI for the composite end point of all-cause mortality and incident MI at 3 years.
In 1879 patients, 257 non-ST-elevation MIs occurred. Application of the 0/1 h algorithm classified 44.5% as rule-out, 20.3% as rule-in, and triaged 35.1% to the observe group. High rule-out safety was confirmed with a sensitivity of 97.7% (95% CI, 95.0%–99.1%) and a negative predictive value of 99.3% (95% CI, 98.4%–99.7%). Rule-in capacity was moderate with a specificity of 88.0% (95% CI, 86.3%–89.6%) and a positive predictive value of 50.8% (95% CI, 45.7%–55.9%). After exclusion of patients with ST-elevation MI the results showed strong prognostic value, even after adjustment for cardiovascular risk factors and comorbidities, with adjusted hazard ratios of 2.51 (95% CI, 1.56–4.04) in the observe and 3.55 (95% CI, 2.18–5.79) in the rule-in group for the composite end point of all-cause mortality and incident MI at 3 years, compared to ruled-out patients.
The ESC 0/1 h algorithm for Access hs-cTnI allows safe and efficient triage of patients with suspected MI and has strong prognostic utility up to 3 years after the initial evaluation.
Introduction
Cardiovascular diseases (CVD), including coronary artery disease (CAD) and myocardial infarction (MI), are one of the main factors for morbidity and mortality in Western populations (1). Additionally, chest pain as a cardinal symptom for acute coronary syndrome and MI is one of the main reasons for presentation to an emergency department (ED) (2, 3). Therefore, the safe and rapid identification of high-risk patients and those suffering MI is crucial for fast and correct treatment of this life-threatening condition. Cardiac troponin is a cornerstone in diagnostic pathways for MI and assay improvement has resulted in substantially increased sensitivity (4, 5). The current generation of high-sensitivity cardiac troponin (hs-cTn) assays is able to detect circulating troponin levels in a majority of even healthy individuals (6). Several rapid 0/1 h algorithms for diagnosis of MI, applying assay specific cutoffs, have been validated and are recommended by current guidelines (7–19).
In 2017, the Access hs-cTnI chemiluminescent immunoassay was developed by Beckman Coulter as an addition to the list of existing assays, fulfilling the criteria for hs-cTn assays (20). In 2018, Greenslade et al. evaluated the rule-out potential of Access hs-cTnI with a single baseline measurement. Application of a very low cutoff of <2 ng/L resulted in both a sensitivity and negative predictive value (NPV) of above 99.0% in a cohort of 1871 patients (21). One year later, a 0/1 h diagnostic algorithm was derived and validated in patients with suspected MI, showing excellent rule-out safety (17). Use of the test in a rule-in capacity also proved to be in line with fast 0/1 h algorithms for other hs-cTn assays, with high specificity and positive predictive value (PPV) (17). Another study evaluated the diagnostic performance using baseline samples for MI at a 99th percentile cutoff in a US population, resulting in appropriate safety and accuracy (22).
As the current 0/1 h algorithm using the Access hs-cTnI assay has only been derived and internally validated in one study, our aim was to externally validate the diagnostic performance of the assay in a second, independent study population. Furthermore, we aimed to determine the possible incremental prognostic value of Access hs-cTnI concentrations.
Materials and Methods
Study Design and Population
We used data from the Biomarkers in Acute Cardiac Care (BACC) study. This study has been described before (10, 23). Briefly, BACC is an observational cohort study with its main goal being the evaluation of cardiac biomarkers, especially cardiac troponin, for the diagnosis of MI and assessment of possible impact on patient outcome. We enrolled patients presenting to the ED of the University Medical Centre Hamburg-Eppendorf with symptoms suggestive of MI. Patients had to be over 18 years and to provide written informed consent. The detailed inclusion criteria as well as the study protocol have been described in previous publications (10, 23). The study is registered at clinicaltrials.gov (NCT02355457) and was conducted in accordance with the declaration of Helsinki and Good Clinical Practice (24). The BACC study was approved by the local ethics committee Hamburg (PV4306) on July 25, 2013.
General Assessment
During routine treatment in the ED, patients received blood withdrawal at presentation and after three 3, measuring hs-cTnT (Elecsys 2010 high-sensitivity troponin T, Roche Diagnostics) as well as a 12-lead electrocardiogram. If indicated, further diagnostic and imaging were performed. Roche hs-cTnT has a 99th percentile of 14 ng/L with dichotomous sex-specific upper reference limits of 9 ng/L in women and 16 ng/L in men. The corresponding coefficient of variation was 10% at 13 ng/L. The limit of detection was determined as 3 ng/L (25).
Study-Specific Measurements
Study-specific blood samples were taken at the same time points as the sampling for general assessment in the ED was performed. Furthermore, an additional sampling after 1 h was performed, at which hs-cTnT was also measured directly from fresh samples in the central laboratory. Study-specific acquisition of blood samples was performed by trained study personnel.
Access hs-cTnI (Access hs-cTnI, Beckman Coulter) was measured in batches from consecutive frozen serum blood samples. The 99th percentile was defined as 18 ng/L, with gender-specific concentrations of 12 ng/L (women) and 20 ng/L (men). The corresponding coefficient of variation was <5%. The limit of blank was determined at 1.7 ng/L and the limit of detection at 2.3 ng/L (26).
