-
PDF
- Split View
-
Views
-
Cite
Cite
Paul Collinson, Janne Suvisaari, Kristin M Aakre, Hannsjörg Baum, Christopher J Duff, Damien Gruson, Angelika Hammerer-Lercher, Kari Pulkki, Sanja Stankovic, Michel R Langlois, Fred S Apple, Päivi Laitinen, for the EFLM Task Group on Cardiac Markers, How Well Do Laboratories Adhere to Recommended Guidelines for Cardiac Biomarkers Management in Europe? The CArdiac MARker Guideline Uptake in Europe (CAMARGUE) Study of the European Federation of Laboratory Medicine Task Group on Cardiac Markers, Clinical Chemistry, Volume 67, Issue 8, August 2021, Pages 1144–1152, https://doi.org/10.1093/clinchem/hvab066
Close - Share Icon Share
Abstract
The CARdiac MARker Guideline Uptake in Europe (CAMARGUE) program is a multi-country audit of the use of cardiac biomarkers in routine clinical practice.
An email link to a web-based questionnaire of 30 multiple-choice questions was distributed via the professional societies in Europe.
374 questionnaires were returned from 39 countries, the majority of which were in northern Europe with a response rate of 8.2%–42.0%. The majority of the respondents were from hospitals with proportionately more responses from central hospitals than district hospitals. Cardiac troponin was the preferred cardiac biomarker, evenly split between cardiac troponin T (cTnT) and cardiac troponin I (cTnI). Aspartate transaminase and lactate dehydrogenase are no longer offered as cardiac biomarkers. Creatine kinase, creatine kinase MB isoenzyme, and myoglobin continue to be offered as part of the cardiac biomarker profile in approximately on 50% of respondents. There is widespread utilization of high sensitivity (hs) troponin assays. The majority of cTnT users measure hs-cTnT. 29.5% of laboratories measure cTnI by a non-hs method but there has been substantial conversion to hs-cTnI. The majority of respondents used ng/L and use the 99th percentile as the upper reference limit (71.9% of respondents). A range of diagnostic protocols are in use.
There is widespread utilization of hs troponin methods. A significant minority do not use the 99th percentile as recommended and there is, as yet, little uptake of very rapid diagnostic strategies. Education of laboratory professionals and clinicians remains a priority.
Introduction
The CARdiac MARker Guideline Uptake in Europe (CAMARGUE) program is a multi-country audit of the use of cardiac biomarkers in routine clinical practice undertaken by the European Federation of Laboratory Medicine cardiac markers task group (EFLM TG-CM). The objective is to benchmark clinical laboratory practice at the time of audit compared with the guidelines in use at the same time point. It therefore aims to describe the evolution of cardiac biomarker practice and to document how well laboratories remain aligned to clinical and laboratory guidelines.
The first audit focused on the introduction of cardiac troponin (cTn) and natriuretic peptides into routine clinical use (1, 2). Subsequent audits covered the evolution of cardiac biomarker testing as the evidence base and assay methodologies underwent progressive improvement (3–6). The most recent audit covered cardiac biomarkers and lipids (7). The major change since the last survey, (undertaken in 2015) has been widespread endorsement of high sensitivity cTn (hs-cTn) assays and their increasing availability from manufacturers. This has been reflected in several documents published since 2015. The International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) task force (now committee) on clinical applications of cardiac biomarkers (IFCC C-CB) made several recommendations on implementing hs-cTn assays in clinical practice. This document covered the 99th percentile upper reference limit and calculating serial changes (8). Specifically, it distinguished between hs-cTn assays (assays which would detect cTn in 50% or more of healthy subjects with the analytical imprecision at the 99th percentile ≤10%) and conventional (contemporary) sensitive assays which would achieve this imprecision goal but not the detection of cTn in 50% or more of healthy subjects. The European Society of Cardiology (ESC) updated the guidelines on diagnosis of non-ST elevation myocardial infarction (NSTEMI) specifically recommending the use of hs-cTn assays and that these could be used with measurements taken on admission and 3 h from admission. In addition, they recommended consideration of rapid diagnostic algorithms based on admission measurement alone or sampling over short time intervals (measurement on admission and 1h from admission) (9). These changes are summarized in Table 1. Finally, the fourth universal definition of myocardial infarction has further updated concepts with commentary on the analytical issues affecting cTn assays and specifically recommended that hs-cTn assays be used for routine diagnosis (10), as has the combined IFCC and Academy of the American Association for Clinical Chemistry (11). This report concentrates on the use of cTn in routine clinical practice with particular emphasis on the use and implementation of high sensitivity assays.
