-
PDF
- Split View
-
Views
-
Cite
Cite
Tarekegn Geberhiwot, Alan F Jones, William A Bartlett, David Husband, Steven C Martin, Laboratory-based Calculation of Coronary Heart Disease Risk, Clinical Chemistry, Volume 47, Issue 3, 1 March 2001, Pages 589–591, https://doi.org/10.1093/clinchem/47.3.589
Close - Share Icon Share
Guidelines for the use of cholesterol-lowering drugs in the primary prevention of coronary heart disease (CHD) now recommend that both the serum cholesterol concentration of the patient and an estimate of future risk of CHD for the patient are considered before treatment is initiated. CHD risk (CHDR) is judged semiquantitatively in the National Cholesterol Education Program guidelines by counting the number of additional nonlipid risk factors (1). In guidelines in the United Kingdom (2), Europe (3), and New Zealand (4), risk is projected in a quantitative manner using a predictive equation derived from the Framingham Heart Study (5). This uses eight weighted risk factors (age, sex, systolic or diastolic blood pressure, total and HDL-cholesterol, and the presence or absence of cigarette smoking, diabetes mellitus, and left ventricular hypertrophy) to calculate an absolute CHDR. The Framingham formula is mathematically complex, and for routine clinical practice, it has been converted into several risk tables (2)(3)(4)(6) that may be inaccurate (7). We previously developed a Framingham-based CHDR calculation system using the laboratory computer (8) and now report our experience of the first 2 years of this program.
The CHDR calculation system was made available to physicians based in Birmingham Heartlands Hospital, a 1300-bed teaching institution, and to all of the 252 primary care physicians working in community-based practices served by the hospital laboratory. Patients were selected for CHDR assessment at the discretion of their own physician. We programmed the laboratory information system software (LIS; TelePath) to use the Framingham CHDR formula. The TelePath LIS is a modular data management system that primarily comprises the operating modes of test ordering and result receipt. In the ordering mode, tests are requested by name. A CHDR request requires that the nonlipid risk factor data, supplied by the physician on a self-adhesive label (Fig. 1 ) attached to the standard pathology request form, be entered into the LIS by reception staff. A nonfasting blood sample is used for measurements of total and HDL-cholesterol. Total serum cholesterol was assayed by the cholesterol esterase/oxidase method (Randox) and HDL-cholesterol by a “direct” method based on the specific solubilization of HDL-cholesterol (Bio-Stat Diagnostics). Assays were performed on a Dax 72 analyzer (Bayer). Between-batch CVs were 2.2% and 4.0% for total cholesterol (mean concentration, 5.34 mmol/L) and HDL-cholesterol (mean concentration, 1.06 mmol/L), respectively. Using the clinical risk factor data and the lipid measurements, the LIS calculates the projected CHDR of an individual over 1, 5, and 10 years, although only the latter is reported. Current United Kingdom guidelines recommend cholesterol-lowering drugs for those with a projected 10-year CHDR of ≥30%.
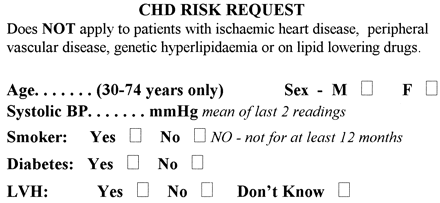
Laboratory CHDR assessment request label.
The clinician completes the details of the patient on a self-adhesive label attached to the clinical chemistry request form. This is sent to the laboratory with a nonfasting blood sample for lipid measurement and CHDR calculation. BP, blood pressure; LVH, left ventricular hypertrophy.
Between December 1997 and March 2000, we received 8334 CHDR assessment requests: 6074 (72.8%) from hospital physicians and the remainder from primary care physicians. The clinical and laboratory characteristics of the patients for whom CHDR requests were received, together with their projected CHDRs are shown in Table 1 . The ethnic distribution (data not shown) was consistent with the demographics of the local population (67.0% were Caucasian, 26.7% were South Asian, 4.6% were Afro-Caribbean, and 1.7% were of other ethnic groups). Nearly 90% of the hospital requests came from the six consultant physicians in diabetology, although 62 of the 160 hospital consultants (32%) sent at least one request to the laboratory. Of the 252 primary care physicians, 55 (21%) submitted CHDR requests. The total number of CHDR requests from hospital physicians was similar in the first and second years of the project (2715 in the first year vs 2552 in the second year), but the number from primary care increased substantially (704 in the first year vs 1169 in the second year). The Framingham risk equation could be applied to 7328 (87.9%) of the requests with complete data, and of the 1006 incomplete requests, 854 (84.9%) were sent by hospital doctors and 152 (15.1%) by primary care physicians (P <0.0001, χ2 test). The blood pressure recording was missing from 606 hospital forms and 112 primary care forms, smoking history was absent from 372 hospital requests and 47 primary care requests, and diabetes history was absent from 345 hospital requests and 33 primary care requests (P <0.0001, χ2 test for all between-group comparisons).