Adjudication of Final Diagnosis
The final diagnosis was adjudicated independently by 2 cardiologists in a blinded fashion according to the 4th Universal Definition of MI (UDMI) considering all available clinical data (i.e., laboratory data including serial measurements of Elecsys hs-cTnT [e.g., baseline, after 1 or 3 h] and imaging data, as well as documented risk factors and pre-existing conditions assessed via the electronic patient record) (27). For the final diagnosis of type 1 MI, as well as type 2 MI, type 4 MI, and acute- and chronic myocardial injury, the exact criteria described in the 4th UDMI were applied using the gender-specific 99th percentile for hs-cTnT concentrations (27). We defined relevant dynamic changes as rise and/or fall of hs-cTnT of >6 ng/L within 3 hours. Diagnosis of type 1 MI was made if a relevant elevation of hs-cTnT was documented in at least one measurement together with a significant dynamic change as defined above as well as one of the following: symptoms suggestive of myocardial ischemia, new ischemic electrocardiogram (ECG) changes, Q-wave development, imaging evidence of loss of vital myocardium, or evidence of intracoronary thrombus. The diagnosis of type 2 MI was made if relevant elevation of hs-cTnT was present with acute dynamic change together with the abovementioned criteria, but without evidence of occlusive CAD or signs of oxygen demand–supply imbalance. In cases of incongruent findings, a third cardiologist was consulted. Neither cardiologist was aware of Access hs-cTnI concentrations. Diagnostic performance of the Access hs-cTnI 0/1 h algorithm was assessed for the composite of type 1 and type 2 non-ST-elevation MI at the time of index presentation.
Follow-Up
Patient follow-up was performed via a structured telephone interview using a predefined questionnaire, assessing occurrence of symptoms, cardiac re-hospitalization, coronary angiography, incident MI, and death. Patients were called up to 4 times on different days and at different time points. If contact was unsuccessful, the questionnaire was sent vial mail and/or the patient’s general practitioner was contacted. If these measures failed, the registration office was contacted to assess if the participant had died during follow-up (including date of death).
Statistical Analyses
For continuous variables, median, 25th, and 75th percentiles are displayed. For binary variables, absolute and relative frequencies are given. P-values were calculated by a Fisher test for categorical variables and by a Mann–Whitney U-test for continuous variables. Patients with a final diagnosis of ST-elevation MI were excluded from analyses. Receiver-operator characteristic (ROC) curves for the final diagnosis of MI (regardless of subtype) were computed for baseline hs-cTnI concentration, absolute hs-cTnI change between 0 and 1 h, and hs-cTnI concentration after 1 h, with corresponding areas under the curve (AUC).
Diagnostic parameters for the ESC 0/1 h algorithm for the diagnosis of MI for Access hs-cTnI were calculated, including 95% CI (18). Additionally, absolute and relative frequencies for the decision categories of rule-in, rule-out, and observe are given. Patients were classified according to the cutoff concentrations mentioned in the ESC guideline (18). Patients were triaged as rule-in if baseline hs-cTnI concentration was >50 ng/L at baseline or 1 h delta was >15 ng/L. Patients were rule-out if baseline hs-cTnI concentration was <4 ng/L and chest pain onset was >3 h or if baseline concentration was <5 ng/L and 1 h delta was <4 ng/L. In other cases, patients were triaged into the observe group. We subsequently performed subgroup analyses for sensitivity, NPV, specificity, and PPV for the following groups: sex (male/female), age (<65/≥65 years), estimated glomerular filtration rate (eGFR), calculated via eGFR-chronic kidney disease epidemiology collaboration (CKD-EPI) formula (<60/≥60 mL/min/1.73m²), and symptom onset time (<1/1 to 3/ ≥3 hours) (28).
We further calculated diagnostic parameters for single baseline measurement with the diagnostic cutoff defined as <4 ng/L. We performed these analyses for (a) the overall population irrespective of symptom onset, (b) after exclusion of all patients with symptom onset <3 h, and (c) using rule-out criteria defined as hs-cTnI <4 ng/L and symptom onset ≥3 h.
Median follow-up time and event rates were calculated by the Kaplan–Meier potential follow-up estimator. Kaplan–Meier curves with strata for categories of rule-in, rule-out, and observe were computed for the composite end point of incident MI and all-cause mortality at 3 years. The log-rank test was used to compare the different strata testing the null hypothesis that all curves are equal vs the alternative hypothesis that at least 2 curves are different.
Cox regression was performed for the defined follow-up period to produce hazard ratios (HR) for rule-in and observe with rule-out as reference group. Additionally, an adjusted regression model was calculated, adjusted for age, sex, documented diabetes, smoking status, documented hypertension, documented hyperlipoproteinemia, history of congestive heart failure and history of CAD, coronary bypass, and percutaneous coronary intervention. All analyses were performed using R-statistics version 4.0.3 (29).
Results
Population
Of 2022 consecutive patients, 1879 subject remained for analyses after exclusion of 143 patients with a final diagnosis of ST-elevation MI and patients without baseline Access hs-cTnI concentrations. Of these patients, 257 were diagnosed as having a non-ST-elevation MI (prevalence of 13.7%), 57 (3.0%) had acute myocardial injury, and 491 (26.1%) received a final diagnosis of chronic myocardial injury. Regarding MI subtypes, 156 (8.3%) were classified as type 1 MI, 98 (5.2%) as type 2 MI, and 3 patients (0.2%) suffered type 4 MI (online Supplemental Table 1).