Comparison of guidelines from the European Society of Cardiology from 2011 to 2017.
| . | ESC guideline 2011 . | ESC guideline 2017 . |
|---|---|---|
| First line biomarker | Cardiac troponin | Cardiac troponin |
| Assay | High sensitivity assay | High sensitivity assay |
| Diagnostic cut-off | 99th percentile upper reference limit (URL) of a normal reference population with 10% CV at the URL | Sex-specific URLs |
| Analytical quality | No methodological recommendations (standardization and analytical performance goals) | CV less than 10% mandatory for hs-Tn. Assays with CVs >20% at the 99th percentile should not be used |
| Point-of-care testing (POCT) | POCT recommended when lab TAT more than 60 min | POCT not recommended as unable to meet high sensitivity performance recommendations |
| Laboratory clinician interaction | No recommendations | “Thus, clinicians must learn about their local assay and should look for reliable information, for example, available on the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) website (http://www.ifcc.org/ executive-board-and-council/eb-task-forces/task-force-on-clinical-applications-ofcardiac-bio-markers-tf-cb/), when they have questions concerning analytical issues.” |
| . | ESC guideline 2011 . | ESC guideline 2017 . |
|---|---|---|
| First line biomarker | Cardiac troponin | Cardiac troponin |
| Assay | High sensitivity assay | High sensitivity assay |
| Diagnostic cut-off | 99th percentile upper reference limit (URL) of a normal reference population with 10% CV at the URL | Sex-specific URLs |
| Analytical quality | No methodological recommendations (standardization and analytical performance goals) | CV less than 10% mandatory for hs-Tn. Assays with CVs >20% at the 99th percentile should not be used |
| Point-of-care testing (POCT) | POCT recommended when lab TAT more than 60 min | POCT not recommended as unable to meet high sensitivity performance recommendations |
| Laboratory clinician interaction | No recommendations | “Thus, clinicians must learn about their local assay and should look for reliable information, for example, available on the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) website (http://www.ifcc.org/ executive-board-and-council/eb-task-forces/task-force-on-clinical-applications-ofcardiac-bio-markers-tf-cb/), when they have questions concerning analytical issues.” |
Comparison of guidelines from the European Society of Cardiology from 2011 to 2017.
| . | ESC guideline 2011 . | ESC guideline 2017 . |
|---|---|---|
| First line biomarker | Cardiac troponin | Cardiac troponin |
| Assay | High sensitivity assay | High sensitivity assay |
| Diagnostic cut-off | 99th percentile upper reference limit (URL) of a normal reference population with 10% CV at the URL | Sex-specific URLs |
| Analytical quality | No methodological recommendations (standardization and analytical performance goals) | CV less than 10% mandatory for hs-Tn. Assays with CVs >20% at the 99th percentile should not be used |
| Point-of-care testing (POCT) | POCT recommended when lab TAT more than 60 min | POCT not recommended as unable to meet high sensitivity performance recommendations |
| Laboratory clinician interaction | No recommendations | “Thus, clinicians must learn about their local assay and should look for reliable information, for example, available on the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) website (http://www.ifcc.org/ executive-board-and-council/eb-task-forces/task-force-on-clinical-applications-ofcardiac-bio-markers-tf-cb/), when they have questions concerning analytical issues.” |
| . | ESC guideline 2011 . | ESC guideline 2017 . |
|---|---|---|
| First line biomarker | Cardiac troponin | Cardiac troponin |
| Assay | High sensitivity assay | High sensitivity assay |
| Diagnostic cut-off | 99th percentile upper reference limit (URL) of a normal reference population with 10% CV at the URL | Sex-specific URLs |