Clinical and laboratory characteristics1 of CHDR requests received from hospital and primary care physicians.
| CHDR requests . | Male . | Female . |
|---|---|---|
| Hospital | ||
| Number | 3289 | 2771 |
| Age, years | 55.4 (12.4) | 56.4 (12.2) |
| Systolic blood pressure, mmHg | 142.8 (20.8) | 145.7 (22.1) |
| Cholesterol, mmol/L | 5.06 (1.34) | 5.29 (1.43) |
| HDL-cholesterol, mmol/L | 1.30 (1.02) | 1.46 (1.04) |
| Diabetes mellitus, % | 88.9 | 87.7 |
| Smoker, % | 25.4 | 15.6 |
| Cholesterol ≥6.5 mmol/L, % | 11.0 | 16.1 |
| Systolic blood pressure ≥160 mmHg, % | 20.7 | 25.8 |
| 10-year CHDR | 19 (9–28) | 15 (7–22) |
| Percentage with 10-year CHDR ≥30% | 17.9 | 8.7 |
| Primary care | ||
| Number | 1283 | 975 |
| Age, years | 54.3 (11.4) | 55.7 (11.2) |
| Systolic blood pressure, mmHg | 144.5 (20.8) | 146.9 (22.3) |
| Cholesterol, mmol/L | 5.64 (1.33) | 5.92 (1.47) |
| HDL-cholesterol, mmol/L | 1.28 (0.80) | 1.53 (0.84) |
| Diabetes mellitus, % | 20.0 | 17.0 |
| Smoker, % | 26.6 | 19.7 |
| Cholesterol ≥6.5 mmol/L, % | 24.5 | 33.5 |
| Systolic blood pressure ≥160 mmHg, % | 24.2 | 30.0 |
| 10-year CHDR | 15 (8–24) | 10 (5–16) |
| Percentage with 10-year CHDR ≥30% | 11.1 | 2.8 |
| CHDR requests . | Male . | Female . |
|---|---|---|
| Hospital | ||
| Number | 3289 | 2771 |
| Age, years | 55.4 (12.4) | 56.4 (12.2) |
| Systolic blood pressure, mmHg | 142.8 (20.8) | 145.7 (22.1) |
| Cholesterol, mmol/L | 5.06 (1.34) | 5.29 (1.43) |
| HDL-cholesterol, mmol/L | 1.30 (1.02) | 1.46 (1.04) |
| Diabetes mellitus, % | 88.9 | 87.7 |
| Smoker, % | 25.4 | 15.6 |
| Cholesterol ≥6.5 mmol/L, % | 11.0 | 16.1 |
| Systolic blood pressure ≥160 mmHg, % | 20.7 | 25.8 |
| 10-year CHDR | 19 (9–28) | 15 (7–22) |
| Percentage with 10-year CHDR ≥30% | 17.9 | 8.7 |
| Primary care | ||
| Number | 1283 | 975 |
| Age, years | 54.3 (11.4) | 55.7 (11.2) |
| Systolic blood pressure, mmHg | 144.5 (20.8) | 146.9 (22.3) |
| Cholesterol, mmol/L | 5.64 (1.33) | 5.92 (1.47) |
| HDL-cholesterol, mmol/L | 1.28 (0.80) | 1.53 (0.84) |
| Diabetes mellitus, % | 20.0 | 17.0 |
| Smoker, % | 26.6 | 19.7 |
| Cholesterol ≥6.5 mmol/L, % | 24.5 | 33.5 |
| Systolic blood pressure ≥160 mmHg, % | 24.2 | 30.0 |
| 10-year CHDR | 15 (8–24) | 10 (5–16) |
| Percentage with 10-year CHDR ≥30% | 11.1 | 2.8 |
Values are mean (SD) except for 10-year CDHR, which is given as median (interquartile range).