The median age of the participants was 63 years and 64.3% were male (Table 1). Cardiovascular risk factors were widely present across the study population with 64.7% of patients having hypertension, 34.2% with documented dyslipidemia, 46.4% ever smokers, and 13.1% with a history of diabetes. Regarding cardiovascular risk factors, included patients with MI as a final diagnosis were significantly older (P < 0.001), more likely to be male (P = 0.007), more frequently had hypertension (P < 0.001), hyperlipoproteinemia (P < 0.001), or were ever smokers (P = 0.049). Of included patients, 29.5% presented within the first 3 h after symptom onset. For extended baseline information, see online Supplemental Table 2.
| . | Overall . | Non-MI . | MI . | P-value . |
|---|---|---|---|---|
| n | 1879 | 1622 | 257 | |
| Age, years | 63.0 [51.0, 75.0] | 62.0 [50.0, 74.0] | 70.0 [59.0, 78.0] | <0.001 |
| Male sex | 1209 (64.3) | 1024 (63.1) | 185 (72.0) | 0.007 |
| Hypertension | 1214 (64.7) | 1010 (62.4) | 204 (79.7) | <0.001 |
| Dyslipidemia | 643 (34.2) | 520 (32.1) | 123 (47.9) | <0.001 |
| Ever smoker | 856 (46.4) | 723 (45.5) | 133 (52.4) | 0.049 |
| Diabetes | 243 (13.1) | 200 (12.5) | 43 (16.9) | 0.066 |
| Congestive heart failure | 218 (11.6) | 174 (10.7) | 44 (17.1) | 0.004 |
| Family history of CAD | 355 (19.6) | 299 (19.2) | 56 (22.4) | 0.274 |
| CAD | 655 (34.9) | 531 (32.7) | 124 (48.2) | <0.001 |
| History of MI | 314 (16.7) | 245 (15.1) | 69 (26.8) | <0.001 |
| Ischemic ECGb | 538 (29.6) | 406 (25.9) | 132 (53.4) | <0.001 |
| Angiography | 487 (25.9) | 297 (18.3) | 190 (73.9) | <0.001 |
| Access hs-cTnI 0h | 5.3 [2.9, 15.8] | 4.5 [2.6, 10.0] | 81.1 [18.4, 520.5] | <0.001 |
| Access hs-cTnI 1h | 5.6 [2.9, 18.1] | 4.6 [2.7, 10.7] | 152.7 [33.3, 655.5] | <0.001 |
| Access hs-cTnI 3h | 5.9 [3.1, 18.7] | 4.9 [2.8, 11.1] | 262.4 [55.0, 1116.5] | <0.001 |
| Symptom onset time | 0.711 | |||
| ȃ<1h | 168 (9.5) | 144 (9.5) | 24 (9.8) | |
| ȃ≥1 h < 3h | 353 (20.0) | 297 (19.5) | 56 (22.9) | |
| ȃ≥3h | 1244 (70.5) | 1079 (71.0) | 165 (67.3) |
| . | Overall . | Non-MI . | MI . | P-value . |
|---|---|---|---|---|
| n | 1879 | 1622 | 257 | |
| Age, years | 63.0 [51.0, 75.0] | 62.0 [50.0, 74.0] | 70.0 [59.0, 78.0] | <0.001 |
| Male sex | 1209 (64.3) | 1024 (63.1) | 185 (72.0) | 0.007 |
| Hypertension | 1214 (64.7) | 1010 (62.4) | 204 (79.7) | <0.001 |
| Dyslipidemia | 643 (34.2) | 520 (32.1) | 123 (47.9) | <0.001 |
| Ever smoker | 856 (46.4) | 723 (45.5) | 133 (52.4) | 0.049 |
| Diabetes | 243 (13.1) | 200 (12.5) | 43 (16.9) | 0.066 |
| Congestive heart failure | 218 (11.6) | 174 (10.7) | 44 (17.1) | 0.004 |
| Family history of CAD | 355 (19.6) | 299 (19.2) | 56 (22.4) | 0.274 |
| CAD | 655 (34.9) | 531 (32.7) | 124 (48.2) | <0.001 |
| History of MI | 314 (16.7) | 245 (15.1) | 69 (26.8) | <0.001 |
| Ischemic ECGb | 538 (29.6) | 406 (25.9) | 132 (53.4) | <0.001 |
| Angiography | 487 (25.9) | 297 (18.3) | 190 (73.9) | <0.001 |
| Access hs-cTnI 0h | 5.3 [2.9, 15.8] | 4.5 [2.6, 10.0] | 81.1 [18.4, 520.5] | <0.001 |
| Access hs-cTnI 1h | 5.6 [2.9, 18.1] | 4.6 [2.7, 10.7] | 152.7 [33.3, 655.5] | <0.001 |
| Access hs-cTnI 3h | 5.9 [3.1, 18.7] | 4.9 [2.8, 11.1] | 262.4 [55.0, 1116.5] | <0.001 |
| Symptom onset time | 0.711 | |||
| ȃ<1h | 168 (9.5) | 144 (9.5) | 24 (9.8) | |
| ȃ≥1 h < 3h | 353 (20.0) | 297 (19.5) | 56 (22.9) | |
| ȃ≥3h | 1244 (70.5) | 1079 (71.0) | 165 (67.3) |
Baseline characteristics of the study population categorized into all, myocardial infarction, and non-myocardial infarction. Diagnoses are according to 4th UDEMI Thygesen et al. (27). Absolute and relative frequencies for binary variables; median, 25th and 75th percentile for continuous ones; relative frequencies in brackets, 25th and 75th percentile in parentheses. P-values calculated by a Fisher test for categorical variables and by a Mann–Whitney U-test for continuous variables comparing patients with MI and non-MI patients. Ischemic ECG defined as at least one of the following findings: T-wave inversion, ST-segment elevation, ST-segment depression.