| Analytical quality | No methodological recommendations (standardization and analytical performance goals) | CV less than 10% mandatory for hs-Tn. Assays with CVs >20% at the 99th percentile should not be used |
| Point-of-care testing (POCT) | POCT recommended when lab TAT more than 60 min | POCT not recommended as unable to meet high sensitivity performance recommendations |
| Laboratory clinician interaction | No recommendations | “Thus, clinicians must learn about their local assay and should look for reliable information, for example, available on the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) website (http://www.ifcc.org/ executive-board-and-council/eb-task-forces/task-force-on-clinical-applications-ofcardiac-bio-markers-tf-cb/), when they have questions concerning analytical issues.” |
Methods
The methodology used has been described in full in previous publications (1–4). Briefly, a web-based questionnaire was developed by the EFLM TG-CM. The questions focused on recommendations of the IFCC C-CB (8), the ESC recommendations on NSTEMI (9), and the universal definition of myocardial infarction (10) for reasons discussed in the introduction. This questionnaire included 30 multiple-choice questions regarding cardiac biomarker testing and covered the analytical methods and manufacturer used, measurement units, decision thresholds, and the use of decision-enhancing comments (Supplemental Table 1). Structured questions were used with the option of adding free text. The questionnaire was implemented using a web-based survey system consisting of a HTML-AJAX interface and a computer-generated imagery (CGI) program storing the results in XML files on a database server. Results from raw data XML files were tabulated and the numbers of different answers for each question were calculated for further analysis using Microsoft Excel, followed by extraction of the data into a Microsoft Access database. The questionnaire was compiled with experience from the 2 previous questionnaires, but also incorporated some modifications in design to allow more in-depth analysis of certain responses. A link to the online questionnaire was sent on March 12019 to EFLM National Societies from 40 European member countries. The questionnaire was open until August 31, 2019. Statistical analysis was performed using the Analyse It (Analyse-It.com) add-in for Excel. Nonparametric statistics were used throughout.
Results
Demographics
A total of 374 questionnaires were returned from 39 countries, fewer than the previous survey (493 responses). Not all questionnaires were fully completed and where a null return (no response to the question) occurred, this is indicated in the subsequent analysis. Where questions are only relevant to hospital practice, nonhospital responders have been excluded from the analysis and this is indicated in the text. Responses were obtained from the majority of European countries (96% of all responses) but there was under-representation of the southern European countries (7.2% of responses) with the exception of Portugal. 84.8% of responses were from hospital laboratories with the breakdown shown in Fig. 1 (no response 6.4%). Proportionately, there were a statistically higher number of central hospitals than district hospitals (203 vs 106) than in the previous survey (219 vs 174, P = 0.009). 68.7% of laboratories reported that they were accredited (no response 0.5%). The majority of accreditation (76%) was to International Organization for Standardization (ISO) 15189. Based on countries where reliable information was available from quality assurance scheme participant numbers, response rate was 8.242.0% with a median of 22%.
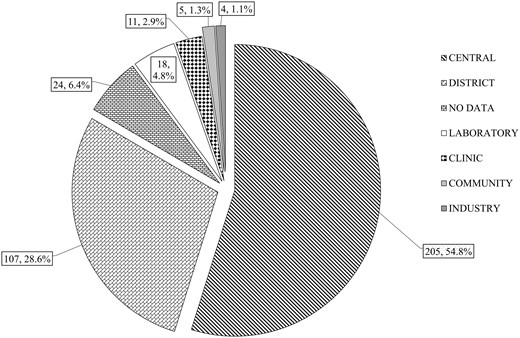
Categories of respondent. Central, University/Teaching hospital or Regional center/Secondary and Tertiary hospital; District, District General or Secondary Care hospital; Laboratory, Free standing laboratory; Clinic, Primary care or Ambulant care facility; Community, Community hospital (nonurgent care).