Clinical and laboratory characteristics1 of CHDR requests received from hospital and primary care physicians.
| CHDR requests . | Male . | Female . |
|---|---|---|
| Hospital | ||
| Number | 3289 | 2771 |
| Age, years | 55.4 (12.4) | 56.4 (12.2) |
| Systolic blood pressure, mmHg | 142.8 (20.8) | 145.7 (22.1) |
| Cholesterol, mmol/L | 5.06 (1.34) | 5.29 (1.43) |
| HDL-cholesterol, mmol/L | 1.30 (1.02) | 1.46 (1.04) |
| Diabetes mellitus, % | 88.9 | 87.7 |
| Smoker, % | 25.4 | 15.6 |
| Cholesterol ≥6.5 mmol/L, % | 11.0 | 16.1 |
| Systolic blood pressure ≥160 mmHg, % | 20.7 | 25.8 |
| 10-year CHDR | 19 (9–28) | 15 (7–22) |
| Percentage with 10-year CHDR ≥30% | 17.9 | 8.7 |
| Primary care | ||
| Number | 1283 | 975 |
| Age, years | 54.3 (11.4) | 55.7 (11.2) |
| Systolic blood pressure, mmHg | 144.5 (20.8) | 146.9 (22.3) |
| Cholesterol, mmol/L | 5.64 (1.33) | 5.92 (1.47) |
| HDL-cholesterol, mmol/L | 1.28 (0.80) | 1.53 (0.84) |
| Diabetes mellitus, % | 20.0 | 17.0 |
| Smoker, % | 26.6 | 19.7 |
| Cholesterol ≥6.5 mmol/L, % | 24.5 | 33.5 |
| Systolic blood pressure ≥160 mmHg, % | 24.2 | 30.0 |
| 10-year CHDR | 15 (8–24) | 10 (5–16) |
| Percentage with 10-year CHDR ≥30% | 11.1 | 2.8 |
| CHDR requests . | Male . | Female . |
|---|---|---|
| Hospital | ||
| Number | 3289 | 2771 |
| Age, years | 55.4 (12.4) | 56.4 (12.2) |
| Systolic blood pressure, mmHg | 142.8 (20.8) | 145.7 (22.1) |
| Cholesterol, mmol/L | 5.06 (1.34) | 5.29 (1.43) |
| HDL-cholesterol, mmol/L | 1.30 (1.02) | 1.46 (1.04) |
| Diabetes mellitus, % | 88.9 | 87.7 |
| Smoker, % | 25.4 | 15.6 |
| Cholesterol ≥6.5 mmol/L, % | 11.0 | 16.1 |
| Systolic blood pressure ≥160 mmHg, % | 20.7 | 25.8 |
| 10-year CHDR | 19 (9–28) | 15 (7–22) |
| Percentage with 10-year CHDR ≥30% | 17.9 | 8.7 |
| Primary care | ||
| Number | 1283 | 975 |
| Age, years | 54.3 (11.4) | 55.7 (11.2) |
| Systolic blood pressure, mmHg | 144.5 (20.8) | 146.9 (22.3) |
| Cholesterol, mmol/L | 5.64 (1.33) | 5.92 (1.47) |
| HDL-cholesterol, mmol/L | 1.28 (0.80) | 1.53 (0.84) |
| Diabetes mellitus, % | 20.0 | 17.0 |
| Smoker, % | 26.6 | 19.7 |
| Cholesterol ≥6.5 mmol/L, % | 24.5 | 33.5 |
| Systolic blood pressure ≥160 mmHg, % | 24.2 | 30.0 |
| 10-year CHDR | 15 (8–24) | 10 (5–16) |
| Percentage with 10-year CHDR ≥30% | 11.1 | 2.8 |
Values are mean (SD) except for 10-year CDHR, which is given as median (interquartile range).
Replicate CHDR requests were received on 1307 patients, 1239 (94.8%) of whom attended the hospital diabetes clinics. Two requests were received on 1307 patients, three requests were received on 173 patients, and four requests were received on 16. The mean (SD) interval between replicate requests was 376 (146) days. The CV for the within-subject 10-year CHDR calculation was 19%, and this was not significantly related to the degree of projected risk. Within-subject CVs for systolic blood pressure, total cholesterol, and HDL-cholesterol were 7.1%, 7.7%, and 12%, respectively. The apparent clinical precision of a projected quantitative risk is misleading. If the method of Fraser and Harris (9) is used, five replicate CHDR requests would be required to allow one to be 95% confident that a projected 10-year CHDR of 30% lay within the interval of 25–35%.