ECG, electrocardiogram.
| . | Overall . | Non-MI . | MI . | P-value . |
|---|---|---|---|---|
| n | 1879 | 1622 | 257 | |
| Age, years | 63.0 [51.0, 75.0] | 62.0 [50.0, 74.0] | 70.0 [59.0, 78.0] | <0.001 |
| Male sex | 1209 (64.3) | 1024 (63.1) | 185 (72.0) | 0.007 |
| Hypertension | 1214 (64.7) | 1010 (62.4) | 204 (79.7) | <0.001 |
| Dyslipidemia | 643 (34.2) | 520 (32.1) | 123 (47.9) | <0.001 |
| Ever smoker | 856 (46.4) | 723 (45.5) | 133 (52.4) | 0.049 |
| Diabetes | 243 (13.1) | 200 (12.5) | 43 (16.9) | 0.066 |
| Congestive heart failure | 218 (11.6) | 174 (10.7) | 44 (17.1) | 0.004 |
| Family history of CAD | 355 (19.6) | 299 (19.2) | 56 (22.4) | 0.274 |
| CAD | 655 (34.9) | 531 (32.7) | 124 (48.2) | <0.001 |
| History of MI | 314 (16.7) | 245 (15.1) | 69 (26.8) | <0.001 |
| Ischemic ECGb | 538 (29.6) | 406 (25.9) | 132 (53.4) | <0.001 |
| Angiography | 487 (25.9) | 297 (18.3) | 190 (73.9) | <0.001 |
| Access hs-cTnI 0h | 5.3 [2.9, 15.8] | 4.5 [2.6, 10.0] | 81.1 [18.4, 520.5] | <0.001 |
| Access hs-cTnI 1h | 5.6 [2.9, 18.1] | 4.6 [2.7, 10.7] | 152.7 [33.3, 655.5] | <0.001 |
| Access hs-cTnI 3h | 5.9 [3.1, 18.7] | 4.9 [2.8, 11.1] | 262.4 [55.0, 1116.5] | <0.001 |
| Symptom onset time | 0.711 | |||
| ȃ<1h | 168 (9.5) | 144 (9.5) | 24 (9.8) | |
| ȃ≥1 h < 3h | 353 (20.0) | 297 (19.5) | 56 (22.9) | |
| ȃ≥3h | 1244 (70.5) | 1079 (71.0) | 165 (67.3) |
| . | Overall . | Non-MI . | MI . | P-value . |
|---|---|---|---|---|
| n | 1879 | 1622 | 257 | |
| Age, years | 63.0 [51.0, 75.0] | 62.0 [50.0, 74.0] | 70.0 [59.0, 78.0] | <0.001 |
| Male sex | 1209 (64.3) | 1024 (63.1) | 185 (72.0) | 0.007 |
| Hypertension | 1214 (64.7) | 1010 (62.4) | 204 (79.7) | <0.001 |
| Dyslipidemia | 643 (34.2) | 520 (32.1) | 123 (47.9) | <0.001 |
| Ever smoker | 856 (46.4) | 723 (45.5) | 133 (52.4) | 0.049 |
| Diabetes | 243 (13.1) | 200 (12.5) | 43 (16.9) | 0.066 |
| Congestive heart failure | 218 (11.6) | 174 (10.7) | 44 (17.1) | 0.004 |
| Family history of CAD | 355 (19.6) | 299 (19.2) | 56 (22.4) | 0.274 |
| CAD | 655 (34.9) | 531 (32.7) | 124 (48.2) | <0.001 |
| History of MI | 314 (16.7) | 245 (15.1) | 69 (26.8) | <0.001 |
| Ischemic ECGb | 538 (29.6) | 406 (25.9) | 132 (53.4) | <0.001 |
| Angiography | 487 (25.9) | 297 (18.3) | 190 (73.9) | <0.001 |
| Access hs-cTnI 0h | 5.3 [2.9, 15.8] | 4.5 [2.6, 10.0] | 81.1 [18.4, 520.5] | <0.001 |
| Access hs-cTnI 1h | 5.6 [2.9, 18.1] | 4.6 [2.7, 10.7] | 152.7 [33.3, 655.5] | <0.001 |
| Access hs-cTnI 3h | 5.9 [3.1, 18.7] | 4.9 [2.8, 11.1] | 262.4 [55.0, 1116.5] | <0.001 |
| Symptom onset time | 0.711 | |||
| ȃ<1h | 168 (9.5) | 144 (9.5) | 24 (9.8) | |
| ȃ≥1 h < 3h | 353 (20.0) | 297 (19.5) | 56 (22.9) | |
| ȃ≥3h | 1244 (70.5) | 1079 (71.0) | 165 (67.3) |
Baseline characteristics of the study population categorized into all, myocardial infarction, and non-myocardial infarction. Diagnoses are according to 4th UDEMI Thygesen et al. (27). Absolute and relative frequencies for binary variables; median, 25th and 75th percentile for continuous ones; relative frequencies in brackets, 25th and 75th percentile in parentheses. P-values calculated by a Fisher test for categorical variables and by a Mann–Whitney U-test for continuous variables comparing patients with MI and non-MI patients. Ischemic ECG defined as at least one of the following findings: T-wave inversion, ST-segment elevation, ST-segment depression.
ECG, electrocardiogram.
Diagnostic Performance
The ROC curves showed a good overall diagnostic performance for Access hs-cTnI for diagnosis of MI with an AUC for baseline concentrations of 0.883, an AUC of 0.886 for the absolute 0/1 h delta, and 0.914 for the 1 h hs-cTnI concentration (Fig. 1).