For the hospitals, median bed number was 600 (range 3–3810, interquartile range 360–1000). Angiography was offered by 56.5% of the participants. 14.2% of laboratories were unaware as to whether their hospital offered angiography. 75.9% of central hospitals and 52.6% of district hospitals offered angiography. Overall, 319/374 (85.3%) offered a 24-h service including 4 clinics and 3 freestanding laboratories (1.6% no response). When the analysis was confined to hospital-affiliated laboratories, 95.6% offered a 24-h service (0.8% no response) and in hospitals providing angiography, 99.4%.
Biomarker Preferences
The preferred cardiac biomarker was cTn, offered overall by 95.2% of all laboratories surveyed, 99.1% of hospital laboratories (although it was not the first line test in 1.3% of those offering cTn), and 100% of those providing angiography, similar to the data from the 2013 survey (98.6% offered cTn). For hospitals not providing cTn, the reasons stated were financial. The median number of cTn measurements annually was 13 500 for hospital laboratories.
The trend of decline of aspartate transaminase (AST), lactate dehydrogenase or its isoenzymes (LD), and creatine kinase (CK) measurement was maintained (Fig. 2 upper panel). Measurement of CK and its the MB isoenzyme of CK (CK-MB) isoenzyme has remained stable from 2013 to 2019 (Fig. 2 lower panel) even when only hospital laboratories were examined (data not shown). Overall, 93.6% of respondents used cTn as a front-line test in suspected acute coronary syndromes (ACS), 97.8% in hospitals, similar to 2013, but 66.6% continue to offer additional cardiac biomarkers for cTn as a routine. 356 laboratories currently measured cTn, 181 cardiac troponin T (cTnT, 50.8%,179 high sensitivity cTnT, hs-cTnT), and 173 cardiac troponin I (cTnI, 48.5%,122 high sensitivity cTnI, hs-cTnI) with 2 unspecified. The major change since 2013 has been the introduction of hs-cTn methods. Currently 84.6% (301/356) of laboratories measure cTn with a high sensitivity method (59.5% hs-cTnT, 34.3% hs-cTnI). The impact of introducing hs-cTn on the laboratory is shown in Fig. 3.
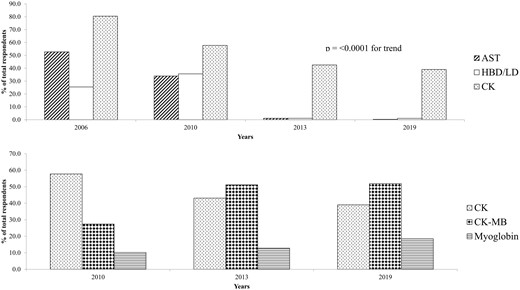
Proportion of laboratories using other biomarker as a percentage of total numbers. Upper panel laboratories using aspartate transaminase (AST), lactate dehydrogenase (LD), or hydroxybutyrate dehydrogenase (HBD). Lower pane laboratories using creatine kinase (CK) or its MB isoenzyme (CK-MB), and myoglobin.
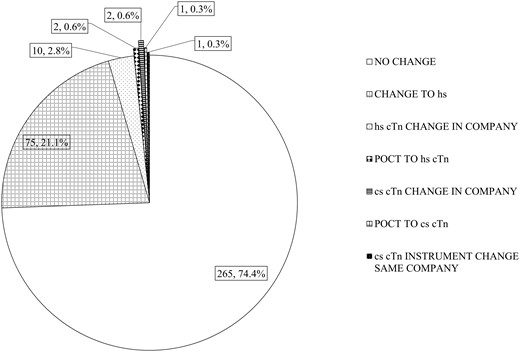
Distribution of laboratories currently utilizing or changing to a high sensitivity troponin method. Change to hs, change to a high sensitivity method supplied by the same company (supplier); hs-cTn change in company, existing user of a high sensitivity method but changing to a different company (supplier); POCT to hs-cTn, change from point-of-care testing to a high sensitivity troponin method; cs-cTn instrument change the same company, continues to use a non-high sensitivity cTn method from the same company (supplier).