Management of hypercholesterolemia has traditionally been governed by the relative CHDR of the patient, using a specific serum cholesterol concentration to determine the need for treatment. Absolute CHDR is of greater importance in assessing the potential clinical benefit from drug therapy and is supplanting relative risk as the basis for treatment. However, a method for CHDR assessment suitable for routine use must be identified. Risk assessment using a qualitative or semiquantitative approach is, not surprisingly, less accurate than using a quantitative method (10). The Framingham CHDR equation has been the most widely used for quantitative risk assessment, but nonetheless it does not account for many risk factors that have become established since the inception of the Framingham study. Furthermore, the Framingham CHDR equation may not apply to all ethnic groups. Nevertheless, the equation appears to represent the best available method and, as such, has been endorsed by most management guidelines.
The Framingham equation is of sufficient mathematical complexity to require the use of a computer; therefore, several authors have constructed risk tables or nomograms based on the equation to avoid formal CHDR calculation (2)(3)(4)(6). These formats inevitably require some simplification of the original equation, and estimated risks may not agree with those derived by calculation (7). Moreover, the risk tables and nomograms may not be easy to use (11). We have argued that direct calculation would be preferable to avoid errors introduced by risk tables (12). Several Framingham-based software programs for personal computers are now available (13)(14), but the clinician must personally enter eight pieces of data, and this may become a disincentive to the use of the software programs.
We have now shown that the laboratory mainframe computer can be used to calculate Framingham risk projections and that this system is practical in routine clinical and laboratory use. At the outset, we were concerned that the quality and completeness of clinical information provided on the request form would preclude risk projections in a substantial number of cases. However, <15% of requests were incomplete, and primary care physicians recorded clinical data much better. The acquisition of the system by diabetes clinics has been good: request numbers indicate that ∼60% of the patients are being tested, which is consistent with our expectations of the proportion of patients eligible for primary prevention of CHD. However, only a minority of primary care practices used the method, although we have seen a large increase during the second year of the project. We feel that this may reflect the relatively low priority given to primary CHD prevention and, in particular, the infrequent use of cholesterol-lowering drugs in the United Kingdom. In March 2000, the United Kingdom Department of Health published its National Service Framework for CHD (15), which now mandates risk assessment for primary prevention. Because our evaluation preceded this national guidance, it is likely that CHDR requests from primary care physicians will increase in the future.
The software modification is now available to all users of TelePath. Because this company has the major share of the United Kingdom pathology software market, the system has the potential to be used widely and has already been adopted by several other laboratories. We believe that LIS software from other suppliers could be readily adapted to incorporate the Framingham CHDR equation, and therefore, the method should be capable of general application. Changing the clinical approach toward the use of drugs in the primary prevention of CHD is unlikely to be a simple matter. We believe that obtaining CHDR projections for individual patients in a straightforward manner is of central importance. A laboratory-based CHDR assessment system is feasible, has been adopted by many physicians, and could be used widely.
References
. Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults. Summary of the second report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel II).
. British Cardiac Society, British Hyperlipidaemia Association, British Hypertension Society. Joint British recommendations on prevention of coronary disease in clinical practice.
Wood D, De Backer G, Faergeman O, Graham I, Mancia G, Pyorala K. Prevention of coronary heart disease in clinical practice: recommendations of the Second Joint Task Force of European and other Societies on Coronary Prevention.
. National Heart Foundation. Clinical guidelines for the assessment and management of dyslipidaemia.
Anderson KM, Odell PM, Wilson PWF, Kannel WB. Cardiovascular disease risk profiles.
Ramsay LE, Haq IU, Jackson PR, Yeo WW, Pickin DM, Payne JN. Targeting lipid-lowering drug therapy for primary prevention of coronary disease: an updated Sheffield table.
Jones AF, Walker J, Jewkes C, Game FL, Bartlett WA, Marshall T, Bayly GR. Comparative accuracy of cardiovascular risk prediction methods in general practice patients.
Bayly GR, Bartlett WA, Davies PH, Husband D, Haddon A, Game FL, Jones AF. Laboratory-based calculation of coronary heart disease risk in a hospital diabetic clinic.
Fraser CG, Harris EK. Generation and application of data on biological variation in clinical chemistry.
Durrington PN, Prais H, Bhatnagar D, France M, Crowley V, Khan J, Morgan J. Indication for cholesterol-lowering medication: comparison of risk-assessment method.
Isles CG, Ritchie LD, Murchie P, Norrie J. Risk assessment in primary prevention of coronary heart disease: randomized comparison of three scoring methods.
Jones AF. Statins and hypercholestrolaemia: UK Standing Medical Advisory Committee Guidelines.
Hingorani AD, Vallance P. A simple computer program for guiding management of cardiovascular risk factors and prescribing.