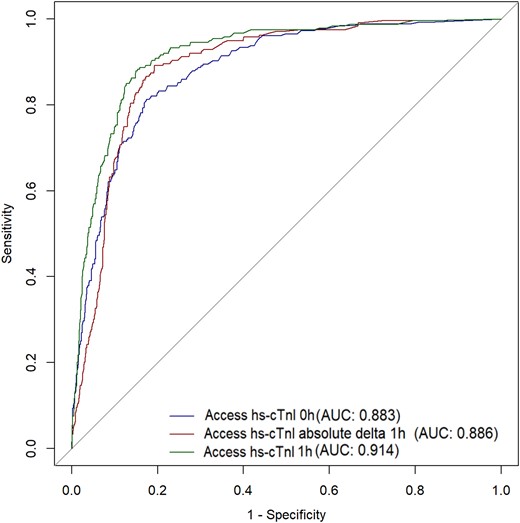
Receiver-operator characteristic curve for Access hs-cTnI for diagnosis of myocardial infarction. Definitions: Hs-cTnI 0 h (Beckman) = baseline concentration of Access hs-cTnI, Hs-cTnI (Beckman); absolute delta = absolute difference between 0 and 1 h concentration of Access hs-cTnI; Hs-cTnI 1 h (Beckman) = 1 h concentration of Access hs-cTnI.
Application of the Access hs-cTnI ESC 0/1 h algorithm resulted in good diagnostic performance, especially for rule-out of MI. A total number of 837 (44.5%) were classified as rule-out with a sensitivity of 97.7% (95% CI, 95.0, 99.1) and a NPV of 99.3% (95% CI, 98.4%–99.7%; online Supplemental Table 3). Only 6 patients with MI were missed applying the ESC 0/1 h algorithm for Access hs-cTnI (MI prevalence 0.7%). Of these patients, 5 received a final diagnosis of type 2 MI, 4 were female, and only 2 had ischemic ECG signs at admission (online Supplemental Table 4). The number of patients ruled-in was 382 (20.3%) with a specificity of 88.0% (95% CI, 86.3%–89.6%) and a PPV of 50.8% (95% CI, 45.7%–55.9%). The remaining 660 (35.1%) patients remained in the observe group and in need of further diagnostic workup. MI prevalence within the observe group was 8.6% (Fig. 2).
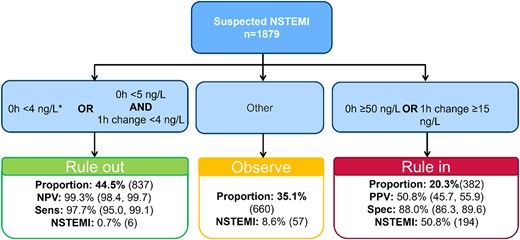
Diagnostic flow diagram of the ESC 0/1 h algorithm. Cutoffs for rule-out, rule-in, and observe as recommended by the ESC 2020 NSTEMI guidelines Collet et al. (18). *, Chest pain onset >3 h. NSTEMI, non-ST-segment elevation myocardial infarction; Sens, sensitivity; Spec, specificity.
Subgroup analyses showed adequate performance within all subgroups regarding sensitivity and NPV value without any significant differences according to gender (Fig. 3, Supplemental Tables 5–8). In patients with impaired renal function, specificity (Spec) was significantly lower (SpecGFR < 60 80.8% vs SpecGFR ≥ 60 90.2%, P < 0.001). The same difference occurred in older patients above 65 years of age, (SpecAge ≥ 65 83.6% vs SpecAge < 65 91.7%, P = 0.023). These differences were not found in the corresponding PPV, although, in women the PPV appeared to be substantially lower than in male participants (PPVmale 57.3% vs PPVfemale 38.0%, P < 0.001) (Fig. 3 and online Supplemental Table 5).

Subgroup analyses of diagnostic parameters. Subgroup analyses of the diagnostic parameters for the ESC 0/1 h algorithm for Access hs-cTnI Collet et al. (18). (A), Sensitivity; (B), negative predictive value; (C), specificity; (D), positive predictive value. Dots mark the resulting value; bars indicate the 95% CI. GFR, estimated glomerular filtration rate.
For a detailed description of the diagnostic performance of baseline hs-cTnI measurement, please see the Supplementary Appendix and online Supplemental Table 9.
Prognostic Implications
Follow-up data was available for almost all patients (1877, 99.9%). Median follow-up time was 4.47 years (95% CI, 4.4–4.52; maximum follow-up time: 7.5 years). The overall rate of death and MI in the study population was 11.3%, with a total of 200 events occurring during the follow-up period of 3 years. In total 178 (10.1%) patients died within the 3-year period and 27 (1.6%) had a non-fatal MI during follow-up. Patients ruled out via the ESC 0/1 h algorithm for Access hs-cTnI appeared to have a significantly better long-term event-free survival compared with those in the rule-in or observe group. The event rate for patients ruled-out was only 3.0% (24 events) opposed to 17.2% (106 events) in the observe group and 19.1% (70 events) in the rule-in group (P < 0.001) (Fig. 4 and online Supplemental Table 10).
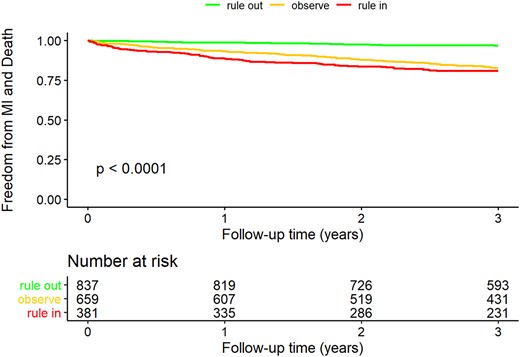
Kaplan–Meier curve for 3-year freedom of death and myocardial infarction. Rule-in, observe, and rule-out according to the ESC 0/1 h algorithm for Access hs-cTnI Collet et al. (18). P-value calculated via the log-rank test (testing the null hypothesis that all curves are equal vs the alternative hypothesis that at least 2 curves are different).