Recent Changes in cTn Assays
In the past 2 years, 74.4% of the laboratories surveyed had not changed the cTn method. Of those who measured cTnT, 147/179 (82.1%) were already using hs-cTnT. Only 2 existing users, both measuring cTnT by point of care (POC), continued to use a non-hs assay. This probably represents service provision restricted to POC testing. Only 10 laboratories switched to hs-cTnT in the previous 2 years, the remaining 22 switched from cTnI or POC (1 laboratory) to hs-cTnT. Roche originally offered a conventional cTnI assay but did not develop an hs-cTnI assay.
Unlike cTnT, where 98.9% of laboratories utilized hs-cTnT currently, 51/173 (29.5%) currently measuring cTnI utilized a non-hs assay. In total, 67/173 (38.7%) of laboratories measuring hs-cTnI reported no change in method. As would be expected, this varied from 95.8% for Abbott to 0% (Roche cTnI). For Roche, conversion to hs-cTnI measurement would have required changing the manufacturer. However, some Roche cTnI users have changed to hs-cTnT. A detailed analysis of the data is available in Supplemental Table 2. The current distribution of methods utilized is shown in Supplemental Fig. 1. Utilization by country was variable (Supplemental Fig. 2) reflecting the different manufacturers’ domestic market position.
Choice of Reporting Units
Current IFCC guidelines recommend criteria for classification of cTn methods and for reporting hs-cTn in nanograms/L and non-high sensitivity assays in micrograms/L (8). The majority of laboratories (338) correctly characterized the methodology they were using, but 17/356 (4.7%) were using an assay that did not meet high sensitivity criteria but reported the assay they were using as high sensitivity (no response 1). Of the 356 laboratories measuring cTn, hs-cTn was measured in 283, 69.6% (197/283) reported in ng/L and 14.5% pg/mL. 9.5% reported in ng/mL and 4.9% µg/L. 1.4% of laboratories reported both units. In the 52 laboratories not utilizing high sensitivity assays, 48.1% (25/52) reported in ng/L with 26.9% in ng/mL and 13.5% in µg/L. 5.9% (21/356) did not respond.
Diagnostic Cut-Offs
There has been a significant shift in the choice of the 99th percentile as the upper reference limit (URL) for acute myocardial infarction (AMI). 71.9% (256/356) utilize the 99th percentile although 8.1% reported not using any decision limit, and no data were obtained in 9.8% (Supplemental Fig. 3). This is significantly different from the previous surveys with the choice of the 99th percentile now dominant (Fig. 4).
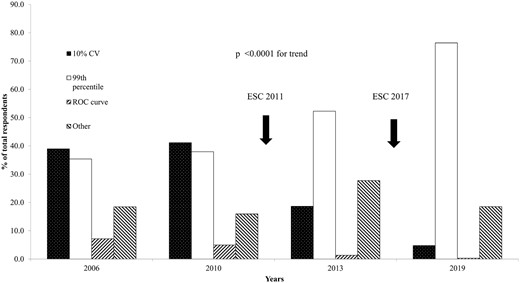
Percentage utilization of decision limits for acute myocardial infarction. Changes to the guidelines of the European Society of Cardiology (ESC) are indicated.
A sex-specific URL was used in only 23% (68/300) of laboratories utilizing hs-cTn assays (10% nonresponders). In addition to the 99th percentile being the dominant discriminant for AMI, there has been a switch to using the correct numeric value. For hs-cTnT, 74.4% now use the recommended value of 14 ng/L, a significant improvement from 2013 (50.2%, P = 0.0001).
For laboratories measuring cTn (n = 356), decision to use the AMI cut-off and choice of cut- off was again dominated by the recommendations of the instruction for use (IFU), the package inset from the manufacturer. This confirmed the trend seen in the past 3 surveys and was statistically significant (P < 0.0001, Supplemental Fig. 4). The choice of AMI cut-off was derived from the IFU in 56.7% (202/356) of responses and from expert recommendations or derived from the literature in 28.7% (nonresponse rate 14.6%). However, when the IFU was used as the source of data for selection of AMI cut-off (202), the 99th percentile was utilized in 85% (172/202), 5% continued to use the 10% CV, with the other 10% using a variety of cut-offs. More interestingly, the reason for selecting the 99th percentile as AMI cut-off (n = 256) was not the IFU alone. The IFU was the prime reason for selecting the 99th percentile in only 67.2% (172/256) of respondents with 28.5% citing clinical or professional reasons (no response in 4.3%).