The finding that patients classified as rule-out via the 0/1 h algorithm show better long-term survival was also supported by the Cox regression models. The 3-year risk for death and MI of patients classified as observe and rule-in was higher than those ruled-out (HRrule-in 7.11 [95% CI; 4.47–11.31]; HRobserve 5.94 [95% CI; 3.82–9.26]; and both P < 0.001; see online Supplemental Table 11). Even after adjustment for common cardiovascular risk factors as well as documented congestive heart failure and CAD, patients in the observe and rule-in groups showed significantly impaired long-term survival for death and MI compared to patients ruled-out, with HRobserve of 2.51 (95% CI, 1.56–4.04) and HRrule-in of 3.55 (95% CI, 2.18–5.79). Therefore, assignment to ESC categories based on the results of Access hs-cTnI at 0 and 1 h is a strong, independent risk factor for death and MI. The risk increase for being classified as observe or rule-in exceeded the impact of each individual risk factor and pre-existing condition adjusted for in our analyses (Table 2). Of the factors adjusted for, each one, except sex, diabetes, and hypertension, appeared to be an independent risk factor for death and MI itself.
Adjusted Cox regression model for death and incident myocardial infarction.a
| . | HR (95% CI) . | P-value . |
|---|---|---|
| Allocated to rule-in group | 3.55 (2.18–5.79) | <0.001 |
| Allocated to observe group | 2.51 (1.56–4.04) | <0.001 |
| Allocated to rule-out group | 1.00 (Reference) | |
| Age, years | 1.05 (1.04–1.06) | <0.001 |
| Male sex | 0.98 (0.71–1.35) | 0.89 |
| Diabetes | 1.38 (0.98–1.95) | 0.069 |
| Smoking | 1.39 (1.02–1.88) | 0.035 |
| Hypertension | 1.42 (0.91–2.20) | 0.12 |
| Dyslipidemia | 0.71 (0.52–0.97) | 0.031 |
| Congestive heart failure | 1.69 (1.21–2.35) | 0.0020 |
| CAD | 1.93 (1.38–2.70) | <0.001 |
| . | HR (95% CI) . | P-value . |
|---|---|---|
| Allocated to rule-in group | 3.55 (2.18–5.79) | <0.001 |
| Allocated to observe group | 2.51 (1.56–4.04) | <0.001 |
| Allocated to rule-out group | 1.00 (Reference) | |
| Age, years | 1.05 (1.04–1.06) | <0.001 |
| Male sex | 0.98 (0.71–1.35) | 0.89 |
| Diabetes | 1.38 (0.98–1.95) | 0.069 |
| Smoking | 1.39 (1.02–1.88) | 0.035 |
| Hypertension | 1.42 (0.91–2.20) | 0.12 |
| Dyslipidemia | 0.71 (0.52–0.97) | 0.031 |
| Congestive heart failure | 1.69 (1.21–2.35) | 0.0020 |
| CAD | 1.93 (1.38–2.70) | <0.001 |
Cox regression for 3-year risk of death and myocardial infarction with rule-out (according to ESC 0/1 h algorithm for Access hs-cTnI for diagnosis of myocardial infarction) as reference group Collet et al. (18).
Adjusted Cox regression model for death and incident myocardial infarction.a
| . | HR (95% CI) . | P-value . |
|---|---|---|
| Allocated to rule-in group | 3.55 (2.18–5.79) | <0.001 |
| Allocated to observe group | 2.51 (1.56–4.04) | <0.001 |
| Allocated to rule-out group | 1.00 (Reference) | |
| Age, years | 1.05 (1.04–1.06) | <0.001 |
| Male sex | 0.98 (0.71–1.35) | 0.89 |
| Diabetes | 1.38 (0.98–1.95) | 0.069 |
| Smoking | 1.39 (1.02–1.88) | 0.035 |
| Hypertension | 1.42 (0.91–2.20) | 0.12 |
| Dyslipidemia | 0.71 (0.52–0.97) | 0.031 |
| Congestive heart failure | 1.69 (1.21–2.35) | 0.0020 |
| CAD | 1.93 (1.38–2.70) | <0.001 |
| . | HR (95% CI) . | P-value . |
|---|---|---|
| Allocated to rule-in group | 3.55 (2.18–5.79) | <0.001 |
| Allocated to observe group | 2.51 (1.56–4.04) | <0.001 |
| Allocated to rule-out group | 1.00 (Reference) | |
| Age, years | 1.05 (1.04–1.06) | <0.001 |
| Male sex | 0.98 (0.71–1.35) | 0.89 |
| Diabetes | 1.38 (0.98–1.95) | 0.069 |
| Smoking | 1.39 (1.02–1.88) | 0.035 |
| Hypertension | 1.42 (0.91–2.20) | 0.12 |
| Dyslipidemia | 0.71 (0.52–0.97) | 0.031 |
| Congestive heart failure | 1.69 (1.21–2.35) | 0.0020 |
| CAD | 1.93 (1.38–2.70) | <0.001 |
Cox regression for 3-year risk of death and myocardial infarction with rule-out (according to ESC 0/1 h algorithm for Access hs-cTnI for diagnosis of myocardial infarction) as reference group Collet et al. (18).
Discussion
We demonstrate in a large patient cohort with suspected MI, that application of the ESC 0/1 h algorithm for Access hs-cTnI resulted in good diagnostic accuracy for diagnosis of MI as well as strong prognostic value for prediction of 3-year all-cause mortality and MI.
With 1879 patients enrolled, our study population allows for subgroup analyses as well as stratification for several risk factors regarding patient outcome, leading to a high validity of our results. Compared to other studies evaluating the performance of hs-cTn assays, the population size in this work is comparable and even exceeds the size of many other cohorts (5, 7–17). The inclusion of unselected patients with different pretest probabilities makes our results generalizable for routine clinical practice in an ED. Regarding the composition of our cohort, with mostly elderly men with high prevalence of cardiovascular risk factors and a substantial proportion of patients with pre-existing cardiovascular conditions, patient characteristics are comparable to other works with similar focus, establishing comparability of results (7–17).