Analysis of alternative clinical use, diagnostic algorithms, sample timings, interpretive comments, and demand management was confined to hospital laboratories measuring cardiac cTn only (314). 45.5% use cTn for other clinical diagnostic purposes than diagnosis of ACS of which the majority was monitoring after cardiac revascularization procedures (Supplemental Fig. 5). Only 1.9% reported using a different diagnostic discriminant when cTn was used for these purposes.
Testing Protocols
48.7% of laboratories used serial testing, with 7.6% using admission rule out plus serial testing up to 3 h and 10.8% the ESC 0/1 h rule out algorithm. 32.5% did not specify a particular testing strategy. Similar results were seen if hs-cTn assays only were considered. Serial testing (n = 153) was most commonly 0–3 h sampling (58.8%, 90/153), followed by 0–6 h sampling (15.7%). Taking all the available data from which sample timings could be extracted (n = 174), diagnosis was expected to be complete by 3 h in 68.0% or 6 h in 21.7% with other strategies in use in only 10.3%. 24% of laboratories provided interpretation as a computer-generated comment and 4% calculated the delta (although absolute or relative was not specified) but the remainder did not indicate that any additional information was provided. 55.4% of respondents documented a derivation of their diagnostic algorithm, but 45.6% did not. The largest single source of derivation of a diagnostic algorithm was the ESC (25.5%). Although laboratories use admission testing plus 0–3 h serial testing or 0–3 h serial testing (n = 104), this strategy was derived from ESC recommendations in only 34.6%.
Of all the hospital laboratories, 66% indicated that they have a procedure in place for regular review of the diagnostic pathway for suspected ACS patients. 46.1% reviewed on an annual basis and in total 84.5% reviewed within 3 years. 38.9% of respondents said test ordering was determined by clinician preference alone. However, 41.1% used structured ordering either an order set plus additional clinician ordering (22.6%), a timed set alone (1.0%), or a combination of a timed order set plus single orders (17.5%). 14.6% of respondents provided no data. Only 3.5% of participants utilized a demand management strategy for cTn testing by limiting the number of cTn orders. No data were available on the average number or timing of cTn orders per patient.
Internal and External Quality Assessment
Overall, of those laboratories who measured cTn (n = 356), 81.2% indicated they used internal quality control, 2.5% that they did not, and 16.3% provided no data. The most frequently used control was around the 99th percentile (66.8%). Distribution of the controls are shown in Supplemental Fig. 6. Only a minority of laboratories (1.7%) used a control close to the limit of detection of the assay. This was confined to hospital laboratories only. 75.3% participated in external quality assessment (proficiency testing) for cTn, 17.7% did not provide any data, and 7% did not participate.
Discussion
There are 6 main findings from this study. First, the measurement of cTn is now the preferred cardiac biomarker and is provided by nearly all laboratories. The only reason for nonprovision was financial. Second, CK, CK-MB, and myoglobin continue to be measured with little change from 2013, with AST and LD no longer measured. Third, there has been widespread adoption of high sensitivity-cTn measurement. Fourth, there has been widespread adoption of the 99th percentile URL as the diagnostic discriminant for AMI, with the main source of information the manufacturers IFU. Fifth, although there is a shift towards use of rapid diagnostic algorithms, they are not yet in use by the majority of hospitals. Sixth, the use of structured ordering protocols occurred in less than 50% although documentation and regular formal review of the diagnostic protocol in use was reported by the majority, but not all, hospitals.