Adjudication of the final diagnosis according to the current 4th UDMI enables analyses considering the recently introduced conditions of acute and chronic myocardial injury and the potential impact on diagnostic accuracy (27). Many previous works evaluating the diagnostic performance of hs-cTn assays adjudicated the final diagnosis to the 3rd UDMI.
The overall diagnostic performance of the ESC 0/1 h algorithm for Access hs-cTnI in our analyses was good which is mirrored in the high AUC values, in line with other hs-cTn assays which have been previously evaluated (7, 16, 17, 30). Our results also show good sensitivity and NPV as well as high specificity, in keeping with previous studies (17, 21, 22).
As a specific example, Boeddinghaus et al. demonstrated a sensitivity of 98.9% (95% CI, 94.3%–99.8%) and a corresponding NPV of 99.8% (95% CI, 98.6%–100.0%) when initially deriving the algorithm which is the basis for the ESC 0/1 h algorithm (17). Rule-out performance was slightly better than in our evaluation; one aspect worth mentioning is the significantly higher prevalence of type 2 MI in our population (5.2% vs 1.6%) (17). This may potentially account for our slightly lower sensitivity and NPV, as the derived cutoffs may not be fitted for detection of type 2 MI as most MI patients in Boeddinghaus’ underlying derivation cohort were type 1 MI (218 of 243 [89.7%]) (17). This hypothesis is supported by the finding that 5 of 6 false-negative patients in the rule-out group as well as the majority of MI patients in the observe group (36/57 [63.2%]) had type 2 MI. The initial work, deriving the tested 0/1 h algorithm, also showed a better rule-in capacity with specificity 95.9% (95% CI, 94.0%–97.2%) and PPV of 73.9% (95% CI, 64.1%–81.8%) (17). Also, the proportion of patients in the observe group was smaller than in our work, with corresponding higher proportions of patients either being classified rule-in or rule-out (17). Besides the comparable sample size, the similar prevalence of MI in both cohorts as well as comparable patient characteristics, one difference in adjudication of the final diagnosis may explain the difference to a certain degree (17). As both works adjudicated the final diagnosis according to the 4th UDMI, in the mentioned work by Boeddinghaus et al. the diagnostic categories of acute and chronic myocardial injury were not considered. This potentially led to a lower number of patients being false-positively classified rule-in, as in our work (188 false-positive patients, 49.2% of rule-in patients). Our subgroup analyses potentially support this hypothesis, as specificity was lower in elderly patients and patients with impaired renal function as well as PPV being significantly lower in women. Previous work showed that these patients in particular more frequently have myocardial injury (31). Yet, we cannot directly compare the proportion of patients with myocardial injury and the potential impact on diagnostic accuracy in both cohorts, as no corresponding data is available (17).
A previous study by Sörensen et al. evaluating the diagnostic performance of a different hs-cTnI assay had the same difficulty with discrimination of acute myocardial injury and MI resulting in a limited PPV when adjudicating diagnoses according to the 4th UDMI (16).
Regarding the possibility of triage of patients with suspected MI with a single baseline hs-cTnI measurement and a low concentration of <4 ng/L, our analyses indicate high rule-out safety, with only a small number of false-negative patients and 39.1% of patients being eligible for immediate rule out and potential discharge from ED. Additional consideration of symptom onset time (Chest pain onset >3 h) resulted in a slight improvement in rule-out safety at the cost of a smaller proportion of patients being identified for potential discharge. Our results are comparable to the results of Greenslade et al., although at a cutoff of <4 ng/L their sensitivity was lower than in our work (94.9% [95% CI, 88.5%–98.3%]), with a slightly higher NPV (99.6% [95% CI, 99.1%–99.9%]) and larger proportions of patients ruled out (65.7%) (21). However, the comparability of both is limited as Greenslade et al. only evaluated diagnostic performance in type 1 MI patients whereas our analyses were performed for MI irrespective of classification (21).
The follow-up analyses in our work show a strong relationship between higher Access hs-cTnI concentrations and significantly impaired long-term outcome for all-cause mortality and MI. Therefore, the ESC 0/1 h algorithm seems not only suitable for fast rule-in or rule-out of patients with suspected MI, but also for rapid risk stratification and identification of high-risk patients in need of further testing and potentially intensified preventive measures. This includes not only patients with MI but also patients with acute and chronic myocardial injury. This is supported by the comparable event rates between the rule-in (18.4%) and observe groups (16.1%), despite a large difference in MI prevalence (observe: 8.6%; rule-in: 50.8%). Patients with acute myocardial injury are often classified as rule-in while patients with chronic myocardial often remain in the observe group; these patients all show hs-cTnI concentrations above the assay-specific 99th percentile and therefore cannot be ruled out by the evaluated algorithm. In addition, as per definition, they do not show relevant hs-cTn dynamics over time and are less likely to be classified as rule-in. Such patients with myocardial injury are known to have impaired outcomes comparable to patients with MI (32). The existing data for 30-day adverse cardiovascular outcomes from the Advantageous Predictors of Acute Coronary Syndrome Evaluation study support our findings, as well as short- and long-term survival data from other hs-cTn assays where in all cases low concentrations of hs-cTn indicate better event-free survival (10, 16, 17, 33).
This work has some limitations. First, as a single-center study, generalizability of results is limited regarding ethnicity and geographic region. Second, though patient enrollment was mainly consecutive, during night hours fewer trained personnel were present in the ED, potentially resulting in an inherent selection bias, as during this time screening and enrollment may not have been performed continuously. Further, we only have a very small number of hemodynamically unstable patients in our cohort, though these patients would be primarily referred for immediate intervention without awaiting the results of serial hs-cTn measurements anyway.