The continued use of CK, CK-MB, and myoglobin is similar to that seen in the previous survey (4). There are several possible reasons. Some respondents may have indicated that they retain these tests as part of a generalized test menu rather than specifically for cardiac disease. This would be consistent with the majority of laboratories reporting that cTn was the first line test. Continued measurement of CK is likely for detection of muscle pathology as it has a defined clinical role and might be combined with cTn measurement for differential diagnosis between cardiac and skeletal muscle injury. As such, it might be considered by laboratories as part of the “cardiac biomarker panel.” Continued measurement of CK-MB and myoglobin may also be specifically used as part of a cardiac panel. However, it is difficult to justify the retention of myoglobin and CK-MB as “cardiac biomarkers.” The diagnostic sensitivity of myoglobin for early detection is superseded by conventional sensitive cTn (12) with hs-cTn assays offering superior diagnostic sensitivity. It is also difficult to justify continued measurement of CK-MB. The usual justifications given are the detection of reinfarction, although this is incorrect (13), and for monitoring after percutaneoous intervention (PCI). CK-MB is used as a marker in PCI trials. It is difficult to justify the retention of CK-MB measurement on financial grounds as cTn is diagnostically superior and now of comparable cost. Where information is available, the reason for retention of CK-MB is stated to be a clinician preference. It is to be hoped that recent recommendations from the ESC will see the retirement of CK-MB (14).
Measurement of cTn by a high sensitivity method is strongly encouraged, although not mandated in current guidelines but has increasingly been adopted. For some methods (cTnT), this has been largely due to the manufacturer replacing a pre-existing assay by a better version with withdrawal of the previous assay version. Manufacturers of cTnI have progressively introduced a high sensitivity version of that assay in response to the perceived clinical need and commercial pressures. The only exception is Roche Diagnostics who already had an hs-cTnT assay. Laboratories are increasingly adopting hs-cTnI, with some manufacturers actively encouraging this transition. Where point-of-care testing is used, where hs-cTn assays are not available, such a transition may be problematic, as was found in this survey, with the only option shifting to a laboratory-based assay.
In accordance with the universal definition, the 99th percentile is now the dominant decision limit for the diagnosis of MI. The main reason will be evolution of cTn assays. The shift from the Roche fourth-generation cTnT assay to the fifth-generation (high sensitivity assay or designated GEN 5 per the FDA in the USA) was accompanied by a significant improvement in assay sensitivity and imprecision. The conventional sensitive cTnI assays previously in use, for the most part, had analytical characteristics that supported the use of the 99th percentile as diagnostic discriminant. The major source of information on the choice of 99th percentile, as well as reference intervals comes from the manufacturers IFU. This trend is even more marked from the previous survey and highlights the need for independent validation of manufacturers claims, as has been discussed previously (3, 4).
The advantage of high sensitivity assays is earlier diagnosis of AMI and in particular the ability to rule out in appropriately selected patients on a single measurement performed on admission to hospital. The clinical utility of rapid diagnosis based on hs-cTn assays is supported by an evidence-based review (15) by the National Institute of Health and Care Excellence (NICE), recently updated with a review of the most recent literature (16, 17). Only a minority of laboratories are using very rapid rule-out algorithms, the majority utilizing 0–3 h or 0–6 h strategies. It is possible that the respondents did not understand the question as phrased or that the diagnostic protocols in use in the emergency department are not agreed with or involve the laboratory. But the results are in agreement with another survey on utilization of rapid diagnosis (18) and the experience of a number of the authors on discussing this subject with clinical colleagues. A possible obstacle is that patients with possible unstable angina pectoris (UAP) still need urgent (within days) diagnosis and follow-up. Hospitals implementing the rapid rule-out algorithms for NSTEMI therefore need to establish an out-patient clinical pathway for rapid follow-up of patients ruled-out for NSTEMI who might have UAP.
The survey continued to highlight deficiencies in communication between the laboratory and clinician users. Some laboratories were unaware whether their hospital offered angiography, a fundamental part of modern clinical cardiology and predicated by cTn measurement. Although there is documentation and review of a diagnostic protocol, there is a lack of structured ordering and demand management. This will contribute to the substantial inappropriate over requesting of cTn testing, degrading diagnostic efficiency (19), and therefore wasting healthcare resources.
Study Limitations
The study was largely confined to European laboratories with variable uptake by country with the best response from northern European countries, although the project team aimed to achieve as wide a geographical representation as possible. The responses were consistent so the conclusions are likely to be valid, but will have been modulated by individual understanding of the questions as formulated. As English is the language of the EFLM, the questionnaire was not translated which might also be a limitation.
In conclusion, the measurement of cTn by high sensitivity methods is now the predominant methodology in use, certainly throughout northern Europe. The adoption of the main benefits of these assays, rapid diagnostic algorithms, appears to lag behind adoption of the methodology itself. There is clearly a need for closer interaction between laboratory and clinical staff to achieve the potential benefits.
Supplemental Material
Supplemental material is available at Clinical Chemistry online.
Author Contributions: All authors confirmed they have contributed to the intellectual content of this paper and have met the following 4 requirements: (a) significant contributions to the conception and design, acquisition of data, or analysis and interpretation of data; (b) drafting or revising the article for intellectual content; (c) final approval of the published article; and (d) agreement to be accountable for all aspects of the article thus ensuring that questions related to the accuracy or integrity of any part of the article are appropriately investigated and resolved.
P.O. Collinson, statistical analysis, administrative support; J. Suvisaari, statistical analysis.
Authors’ Disclosures or Potential Conflicts of Interest: Upon manuscript submission, all authors completed the author disclosure form. Disclosures and/or potential conflicts of interest:
Employment or Leadership: P.O. Collinson, The Journal of Applied Laboratory Medicine, AACC; C. Duff, University Hospitals of North Midlands NHS Trust, Keele University, Association for Clinical Biochemistry and Laboratory Medicine (ACB); F.S. Apple, Clinical Chemistry, AACC.
Consultant or Advisory Role: K.M. Aakre, Roche Diagnostics; F.S. Apple, Instrumentation Laboratory, Siemens, Lumiradx, Qorvo.
Stock Ownership: None declared.
Honoraria: S.D.J. Stankovic, Abbott, Roche; F.S. Apple, Siemens Healthcare, Abbott Diagnostics.
Research Funding: None declared.
Expert Testimony: None declared.
Patents: None declared.
Other Remuneration: K.M. Aakre, personal fees from Siemens Healthineers; F.S. Apple, HyTest.
Role of Sponsor: No sponsor was declared.
References
Diagnostic Guidance 15 [DG15] Diagnostics Assessment Committee National Institute for Health and Care Excellence. Myocardial infarction (acute): early rule out using high-sensitivity troponin tests (Elecsys Troponin T high-sensitive, ARCHITECT STAT High Sensitive Troponin-I and AccuTnI+3 assays). NICE. 1-10-2014. Doi: 10.1093/eurheartj/ehaa1079.
Diagnostic Guidance 40 [DG40] Diagnostics Assesment Committee National Institute for Health and Care Excellence. High-sensitivity troponin tests for the early rule out of NSTEMI. NICE. 26-8-2020. https://www.nice.org.uk/guidance/dg40/resources/highsensitivity-troponin-tests-for-the-early-rule-out-of-nstemi-pdf-1053804469957 (Accessed April 6, 2021).
Nonstandard Abbreviations:
- CAMARGUE
CARdiac MARker Guideline Uptake in Europe
- EFLM TG-CM
European Federation of Laboratory Medicine cardiac markers task group
- cTn
cardiac troponin
- hs-cTn
high sensitivity cTn
- IFCC
International Federation of Clinical Chemistry and Laboratory Medicine
- IFCC C-CB
IFCC clinical applications of cardiac biomarkers
- ESC
European Society of Cardiology
- NSTEMI
non-ST elevation myocardial infarction
- CGI
computer-generated imagery
- AST
aspartate transaminase
- LD
lactate dehydrogenase
- CK
creatine kinase
- ISO
International Organization for Standardization
- ACS
acute coronary syndromes
- POC
point-of-care
- hs-cTnT
high sensitivity cardiac troponin T
- hs-cTnI
high sensitivity cardiac troponin I
- IFU
instruction for use
- URL
upper reference limit
- PCI
percutaneous invention
- NICE
National Institute of Health and Care Excellence
- UAP
unstable angina pectoris