In conclusion, application of the ESC 0/1 h algorithm for the Access hs-cTnI assay results in good diagnostic performance especially for safe rule-out of MI as well as having high prognostic value for long-term risk of all-cause mortality and MI.
Supplemental Material
Supplemental material is available at Clinical Chemistry online.
Nonstandard Abbreviations
hs-cTn, high-sensitivity cardiac troponin; MI, myocardial infarction; ESC, European Society of Cardiology; CAD, coronary artery disease; ED, emergency department; NPV, negative predictive value; PPV, positive predictive value; UDMI, Universal Definition of MI; AUC, area under the curve; CI, Confidence interval; eGFR, estimated glomerular filtration rate; HR, hazard ratio.
Author Contributions
All authors confirmed they have contributed to the intellectual content of this paper and have met the following 4 requirements: (a) significant contributions to the conception and design, acquisition of data, or analysis and interpretation of data; (b) drafting or revising the article for intellectual content; (c) final approval of the published article; and (d) agreement to be accountable for all aspects of the article thus ensuring that questions related to the accuracy or integrity of any part of the article are appropriately investigated and resolved.
J. Lehmacher, Data curation-Equal, Investigation-Equal, Writing—original draft-Lead; B. Toprak, Data curation-Equal, Investigation-Equal, Writing—review & editing-Equal; N. A. Soerensen, Data curation-Equal, Investigation-Equal, Methodology-Equal, Project administration-Equal, Supervision-Equal, Writing—review & editing-Equal; R. Bei der Kellen, Formal analysis-Lead, Visualization-Lead, Writing—review & editing-Equal; A. Goßling, Formal analysis-Supporting, Visualization-Supporting, Writing—review & editing-Equal; T. S. Hartikainen, Data curation-Equal, Investigation-Equal, Writing—review & editing-Equal; P. M. Haller, Data curation-Equal, Investigation-Equal, Writing—review & editing-Equal; A. Schock, Data curation-Equal, Investigation-Equal, Writing—review & editing-Equal; R. Twerenbold, Writing—review & editing-Equal; T. Zeller. Writing—review & editing-Equal; S- Blankenberg, Supervision-Equal, Writing—review & editing-Equal; D. Westermann, Writing—review & editing-Equal; and J. T. Neumann, Conceptualization-Equal, Data curation-Equal, Investigation-Equal, Methodology-Equal, Project administration-Equal, Supervision-Equal, Writing—review & editing-Equal.
Authors’ Disclosures or Potential Conflicts of Interest
Upon manuscript submission, all authors completed the author disclosure form. Disclosures and/or potential conflicts of interest:
Employment or Leadership
S. Blankenberg, DGK Board, UHZ Medical Director, Faculty Member UHZ.
Consultant or Advisory Role
R. Twerenbold, Abbott, Amgen, Astra Zeneca, Roche, Siemens, and Singulex; S. Blankenberg, Bayer, Novartis, and Thermo Fisher.
Stock Ownership
None declared.
Honoraria
D. Westermann, Abiomed, Astra Zeneca, Bayer, Berlin-Chemie, Edwards, Novartis, and Medtronic; N. Arne Sörensen, Siemens Healthineers GmbH; R. Twerenbold, Abbott, Amgen, Roche, and Siemens; S. Blankenberg, Abbott, Astra Zeneca, Bayer, AMGEN, Medtronic, Pfizer, Siemens, and Thermo Fisher; P.M. Haller, Thieme Media and CDC; J.T. Neumann, Siemens Healthineers, Roche, and PHC.
Research Funding
The Biomarkers in Acute Cardiac Care study is supported by the German Center for Cardiovascular Research and received an unrestricted grant from Abbott Diagnostics (Abbott Laboratories). T. Zeller is funded by the German Centre for Cardiovascular Research (DZHK, 81Z0710101 and 81Z0710102) and the German Ministry of Education and Research. J. Lehmacher, Ernst und Berta Grimmke Stiftung; N. Arne Sörensen, German Heart Foundation; R. Twerenbold, the Swiss National Science Foundation (Grant No. P300PB_167803), the Swiss Heart Foundation, the Swiss Society of Cardiology, the Cardiovascular Research Foundation Basel, the University of Basel, and the University Hospital Basel; S. Blankenberg, Abbott Diagnostics, Bayer, SIEMENS, Singulex, and Thermo Fisher; P.M. Haller, University of Hamburg, Faculty of Medicine; B. Toprak, German Foundation of Heart Research and Ernst und Berta Grimmke Stiftung.
Expert Testimony
None declared.
Patents
T. Zeller, S. Blankenberg, R. Twerenbold, and J.T. Neumann are listed as co-inventors of an international patent on the use of a computing device to estimate the probability of myocardial infarction (International Publication Number WO2022043229A1). R. Twerenbold, co-inventor and co-applicant of a Taiwanese patent on the use of a computing device to estimate the probability of myocardial infarction (Taiwanese Publication Number 202219980).
Other Remuneration
S. Blankenberg, support for attending meetings and/or travel from Abbott, Astra Zeneca, Bayer, AMGEN, Medtronic, Pfizer, Siemens, and Thermo Fisher; P.M. Haller, support for attending meetings and/or travel from the Association for the Research on Atherosclerosis, Thrombosis and Vascular Biology (ATVB), Vienna, and the German Center for Cardiovascular Research.
Role of Sponsor
The funding organizations played no role in the design of study, choice of enrolled patients, review and interpretation of data, preparation of manuscript, or final approval of manuscript.
References
R Core Team.
Author notes
Jonas Lehmacher and Betül Toprak contributed equally to this work.
Present address: Department of Cardiology, University Heart Center Freiburg, Bad Krozingen, Germany.



